Abstract
The terrestrial environment of the High Arctic consists of a mosaic of habitat types. In addition to the natural habitat diversity, various human-influenced types may occur. For the resident invertebrate fauna, these anthropogenic habitats may be either unusually favourable or detrimental. In the town of Barentsburg, Svalbard, soils were imported for the greenhouses from southern Russia. These soils were subsequently discarded outside the greenhouses and have become augmented with manure from the cowsheds. Both the greenhouse and the cowsheds are now derelict. This site represents an unusually nutrient-rich location with considerable development of organic soils, in stark contrast to the naturally forming organic soils in Svalbard, which are typically thin and nutrient poor. Few previous studies have examined the soil invertebrate communities of human-disturbed or -created habitats in the Arctic. In an often nutrient-poor terrestrial environment, it is unclear how the invertebrate fauna will react to such nutrient enhancement. In these soils, 46 species of invertebrates were determined. Eleven species have not been recorded from other habitats in Svalbard and are hence likely to have been introduced. The native species assemblage in the anthropogenic soils was not atypical for many natural sites in Svalbard. Despite the enriched organic soils and highly ameliorated winter temperature conditions, the soil invertebrate fauna biodiversity does not appear to be enhanced beyond the presence of certain probably introduced species.
The tundra environment of the Arctic is diverse, ranging from sedge–moss mires, grass–moss low shrub and cushion plants/cryptograms to polar deserts with extremely low plant cover (Rønning Citation1979; Bliss & Matveyeva Citation1992; Cooper Citation2011). This vegetation is often extremely patchy, comprising a mosaic of habitat types which often vary over a scale of a few metres (Jónsdóttir Citation2005). The heterogeneity is reflected in the soil microarthropod fauna (Coulson et al. Citation2003), where clear relationships can often be observed between vegetation cover and invertebrate species diversity. In a few locations, this habitat diversity is enhanced by human activities in the settlements or at industrial sites, often resulting from mineral extraction. One such habitat is associated with the anthropogenic soils beneath the abandoned cowsheds in the Russian mining town of Barentsburg in Svalbard.
The principal islands of the Svalbard Archipelago lie in the European High Arctic. Most of the ice-free regions are along the coastal margins and are composed of either polar desert or tundra heath vegetation (Jónsdóttir Citation2005). Invertebrate studies in Svalbard commenced at the start of the 20th century and there are to date close to 600 articles describing the terrestrial and freshwater invertebrate fauna of Svalbard, yet our understanding of the system is still patchy (Coulson Citation2007Coulson Citation2013). Most articles focus on the same two regions, the vicinities of Ny-Ålesund and Longyearbyen, and hence the inventory of the archipelago is far from complete. Furthermore, there is considerable taxonomic confusion in some groups. For example, the inventories of Coulson & Refseth (2004) and Coulson (2007) cite 18 species of gamasid mite. This has recently been revised to 22 based on freshly collected material and recent publications (Ávila-Jiménez et al. Citation2011) but subsequently three additional species have been identified (Gwiazdowicz et al. Citation2012; Coulson et al. Citation2013) bringing the total recorded to 25.
The natural colonization of islands is an accidental process and depends on numerous factors such as size, altitude, age, climate of the island, distance to the source area, possible stepping stones, currents, wind, dispersal ability of an organism, preadaptations, time and location of arrival, suitable environmental conditions such as vegetation, food or predators. The agents which are normally responsible for the geographical distribution of larger animals or plants are not necessarily the same for small organisms (Jacot Citation1934). The currently observed flora and fauna of the Svalbard Archipelago are generally thought to be the result of recent immigration during the 10 000 years subsequent to the retreat of the ice following the last glacial maximum (Brochmann et al. Citation2003; Alsos et al. Citation2007; Ávila-Jiménez & Coulson Citation2011). The fauna therefore consists largely of Holarctic species but includes a number of possible endemics such as the aphid Acyrthosiphon svalbardicum Heikinheimo 1968 or the gamasid mite Amblyseius magnanalis (Thor 1930). However, such apparent endemism may be a reflection of a lack of sampling effort in other Arctic and northern boreal regions.
In some restricted localities, for example, under bird cliffs, deep ornithogenic soils may accumulate (Klekowski & Opalinski Citation1986; Zmudczynska et al. Citation2012), sites that are also often botanically diverse (Odasz Citation1994; Zmudczynska et al. Citation2009). In areas around the settlements, humans may also have created similar suitable rich organic soils. One example is adjacent to the abandoned cowsheds in the Russian mining town of Barentsburg, Grønfjord. Chernozem-type soil was imported for the greenhouses some time around 1980. Chernozem soils are distributed in forest steppe and steppe zones. They contain a high humus content and are agriculturally fertile soils. The precise origin is unclear but the soil is most likely to have been sourced from the southern European parts of Russia. These soils were subsequently discarded outside the greenhouses and have since been augmented by mature manure from the cow- and pig-sheds to create organic soils up to several metres deep lying along a westerly facing slope on the outskirts and below the town. These soils create an unusually rich and deep organic substrate for plants and soil-dwelling invertebrates within the typically nutrient-poor tundra environment of the archipelago. The plant community at this location includes 44 non-native taxa (Liška & Soldán Citation2004). In total, there are approximately 100 species of alien vascular plant recorded in Svalbard and alien species represent more than 30% of the total vascular plant species recorded for the archipelago (Inger Alsos, pers. comm.), although whether these alien species have naturalized or spread beyond the locale of human habitation is often unknown. However, apart from Coulson et al. (2013), there is no information about the invertebrate fauna, native or introduced, of these anthropogenic soils. We therefore surveyed the invertebrate fauna of these anthropogenic soils during summer 2011 to describe the community present.
Materials and methods
The principal islands of the Svalbard Archipelago lie in the European Arctic between 74 and 81°N and 10 to 35°E (). The land area comprises 61 200 km2 with a maximum elevation of 1700 m (Hisdal Citation1985) and of which some 60% is under permanent snow and ice. Mean annual air temperature in Barentsburg is −6.1°C but monthly mean air temperatures are above 0°C between June and September, peaking in July at +5.5°C (Norwegian Meteorological Institute Citation2012; ). Mean annual precipitation is 525 mm, most of which falls during the winter as snow. The period of the midnight sun lasts 126 days, from 20 April until 23 August. Conversely, the duration of the polar night is 81 days, from 11 November to 30 January.
Fig. 1 The location of Svalbard and the positions of the two settlements of Barentsburg and Longyearbyen.
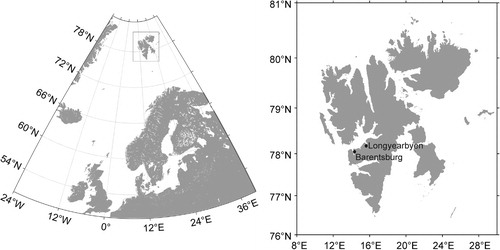
Fig. 2 Average monthly air temperature in Barentsburg (solid line), Longyearbyen (broken line) and precipitation in Barentsburg (bars) for the period 1961–1990.
A total of 15 10×10×5 cm-thick soil samples were collected from the bottom and sides of a gully formed in the organic soils accumulated down a westerly facing slope under the abandoned cowsheds in Barentsburg in Grønfjord (78° 04.3N, 014° 12.09 E; ) on 8 July 2011. The sampling site (, location A) can be readily identified by the comparatively lush green vegetation to the left of the derelict cowsheds. The soils formed layers several metres thick, created from a mixture of discarded greenhouse soil and manure from the animal houses. Some coal dust is also present. The flora here is rich in introduced species (Liška & Soldán Citation2004), including species, such as Achillea millefolium L. and Anthriscus sylvestris (L.) Hoffm. ().
It has proved difficult to determine with certainty the origin of the soils imported to Barentsburg. Despite the willingness to assist by the mining company in Barentsburg, Trust Arktikugol, records of the origin of the soils after over 30 years were not available. Liška & Soldán (2004) were similarly unable to determine precisely the source of the fodder for the animals as it was at that time considered to be of strategic importance by the then Soviet authorities. There was a network of suppliers of soil for greenhouses in the former USSR. The Murmansk Company, Cvety Zapolyarya, bought the soils from various companies in the Moscow and Leningrad regions, Republic of Udmurtia. It is known that the soils were imported from the central or southern European parts of Russia in the 1980s. After approximately 30 years, it has proved impossible to obtain more precise information as to the precise geographical source of the soils.
Annelida extraction
Lumbricidae were extracted from the soil on-site by the conventional formalin method (Raw Citation1959; Satchell Citation1969). Formalin solution (0.2%) was poured over two 50×50 cm plots with two applications each. Emerging worms were immediately washed in water and placed in 96% ethanol. A total of 21 specimens were collected, the majority were juvenile and therefore could only be tentatively identified on the basis of morphological features. Five individuals were successfully DNA-barcoded at the University of Gothenburg. The mitochondrial COI gene was sequenced using standard protocols (see James et al. Citation2010) and the barcodes BLASTED against GenBank and compared to COI sequences of an extensive Scandinavian collection of Lumbricidae (Erséus, unpubl. data). Specimens are lodged at the Department of Biology and Environmental Sciences, University of Gothenburg, Sweden.
The Enchytraeidae were wet-extracted from three soil samples using the method of O'Connor (O'Connor Citation1955) at the University of A Coruña. Extraction time was 6 h and worms were identified to species in vivo. Specimens selected for morphological investigations and for the voucher collection were fixed in 70°C hot Bouin's fluid and preserved in 70% ethanol. Results presented here are based on identification in vivo. The voucher collection is in the personal collection of R.M. Schmelz (Department of Animal Biology, University of A Coruña, Spain). Additional specimens are deposited in the invertebrate collection at the University Centre in Svalbard (UNIS).
Microarthropod extraction
Twelve soil samples, 10×10 cm and 5 cm deep, were taken on 8 July 2011 and immediately placed into Tullgren soil extractors (Burkard Scientific Ltd., Uxbridge, UK) at the research station of the Kola Scientific Centre of Russian Academy of Sciences in Barentsburg. The microarthropod fauna was extracted from these samples into 96% alcohol for 4 days until the soil was completely dried out.
The Collembola and mites were identified to species by morphological taxonomic techniques. Specimens of the Collembola are deposited at the Department of Biology, UNIS, Longyearbyen, Svalbard. The identified mites are deposited at the Department of Forest Protection, Poznan University of Life Sciences, Poland and UNIS (Gamasida) and at the Ecological and Environmental Change Research Group, University of Bergen, UNIS and in the personal collection of L. Miko (Oppiidae in part).
Diptera extraction
Diptera larvae were extracted along with the Collembola and mites. Collected individuals were fixed in 96% ethanol. Mid-sections of each chironomid morphotype were dissected and the tissue was sent to the Canadian Centre for DNA Barcoding at the University of Guelph, Canada, for DNA extraction and sequencing of partial COI-genes using standard protocols. All data were entered in the Barcode of Life Data Systems (Ratnasingham & Hebert Citation2007) and compared with DNA barcodes from adult Diptera recorded from the Holarctic through other projects using genetic similarity and taxon identification-trees based on the neighbour joining method. Specimens are deposited at the Department of Natural History, University Museum, Norwegian University of Science and Technology. A corresponding procedure was used for the Sciaridae larvae. Identification was accomplished by comparing the DNA barcodes of adult male sciarids in the collection at the Zoology Museum in Oslo sampled previously from Svalbard with the DNA barcodes of the larvae from Barentsburg. The Sciaridae specimens are deposited in the Natural History Museum, University of Oslo, Norway.
Soil temperatures and climate
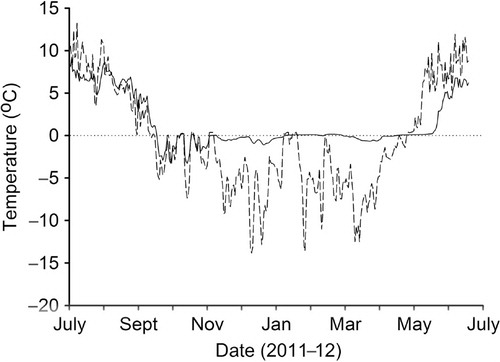
Soil temperatures were recorded at the cowshed soils under A. sylvestris (location A, ) and, to obtain representative temperatures from natural non-anthropogenic Svalbard soils, in the grass-dominated vegetation below the Barentsburg bird cliffs (location B, ) less than 1 km from the anthropogenic site. Two TGP-4020 Tinytag dataloggers (Gemini, Chichester, West Sussex, UK) fitted with PB-5009 external thermistor probes were deployed. Loggers recorded soil temperatures at a depth of approximately 1 cm every hour in the period 19 July 2011–14 July 2012. Mean daily soil temperatures were calculated as the average of the 24-h observations so as to agree with the calculation for the official mean daily air temperatures. Climate and daily air temperature data for Barentsburg and Longyearbyen were obtained from the Norwegian Meteorological Institute (Norwegian Meteorological Institute Citation2012). Daily mean air temperatures during the campaign were not available from Barentsburg. Comparison of the summer mean monthly air temperatures in Barentsburg and at Svalbard Airport, Longyearbyen, for the period 1961–1990 revealed only small differences between the locations (). Therefore, daily air temperatures from Longyearbyen were used to describe air temperatures in Barentsburg.
Results
The invertebrate fauna of the cowshed soils is presented in . In total, 46 species were recorded, 11 of which have not been recorded from other locations in Svalbard.
Table 1 The invertebrate community sampled from the cowshed soils. Species marked with an asterisk have not been recorded in Svalbard outside the anthropogenic soils in Barentsburg. The remaining species are generally widely distributed in Svalbard.
Eight species of Enchytraeidae were identified from the cowshed samples ().
The Lumbricidae community of the cowshed soils consisted of two species: Dendrodrilus rubidus (Savigny, 1826) sensu Sims & Gerard (Citation1985) and Dendrobaena hortensis (Michaelsen, 1890). In addition, the BLAST search gave a 99% COI match with two unpublished barcodes (GenBank accession numbers: HQ562578.1 and HQ562579.1). According to the collector, they originate from an Eisenia-like species collected from a compost bin in Curitiba, southern Brazil (Samuel W. James, unpubl. data).
The oribatid mite fauna consisted of eight species (). Five species of gamasid mite were collected. Only one individual spider was collected, a male Erigone arctica (White, 1852). Eighteen species of Collembola were present. The extracted Chironomidae larvae could be positively associated by barcodes to adult females of the genus Smittia, which have been collected in other on-going projects in the Arctic (Stur & Ekrem, unpubl. data). Two species were recognized, both previously known only as females from the Arctic. The larvae of the other species could be associated with females of Smittia sp. 2, presumably an undescribed species from Spitsbergen (Adventdalen) and Greenland. As with the Chironomidae, the extracted sciarid larvae could be associated by barcodes with adult individuals of the genus Lycoriella, subgenus Hemineurina. Despite some variation between the barcodes (approximately 1%), the originating cluster within the larvae is likely to represent one species. However, at present the studied adult individuals cannot be identified to species with certainty. Only one species of beetle was collected, the staphylinid Atheta graminicola (Gravenhorst, 1806).
In the natural soils, the temperature at 1 cm depth varied greatly and rapidly, especially during winter, with temperatures swinging from close to 0° to below -14°C on several occasions (). The cowshed soils tracked the temperature decline of the natural soils during the autumn but showed a slight delay in the rate of cooling and smaller diurnal fluctuations. During September and October, the cowshed soils reached an absolute minimum temperature of -3.3°C, after which temperatures increased and remained close to 0°C until late May (). There was a delay of some two weeks between the spring rise in temperature of the cowshed soils compared to the natural soils. Moreover, the cowshed soils remained cooler than the natural soils throughout the campaign period in the summer of 2012 and diurnal fluctuations were less marked.
Discussion
There is a gradually increasing knowledge of the soil invertebrate fauna of Svalbard, especially from locations on the west coast (Coulson Citation2007Coulson Citation2013). However, there have been no investigations of the invertebrate fauna inhabiting anthropogenic sites in the archipelago such as the cowshed soils in Barentsburg.
The enchytraeid species collected from the Barentsburg soils are all Holarctic or cosmopolitan. Two species, Enchytraeus dichaetus and Cognettia glandulosa, are not known from other locations in Svalbard and are considered to be recent human introductions with the imported soils (Coulson et al. Citation2013). In total, more than 30 species of enchytraeids have been recorded from all types of soil habitats in Svalbard (Nurminen Citation1965; Dózsa-Farkas Citation1999) and abundance can exceed 50 000 individuals per square metre under favourable conditions (Birkemoe Citation1995). Excluding the two introduced species, six species were identified, similar to species diversity at other locations around Svalbard (Dózsa-Farkas Citation1999; Birkemoe et al. Citation2000). There are some indications that enchytraeid diversity is positively related to plant diversity and the development of organic soil. Birkemoe & Dózsa-Farkas (Citation1994) observed the greatest enchytraeid diversity, 10 species, in wet moss with grasses, while only one species was present in samples consisting predominantly of detritus from black-legged kittiwake (Rissa tridactyla) nests or dominated by moss. The community observed in the Barentsburg soils does not show a unique or unusual assemblage of species compared to natural sites in Svalbard. This may be since the anthropogenic habitat was created too recently for colonization by enchytraeids from other locations in Svalbard. However, the soils are likely to be heavily contaminated with coal dust and also have reduced pH values. Such effects may be especially challenging for animals such as Lumbricidae or Enchytraeidae in contact with the soil solution (Frouza et al. Citation2011).
Both the lumbricids are only known from the Barentsburg soils (Coulson et al. Citation2013) and Lumbricidae otherwise appear to be largely absent from the natural environment in Svalbard (Coulson Citation2007Coulson Citation2012). The Lumbricidae recorded here are globally widespread, including with records from the Southern Hemisphere. While D. rubidus can be considered as resident in large parts of the Holarctic, D. hortensis has only been occasionally found in Scandinavia. Stöp-Bowits (Citation1969) recorded D. hortensis from a “heap of foliage manure” in the University Botanical Garden in Oslo and Julin (Citation1949) considered it (as “Eisenia veneta, var. hortensis”) an accidental guest in Sweden based on isolated records from gardens in the Swedish province of Uppland. Erséus (unpubl. data) recently collected and barcoded specimens of D. hortensis from Västergötland (south-western Sweden) and Gotland (an island in the Baltic Sea). The record from Gotland is a worm with the same COI haplotype as all the barcoded D. hortensis from Svalbard (Erséus, unpubl. data). Nurminen (1965) reported finding one immature lumbricid specimen in samples taken from clayey soils close to the river in Adventdalen (close to Longyearbyen) but the specimen was too badly damaged to be identifiable. Nurminen merely states that the arrangement of the setae prohibit the animal being Dendrobaena octaedra (Sav.) which in Finland can occur above the tree line. D. hortensis is widely distributed but not so often reported, which may be partly due to the fact that it has been erroneously identified as either Dendrobaena veneta or one of the various “compost worms” belonging to Eisenia. There is little doubt, however, that it is an anthropochorous species and that it is likely that it was recently introduced in Svalbard. Similarly, Rundgren (Citation2007) speculated that the lumbricid fauna of Iceland might be in part due to non-native species imported with ballast soils. D. rubidus on the other hand could, in some areas, be a natural component of the sub-Arctic and Arctic terrestrial fauna. For instance, it was recently found at 70°N in Vardø (eastern Finnmark, Norway), and appears to be common throughout northern mainland Norway (Erséus, unpubl. data). It is also abundant in greenhouses in northern settlements of north-east Russia (Yakutia, Magadan oblast and Chukotka). Up to 120 000 egg cocoons of D. rubidus tenuis per square metre of greenhouse soil were recorded by Berman et al. (Citation2010) in these areas. In winter, these greenhouses are not heated and the temperature may decline to below-40°C. Nonetheless, the cocoons in spring are viable, thereby demonstrating significant cold hardiness as seen in other species of earthworms (Holmstrup Citation2003).
Of the five species of gamasid mite collected, two species are not recorded elsewhere in Svalbard, Paragamasus insertus and Vulgarogamasus remberti (Ávila-Jiménez et al. Citation2011). The remaining three species Antennoseius oudemansi, Arctoseius haarlovi and Arctoseius multidentatus are widespread in Svalbard and recorded from a variety of habitats (Ávila-Jiménez et al. Citation2011; Gwiazdowicz & Coulson Citation2011) including Salix and Dryas tundra, Cassiope heaths and under bird cliffs. A. multidentatus is one of the most widespread gamasid mite species occurring at 10 of the 18 locations throughout Svalbard investigated by Ávila-Jiménez et al. (2011). A. oudemansi and A. haarlovi are likewise almost as prevalent, found at seven and five of the 18 locations, respectively. Excluding the two introduced species of gamasid mite (Coulson et al. Citation2013), the overall gamasid diversity observed in the cowshed soils is not very much different from other vegetation types in Svalbard (Ávila-Jiménez et al. Citation2011; Gwiazdowicz & Coulson Citation2011).
The eight species of observed oribatid observed are all previously known from Svalbard (Coulson & Refseth Citation2004; Bayartogtokh et al. Citation2011) with wide distributions throughout the Palaearctic or Holarctic (Bayartogtokh et al. Citation2011). The species number is very poor and the composition representing species with broad ecological tolerance and often present in diverse habitat types. It is most probable that the oribatid mites sampled have colonized the cowshed soils from the adjacent localities and are populations native to Svalbard. However, it is possible that some populations of Tectocepheus velatus, Oppiella nova, Liochthonius brevis, Liochthonius sellnicki, Oribatula tibialis and Oppiella neerlandica collected here may also have been imported to Svalbard together with the greenhouse soil as they occur in chernozem soils in the European part of Russia, where the soils are thought to have been sourced. The range of Diapterobates notatus is mainly in the Arctic–boreal zone (Melekhina Citation2011). Identification of the oribatid mites in this study was done by morphological taxonomy. Hence, we cannot conclude if the species identified here, but already present in the invertebrate inventory of Svalbard, have been introduced with the soils or not. However, it is appreciated that there can be marked genetic divergence within species of oribatid mites (Schäffer et al. Citation2010). Hence, the possibility of the introduction of cryptic species should not be discounted. The composition and structure of the oribatid species found in our material is unusual compared to samples from natural habitats in Svalbard (Solhøy et al. unpubl. data). Of the 761 specimens recovered, the Oppiidae accounted for 57% and the Brachychthoniidae for 42%. The specimens of T. velatus (1), O. tibialis (2) and D. notatus (3) made up only 1% of the community represented by stray individuals. This could indicate that the Oppiidae (O. neerlandica and O. nova) and the Brachychthoniidae (at least L. brevis and L. sellnicki) were introduced by human activity. They are all parthenogenetic and probably establish more easily than sexual species (Norton & Palmer Citation1991).
The majority of the 18 species of Collembola are widespread outside the Arctic (Fjellberg Citation1998, Citation2007) except for Friesea quinquespinosa which is an exclusively Arctic species. The five species only recorded from Barentsburg (Coulson et al. Citation2013) are typical members of a fauna associated with soils having a high organic content such as compost, garden soil and wrack beds along seashores and stream banks. It is likely that these five species of Collembola are all human imports. The remaining 13 species are common in a variety of habitats throughout Svalbard (Coulson Citation2007) and do not represent an unusual species assemblage.
In the ornithogenic soils from bird cliffs, predominantly little auk (Alle alle), Zmudczynska et al. (2012) observed seven species of Collembola. Few other studies in Svalbard have targeted the invertebrate communities in such habitats but in a study that included the Florabukta bird cliffs and polar desert-type vegetation in the vicinity of Kinnvika, Nordaustlandet (north-east Svalbard), Coulson et al (Citation2011) recorded 24 species of Collembola. Similarly, Fjellberg (Citation1997) identified 26 species of Collembola from Florabukta and noted the strong relationship between floral diversity and cover with Collembola species diversity. Interestingly, Fjellberg (1997) also observed that while amongst the Nordaustlandet habitats surveyed species diversity was greatest at Florabukta, the number of species per soil sample was not particularly great. Fjellberg also noted that sites with a lower total species diversity often had the greatest densities of individuals. Studies using chronosequences across proglacial margins have also revealed a relationship between vegetation cover/diversity and collembolan biodiversity (Hodkinson et al. Citation2004). Nonetheless, despite the organic soil and high plant diversity (Liška & Soldán Citation2004), the soils examined here do not appear to have a distinct collembolan community from other locations in Svalbard, excluding the introduced species. Thirteen species of Collembola in the samples are not untypical for habitats in the archipelago and the species observed are all widely distributed throughout the region (Coulson Citation2007Coulson Citation2012)
The chironomid larvae extracted from the Barentsburg soil were associated with two species of the genus Smittia only known as females and of which one probably is new to science. Smittia brevipennis (Boheman 1865) was described from Spitsbergen more than 150 years ago. This species has not been recorded from the European continent but is known from Arctic Siberia (Sæther Citation2004). It is not unlikely that these species are parthenogenetic (or at least facultative parthenogenetic) as reproduction by parthenogenesis has been documented from Smittia and is known to be more frequent in the Arctic than at lower latitudes (Oliver Citation1970). Both species of Chironomidae have previously been found in the Arctic. Concerning the Sciaridae, four species belonging to the subgenus Hemineurina have previously been recorded from Spitsbergen (Coulson & Refseth Citation2004). Hence, the larvae from Barentsburg may well represent a resident species. The staphylinid beetle, A. graminicola, is widespread in Svalbard and particularly so under bird cliffs preying on the often abundant collembolan Megaphorura arctica (Coulson pers. obs). However, it should be recognized that the sampling and extraction techniques are not efficient for these last groups and hence, it is likely that not all species of Diptera, Coleoptera or Araneae were collected.
The composition of the soils, especially the significant organic enhancement from the cowshed manure, and the westerly facing aspect of the site in Grønfjord create an unusual habitat in Svalbard. The open aspect and open water in front of the site enable continual insolation during the summer months. The deep and rich organic soils provide a habitat with excellent water-holding potential throughout the summer. This in particular may explain the persistence of the Lumbricidae observed in these soils. Where snow cover is thin, the soil temperature may track air temperature more closely and decrease to below -20°C (Coulson et al. Citation1995) but under a snow blanket soil temperatures may not decline much below -5°C. Air temperatures during winter 2011–12 were highly atypical. Monthly mean monthly air temperatures in January and February were -3.4 and -5.5°C, respectively, some 11.9 and 10.7°C above long-term normals (Norwegian Meteorological Institute Citation2012). Nonetheless, the lack of deep snow cover on natural soils resulted in soil temperatures closely tracking air temperatures, with large variation in temperatures. In stark contrast, the cowshed soils remained at close to 0°C throughout the entire winter. This is in part due to the thick litter layer accumulated from plants such as A. sylvestris but there may also be some thermogenic activity due to the slow decomposition of the large quantities of organic matter in the soils. This extremely ameliorated winter environment may explain in part the persistence of some of the exotic species identified in these soils (Coulson et al. Citation2013). Although, compared to the natural soil site, the cowshed soils are warmer during the winter, these soils warmed later in the following spring and remained at lower temperatures than the natural soil site. This is likely to be due to the shaded location of the site, the presence of accumulated litter and the tall plants hindering the warming of the soil surface by solar insolation.
In contrast to the natural soils, the anthropogenic soils provide a nutrient-rich, organic soil with excellent water-holding properties and with a cool moist environment during the summer but an extremely ameliorated habitat during the winter. However, soil pollution levels, for example, PCB7 (polychlorinated biphenyl) and the insecticide dichlorodiphenyltrichloroethane (DDT), in Barentsburg are generally high (Jartun et al. Citation2009). In the area sampled, PCB7 levels are up to 0.5 mg/kg (Geological Survey of Norway Citation2010) and the pollution report publicised by the Russian Federal Service of Hyrometeorology and Environmental Monitoring (FSHEM Citation2009) found DDT levels of 1.3 µg/g in soils close to the cowsheds. Coal dust is also present. Such dust, and associated heavy metals contaminants such as lead and arsenic (FSHEM Citation2009), may also have toxic or repellent effects on the invertebrate fauna (Frouza et al. Citation2011). Hence, the resident Svalbard invertebrate fauna, not generally challenged by high pollutant levels, may have difficulties fully exploiting the new habitat perhaps partly explaining the diversity observed.
In conclusion, the soil invertebrate fauna observed in these anthropogenic soils is a mixture of species widespread throughout Svalbard , including a few probably introduced species (Coulson et al. Citation2013), and is likely to represent a unique community in the archipelago. However, excluding the introduced species, the invertebrate fauna does not form an especially atypical community. On the contrary, the community shows strong similarities with other habitats in Svalbard, both nutrient poor and enhanced. The soils therefore may well provide a habitat for introduced species, possibly in some cases the only location in Svalbard that is suitable, but, as yet, do not seem to furnish a particularly favourable site when considering overall invertebrate species diversity.
Acknowledgements
This article is dedicated to Torstein Solhøy, who died shortly after it was accepted. The authors extend their thanks to Vitaly Kuleshov, the Kola Scientific Centre of Russian Academy of Sciences (KSC RAS) research station manager in Barentsburg, and Alexander Roskulyak (Polar Geophysical Institute, KSC RAS) for assistance during their fieldwork, Trust Arktikugol and G.A. Tarasov (Murmansk Marine Biological Institute) for information on the origin of the imported soils, Vladimir Gusarov (Natural History Museum, Oslo) for the identification of A. graminicola and Paul Hebert and the Canadian Centre for DNA Barcoding team (University of Guelph) for barcoding our chironomid specimens. The sequencing and databasing was funded by a grant from Genome Canada to Paul Hebert and a grant from Artsdatabanken (Artsprosjektet), Norway, to CE. RMS's work was financially supported by the research funding programme Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz of Hesse's Ministry of Higher Education, Research, and the Arts. They also thank Anna Sjöblom for advice on calculating daily mean temperatures and Malin Daase (Norwegian Polar Institute) for preparing . The fieldwork was funded via Norwegian Research Council project Avian Vectors of Invertebrate Faunas (project no. 6172/S30).
References
- Alsos I.G, Eidesen P.B, Ehrich D, Skrede I, Westergaard K, Jacobsen G.H, Landvik J.Y, Taberlet P, Brochmann C. Frequent long-distance plant colonization in the changing Arctic. Science. 2007; 316: 1601–1608.
- Ávila-Jiménez M.L, Coulson S.J. A Holarctic biogeographical analysis of the Collembola (Arthropoda, Hexapoda) unravels recent post-glacial colonization patterns. Insects. 2011; 2: 273–296.
- Ávila-Jiménez M.L, Gwiazdowicz D.J, Coulson S.J. On the gamasid (Acari: Parasitiformes) mite fauna of Svalbard: a revised checklist of a High Arctic archipelago. Zootaxa. 2011; 3091: 33–41.
- Bayartogtokh B, Schatz H, Ekrem T. Distribution of the soil mites of Svalbard with redescriptions of three known species (Acari: Oribatida). International Journal of Acarology. 2011; 37: 467–484.
- Berman D.I, Meshcheryakova E.N, Leirikh A.N. Egg cocoons of the earthworm Dendrodrilus rubidus tenuis (Lumbricidae, Oligochaeta) withstand the temperature of liquid nitrogen. Doklady Biological Sciences. 2010; 434: 347–350.
- Birkemoe T. Population dynamics of Enchytraeidae at the Arctic tundra at Spitsbergen, Svalbard. Newsletter Enchytraeidae. 1995; 4: 45–52.
- Birkemoe T, Coulson S.J, Sømme L. Life cycles and population dynamics of Enchytraeids (Oligochaeta) from the High Arctic. Canadian Journal of Zoology. 2000; 78: 2079–2086.
- Birkemoe T, Dózsa-Farkas K. New records of Enchytraeidae (Oligochaeta) from Spitsbergen, Svalbard. Fauna Norvegica Series A. 1994; 15: 35–44.
- Bliss L.C, Matveyeva N.V, Chapin F.S III, etal. Circumpolar Arctic vegetation. Arctic ecosystems in a changing climate. An ecophysiological perspective. 1992; San Diego: Academic Press. 59–89.
- Brochmann C, Gabrielsen T.M, Nordal I, Landvik J.Y, Elven R. Glacial survival or tabula rasa? The history of the North Atlantic biota revisited. Taxon. 2003; 52: 417–450.
- Cooper E.J. Polar desert vegetation and plant recruitment in Murchisonfjord, Nordaustlandet, Svalbard. Geografiska Annaler Series A. 2011; 93: 243–252.
- Coulson S.J. The terrestrial and freshwater invertebrate fauna of the High Arctic archipelago of Svalbard. Zootaxa. 2007; 1448: 41–58.
- Coulson S.J. Checklist of the terrestrial and freshwater invertebrate fauna of Svalbard. 2012. Accessed on the internet at http://www.unis.no/35_STAFF/staff_webpages/biology/steve_coulson/default.htm on various dates in June 2012.
- Coulson S.J. The terrestrial invertebrate fauna of the Svalbard Archipelago in a changing world: history of research and challenges. Canadian Entomologist. 2013; 145: 131–146.
- Coulson S.J, Fjellberg A, Gwiazdowicz D.J, Lebedeva N.V, Melekhina E.N, Solhøy T, Erséus C, Maraldo K, Miko L, Schatz H, Schmelz R.M, Søli G, Stur E. Introduction of invertebrates into the High Arctic via imported soils: the case of Barentsburg in the Svalbard. Biological Invasions. 2013; 15
- Coulson S.J, Fjellberg A, Snazell R, Gwiazdowicz D.J, Avila-Jimenez M.L. On the Collembola, Araneae and Gamasida from the Kinnvika region of Nordaustlandet, Svalbard. Geografiska Annaler. 2011; 93: 253–257.
- Coulson S.J, Hodkinson I.D, Strathdee A.T, Block W, Webb N.R, Bale J.S, Worland M.R. Thermal environments of Arctic soil organisms during winter. Arctic and Alpine Research. 1995; 27: 365–371.
- Coulson S.J, Hodkinson I.D, Webb N.R. Microscale distribution patterns in High Arctic soil microarthropod communities: the influence of the vegetation mosaic. Ecography. 2003; 26: 801–809.
- Coulson S.J, Refseth D, Prestrud P, etal. The terrestrial and freshwater invertebrate fauna of Svalbard (and Jan Mayen). A catalogue of the terrestrial and marine animals of Svalbard. 2004; Tromsø: Norwegian Polar Institute. 57–122.
- Dózsa-Farkas K. Taxonomical problems in enchytraeids (Oligochaeta) from Spitsbergen. Newsletter on Enchytraeidae. 1999; 6: 21–30.
- Fjellberg A. Collembola from Nordaustlandet, Svalbard. Fauna Norvegica Series B. 1997; 44: 71–75.
- Fjellberg A. The Collembola of Fennoscandia and Denmark. Part I: Poduromorpha. Fauna Entomologica Scandinavica 35. 1998; Leiden: Brill.
- Fjellberg A. The Collembola of Fennoscandia and Denmark. Part II: Entomobryomorpha and Symphypleona. Fauna Entomologica Scandinavica 42. 2007; Leiden: Brill.
- Frouza J, Hrcková K, Lána J, Krišt?fek V, Mudrák O, Lukešová A, Mihaljevic M. Can laboratory toxicity tests explain the pattern of field communities of algae, plants, and invertebrates along a toxicity gradient of post-mining sites?. Applied Soil Ecology. 2011; 51: 114–121.
- FSHEM (Federal Service for Hydrometeorology and Environmental Monitoring). Oversikt over forurensning av naturmiljøet, basert på resultater av bakgrunnsmiljøovervåkning og lokal miljøovervåkning, gjort i næringslokalitetene til de russiske bedriftene i Spitsbergen-arkipelet (bygda Barentsburg og de tilgrensende strøk) i 2008. (Overview of the pollution in the natural environment based on the results of background and local environmental monitoring carried out in the industrial areas of the Russian companies in the Spitsbergen archipelago [buildings in Barentsburg and neighbouring areas] in 2008.). 2009; St. Petersburg: Federal Service for Hydrometeorology and Environmental Monitoring. Translated from Russian to Norwegian by the Office of the Governor of Svalbard, Longyearbyen.
- Geological Survey of Norway. PCB fra locale kilder på Svalbard—overflatejord og produkter 2007–2009. (PCB from local sources in Svalbard—soil and products.). 2010; Trondheim: Geological Survey of Norway. NGU Rapport 2010.038.
- Gwiazdowicz D.J, Coulson S.J. High Arctic gamasid mites (Acari, Mesostigmata); community composition on Spitsbergen, Svalbard. Polar Research. 2011. 30, article no. 8311.
- Gwiazdowicz D.J, Solhøy T, Coulson S.J, Lebedeva N, Melekhina E. First record of Vulgarogamasus immanis (Acari, Mesostigmata) in Svalbard. Polish Polar Research. 2012; 33: 35–39.
- Hisdal V. Geography of Svalbard. 1985; Oslo: Norwegian Polar Institute.
- Hodkinson I.D, Coulson S.J, Webb N.R. Invertebrate community assembly along proglacial chronosequences in the High Arctic. Journal of Animal Ecology. 2004; 73: 556–568.
- Holmstrup M. Overwintering adaptations in earthworms. Pedobiologia. 2003; 504–510. 5–6.
- Jacot A.P. Some Hawaiian Oribatoidea (Acarina). Bulletin of the Bernice P. Bishop Museum Bulletin 121. 1934; Honolulu: Bernice P. Bishop Museum.
- James S.W, Porco D, Decaëns T, Richard B, Rougerie R, Erséus C. DNA barcoding reveals cryptic diversity in Lumbricus terrestris L., 1758 (Clitellata): resurrection of L. herculeus (Savigny, 1826). PLoS One. 2010; 5: 15629.
- Jartun M, Ottesen R.T, Volden T, Lundkvist Q. Local sources of polychlorinated biphenyls (PCB) in Russian and Norwegian settlements on Spitsbergen island, Norway. Journal of Toxicology and Environmental Health, Part A. 2009; 72: 284–294.
- Jónsdóttir I.S. Terrestrial ecosystems on Svalbard: heterogeneity, complexity and fragility from an Arctic island perspective. Proceedings of the Royal Irish Academy. 2005; 105: 155–165.
- Julin E. De svenska daggmaskarterna. (The Swedish earthworms.) Arkiv för Zoologi 42A. 1949; Stockholm: Royal Swedish Academy of Sciences.
- Klekowski R.Z, Opalinski K.W. Matter and energy flow in Spitsbergen ornigenic tundra. Polar Research. 1986; 4: 187–197.
- Liška J, Soldán Z. Alien vascular plants recorded from the Barentsburg and Pyramiden settlements, Svalbard. Preslia. 2004; 76: 279–290.
- Melekhina E.N. Taxonomic diversity and areology of Oribatid mites (Oribatei) of the European north of Russia. Izvestiya Komi nauchnogo centra UrO RAN. 2011; 2: 30–37.
- Norton R.A, Palmer S.C, Schuster R, Murphy P.W. The distribution, mechanisms and evolutionary significance of parthenogenesis in oribatid mites. The Acari—reproduction, development and life-history strategies. 1991; New York: Chapman and Hall. 107–136.
- Norwegian Meteorological Institute. Klimastatistikk Svalbard. (Climate statistics for Svalbard.). 2012. Accessed on the internet at http://retro.met.no/observasjoner/svalbard/ on various dates in June 2012. (This service has been discontinued; the data are now available from http://www.eklima.no.).
- Nurminen M. Enchytraeid and lumbricid records (Oligochaeta) of Spitsbergen. Annales Zoologici Fennici. 1965; 2: 1–10.
- O'Connor F. Extraction of enchytraeid worms from a coniferous forest soil. Nature. 1955; 175: 815–816.
- Odasz A.M. Nitrate reductase-activity in vegetation below an Arctic bird cliff, Svalbard, Norway. Journal of Vegetation Science. 1994; 5: 913–920.
- Oliver D.R. Designation and description of lectotypes of the six Greenland Orthocladiinae (Diptera, Chironomidae) described by Lundbeck in 1898. Entomologica Scandinavica. 1970; 1: 102–108.
- Ratnasingham S, Hebert P.D.N. Bold: the barcode of life data system (www.barcodinglife.org). Molecular Ecology Notes. 2007; 7: 355–364.
- Raw F. Estimating earthworm populations by using formalin. Nature. 1959; 184: 1661–1662.
- Rønning O.I. Svalbards flora. 1979; Oslo: Norwegian Polar Institute.
- Rundgren S. Lumbricidae in Iceland. Insect Systematics and Evolution. 2007; 61: 121–159.
- Sæther O.A. The chironomids described by Lundström (1918) from Arctic Siberia (Diptera, Chironomidae) with a redescription of Derotanypus sibiricus (Kruglova and Chernovskii). Zootaxa. 2004; 595: 1–35.
- Satchell J.E. Methods of sampling earthworm populations. Pedobiologia. 1969; 9: 20–25.
- Schäffer S, Pfingstl T, Koblmüller S, Winkler K.A, Sturmbauer C, Krisper G. Phylogenetic analysis of European Scutovertex mites (Acari, Oribatida, Scutoverticidae) reveals paraphyly and cryptic diversity: a molecular genetic and morphological approach. Molecular Phylogenetics and Evolution. 2010; 55: 677–688.
- Sims R.W, Gerard B.M. Earthworms. Synopsis of the British fauna. Vol. 31. 1985; London: Brill & Backhuys.
- Stöp-Bowits C. A contribution to our knowledge of the systematics and zoogeography of Norwegian earthworms (Annelida Oligochaeta: Lumbricidae). Nytt Magasin för Zoologi. 1969; 17: 169–280.
- Zmudczynska K, Olejniczak I, Zwolicki A, Iliszko L, Convey P, Stempniewicz L. The influence of allochtonous nutrients delivered by colonial seabirds on soil collembolan communities on Spitsbergen. Polar Biology. 2012; 35: 1233–1245.
- Zmudczynska K, Zwolicki A, Barcikowski M, Barcikowski A, Stempniewicz L. Spectral characteristics of the Arctic ornithogenic tundra vegetation in Hornsund area, SW Spitsbergen. Polish Polar Research. 2009; 30: 249–262.