Abstract
We carried out a dietary overlap analysis between notothenioid species by examining the stomach contents of more than 900 specimens collected in a fish assemblage at the Danco Coast, western Antarctic Peninsula, in the summer of 2000. Prey reoccurrences among fish species were 32.2%, with krill Euphausia superba, salps and the gammaridean Prostebeingia longicornis the most reoccurring prey. The diet similarity between species pairs was lower than 55%, in accordance with similar fish assemblages in the South Orkney Islands, the South Shetland Islands and the Antarctic Peninsula. Whereas at those localities the higher prey overlap was between krill-feeding fish species, at the Danco Coast it was between Trematomus bernacchii and Lepidonotothen nudifrons, Notothenia coriiceps and Notothenia rossii, Notothenia coriiceps and Parachaenichthyis charcoti, and Trematomus newnesi and Notothenia rossii, which shared primarily gammaridean amphipods, algae, fish and krill, respectively. Krill is normally the main prey of fish in summer in inshore waters of the western Antarctic Peninsula, but its density in January/February 2000 was notably lower than in previous years. Therefore, at the Danco Coast, under conditions of krill shortage, most of the notothenioid species foraged more intensively on alternative prey, such as gammarideans, fish and algae. The difference between areas in the pattern of dietary overlap might be related to differences in prey availability between years and to the degree of competition for targeted prey.
Diet overlap may reflect competition under conditions of limited resource availability (Odum Citation1971). In the Antarctic marine ecosystem, the Notothenioidei fish occupy different food niches to reduce food overlap by mechanisms that range from taking different portions of the same prey groups and feeding on different organisms along a vertical distributional axis, to specialized or generalized feeding (Kock Citation1992; Gröhsler Citation1994). Examples of trophic specialization in fish species in the Antarctic Ocean, mainly for the Scotia Arc region, are summarized in Barrera-Oro (Citation2003).
Several studies deal with food competition and resource partitioning among vertebrates in the Antarctic Ocean (e.g., Croxall et al. Citation1997; Lynnes et al. Citation2002; Wilson Citation2010; Casaux et al. Citation2011). These types of studies are essential for the understanding of ecosystem functioning as well as predator–prey interactions. However, research on dietary overlap in Antarctic fish is scarce. A few studies are focused on pairs of fish species in the South Shetland Islands (Moreno & Bahamonde Citation1975) and the Ross Sea (Vacchi et al. Citation1994; La Mesa et al. Citation1997), while others analyse food overlap between multiple species in fish assemblages of the South Shetlands and western Antarctic Peninsula (Rakusa-Suszczewski & Piasek Citation1973; Daniels Citation1982; Barrera-Oro Citation2003), the South Orkney Islands (Targett Citation1981) and the Weddell Sea (Schwarzbach Citation1988).
The aim of this study is to provide new insights on food overlap and potential competition among fish species of the Danco Coast, a less investigated area of the western Antarctic Peninsula, using diet data of fish sampled in a summer campaign in 2000 (see Casaux et al. Citation2003), a year of unusually high sea-surface temperature (Xavier et al. Citation2013) and low krill availability (Siegel et al. Citation2004) in the region.
Material and methods
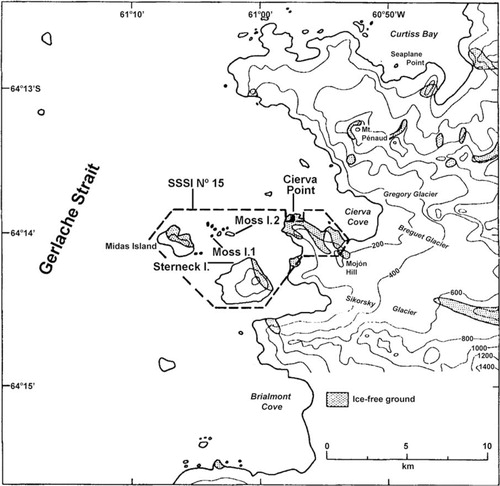
A total of 1103 fish were caught by means of trammel nets in four sampling sites close to Cierva Point (64°09′S, 60°57′W, )—Moss Island 1, Moss Island 2, Sterneck Island and Leopardo Island (), Danco Coast, Antarctic Peninsula—between 2 February and 31 March 2000. The distance between the farthest sampling sites is 7.5 km. The nets (length 25 m, width 1.5 m, inner mesh 2.5 cm, outer mesh 12 cm) were fastened to rocks on the coast and deployed on the bottom at depths from 20 to 70 m for periods of 48–96 hours. The seabed in the sampling area likely consists of rocky bottoms with algae beds (see Casaux et al. Citation2003).
Fig. 2 Location of the sampling sites in the proximity of Cierva Point, Danco Coast, western Antarctic Peninsula.
Using the mixed method of Hureau (Citation1970), 925 stomach contents were examined. Data are expressed in terms of importance by number (N%) and by mass (M%). To estimate the percentage by number of algae, the number of algae species represented in each stomach content was considered as the number of specimens represented in the sample.
The dietary overlap among species was estimated following Tyler (Citation1972), where the reoccurrence of prey, or percentage overlap, among predator species is the number of reoccurrences of preys among predators divided by the number of possible reoccurrences. One reoccurrence means that a prey occurs in two predator species. The total number of reoccurrences possible is the number of predators minus 1 multiplied by the number of prey.
Prey overlap between pairs of fish species was estimated according to the diet similarity index S (Linton et al. Citation1981) as follows:
where Pxi x and Pyi y are the proportions in the diets of fish species x and y, respectively, of prey i. S ranges from 0, when no prey are shared, to 100, when the diet of two fish species is identical. S was estimated by considering the contribution of the different prey to the diet by mass.
Fish nomenclature follows Gon & Heemstra (Citation1990).
Results
All of the fish species caught belonged to the dominant endemic coastal demersal group, the suborder Notothenioidei. Species of the family Notothenidae (471 individuals of Notothenia coriiceps, 265 Trematomus newnesi, 215 Gobionotothen gibberifrons, 45 Trematomus bernacchii, 28 Lepidonotothen nudifrons, three Notothenia rossii and three Trematomus hansoni) prevailed in the samples, whereas the families Bathydraconidae (12 Parachaenichthys charcoti) and Channichthyidae (eight Chaenocephalus aceratus) were scarcely represented (see details in Casaux et al. Citation2003). The stomachs of T. hansoni and C. aceratus specimens were empty; therefore, these species were not included in the analysis.
The diet diversity was wide in N. coriiceps and G. gibberifrons but limited in the remaining species (). Notothenia coriiceps ate mainly algae and gammaridean amphipods. Gammarideans predominated in the diet of G. gibberifrons, L. nudifrons and T. bernacchii. The krill (Euphausia superba) was the main prey of N. rossii (together with algae) and T. newnesi, whereas P. charcoti fed mainly on fish.
Table 1 Diet composition of the fish sampled at the Danco Coast, western Antarctic Peninsula
The reoccurrence of prey among fish species was 32.2% (). Krill, salps and the gammaridean Prostebeingia longicornis were the most reoccurring prey. The dietary similarity index S fluctuated widely between fish of different species in pair-wise comparisons (). The highest value of prey overlap recorded was 54.8% between L. nudifrons and T. bernacchii, whereas the lowest was 0.3–0.6% between P. charcoti and the species L. nudifrons, N. rossii, T. bernacchii and N. coriiceps.
Table 2 Reoccurrence of prey (Tyler's method) among seven fish species at the Danco Coast, western Antarctic Peninsula.
Table 3 Percentage dietary overlap by weight (index S) between fish species pairs at the Danco Coast, western Antarctic Peninsula.
Discussion
The composition of the diet and the main prey of the fish sampled at the Danco Coast showed general agreement with those reported for the same species in other Antarctic areas (see Moreno & Bahamonde Citation1975; Richardson Citation1975; Daniels Citation1982; Burchett Citation1983; Linkowski et al. Citation1983; Casaux et al. Citation1990; Vacchi et al. Citation1994; Barrera-Oro Citation1996; La Mesa et al. Citation2000). Although some species were predominantly benthos (N. coriiceps, G. gibberifrons, L. nudifrons and T. bernacchii) or plankton/water-column feeders (T. newnesi, N. rossii and P. charcoti), all of them preyed on both benthic–demersal and pelagic organisms, evidence of their foraging plasticity. Except for N. coriiceps and G. gibberifrons, most of the species foraged on a limited spectrum of prey (). Gammaridean amphipods were largely the main prey of G. gibberifrons, L. nudifrons and T. bernacchii; krill was the main food of T. newnesi and N. rossii (together with algae); P. charcoti foraged mainly on fish; whereas algae and fish constituted the bulk of the diet of N. coriiceps. Grazing has been reported as an important feeding strategy in some Antarctic demersal fish, and it has been demonstrated that algae are actively selected and deliberately consumed by fish (Barrera-Oro & Casaux Citation1990; Casaux et al. Citation1990; Iken et al. Citation1997). Therefore, some notothenioid fish species could be considered as omnivorous (Barrera-Oro Citation2002).
Whereas in our study algae and fish constituted the bulk of the diet of N. coriiceps, gammarideans (Casaux et al. Citation1990), krill (Permitin & Tarverdiyeva Citation1978; Barrera-Oro & Casaux Citation1990) or salps (Linkowski et al. Citation1983) were their main prey at other localities of the western Antarctic Peninsula. This supports the fact that N. coriiceps is a euryphagous and opportunistic feeder, and that its diet reflects the food availability at feeding grounds (Barrera-Oro & Casaux Citation1990). Although the diet composition of the remaining fish species indicated that gammarideans, krill and salps were available and abundant around Cierva Point, N. coriiceps nevertheless preyed more intensively on algae and fish. This suggests that the diet composition of fish within an assemblage is determined not only by food availability but also, to some extent, by factors related to interspecific competition for food. This is in line with the premise expressed above that, in order to reduce interspecific food competition, fish species forage on alternative prey or on different amounts of the same prey (see also Klemetsen Citation1993; La Mesa et al. Citation1997; Høines & Bergstad Citation1999, Citation2002).
The reoccurrence of prey among fish species observed in this study (32.2%) was similarly low to that also reported in Barrera-Oro (Citation2003) for summer at two localities of King George Island (Isla 25 de Mayo) in the South Shetland Islands, Potter Cove (33.0%) and Admiralty Bay (25.0%). However, in those fish assemblages the reoccurrence of prey might have been lower than that registered at the Danco Coast given that in that study, only main and secondary prey (according to the Q index of Hureau Citation1970) were considered, and these food items were grouped into categories broader than species. This suggests that the dietary overlap among notothenioid species and therefore potential competition in the fish assemblage at the Danco Coast was higher than that at King George Island, at least during the sampling period of this work.
As in our study, the food overlap between pairs of species in similar fish assemblages in the South Orkney Islands (Targett Citation1981), King George Island (Barrera-Oro Citation2003) and the Antarctic Peninsula (Daniels Citation1982) was usually below 50%. Whereas at those localities the higher prey overlaps were between krill feeder fish species, at the Danco Coast it was between T. bernacchii and L. nudifrons, N. coriiceps and N. rossii, N. coriiceps and P. charcoti, and T. newnesi and N. rossii, which mainly shared gammaridean amphipods, algae, fish and krill, respectively. Considering that some of these species forage intensively on krill when it is available, the difference between areas in the pattern of prey overlap might be related to differences in prey availability and to the degree of competition for targeted prey. In fact, there is information indicating that during our study period krill availability at the Danco Coast was comparatively lower than in previous seasons and in nearby areas in other years. Results from the Commission for the Conservation of Antarctic Marine Living Resources Survey in January/February 2000 indicated that the area of the western Antarctic Peninsula had the lowest krill densities, with a remarkable scarcity of juvenile krill (<5 cm TL; Siegel et al. Citation2004), which is normally the main prey of fish in summer in inshore waters of the region. This indicates that, under conditions of krill shortage, to reduce food competition, some fish species forage more intensively on alternative prey such as gammarideans, fish and algae. Concurrently, Casaux et al. (Citation2011) observed that at the Danco Coast, leopard (Hydrurga leptonyx), Antarctic fur (Arctocephalus gazella) and Weddell seals (Leptonychotes weddellii) competed for krill and that to reduce such competition these pinnipeds preyed also on fish to a different degree. They concluded that fish are important to buffer interspecific resource competition among seals, particularly in years of low krill availability. Interestingly, except for N. rossii, whose geographical distribution barely reaches the Danco Coast area, the fish species included in this study were also represented in the diet of the seal species in question (see Casaux et al. Citation2011). Hence, it is also possible that the differences between localities in the pattern of prey overlap among fish are, to some extent, also related to competition with seals and to feeding strategies displayed by fish to reduce predation risk while feeding on krill. In this sense, to interpret interspecific foraging relationships among fish and to improve our knowledge on their foraging behaviour, the analysis should exceed the scale of the fish assemblage by also considering prey availability and interactions of fish with predators. On the other hand, the information from the Danco Coast suggests that the decrease in krill populations, caused either by global warming or overfishing, may have a negative cascade effect on many of the predators species constituting the coastal ecosystem (see also Forcada Citation2008). This process not only affects krill-dependent species, which could be forced to prey on less profitable resources, but also generalist predators given that the availability of many of their prey might be also negatively affected.
Based on evidence of food resource partitioning among fish, several authors reported that there was no substantial competition for food among the species integrating some non-Antarctic assemblages (e.g., Klemetsen Citation1993; Høines & Bergstad Citation1999, Citation2002). In the Antarctic Ocean, the diversity of the notothenioids is limited compared with the large size of the ecosystem, and therefore some potential ecological niches of fish are not fully occupied as, for example, in tropical seas with many shallow reef-related niches and high fish diversity (Eastman Citation1995, Citation2005). The relatively loose packing of niches, in conjunction with the existence of fish strategies tending to partition food resources and feeding areas, is essential in minimizing the effects of competition for the most desirable prey and for the co-existence of the species composing the fish assemblages.
Acknowledgements
We thank A. Baroni, A. Ramón, M. Favero and P. Silva for their help in the field, G. Alonso for amphipod identifications, the staff of Primavera Station in summer 2000 for logistic support and two anonymous referees whose comments improved the manuscript. This is contribution no. 97 to the Laboratorio de Investigaciones en Ecología y Sistemática Animal.
References
- Barrera-Oro E. Ecology of inshore demersal fish (Notothenioidei) from the South Shetland Islands. 1996; Germany: University of Bremen. PhD thesis.
- Barrera-Oro E. The role of fish in the Antarctic marine food web: differences between inshore and offshore waters in the southern Scotia Arc and west Antarctic Peninsula. Antarctic Science. 2002; 14: 293–309.
- Barrera-Oro E. Analysis of dietary overlap in Antarctic fish (Notothenioidei) from the South Shetland Islands: no evidence of food competition. Polar Biology. 2003; 26: 631–637.
- Barrera-Oro E., Casaux R. Feeding selectivity in Notothenia neglecta, Nybelin, from Potter Cove, South Shetland Islands, Antarctica. Antarctic Science. 1990; 2: 207–213.
- Burchett M.S. Food, feeding and behaviour of Notothenia rossii nearshore at South Georgia. British Antarctic Survey Bulletin. 1983; 61: 45–51.
- Casaux R., Barrera Oro E., Baroni A., Ramón A. Ecology of inshore notothenioid fish from the Danco Coast, Antarctic Peninsula. Polar Biology. 2003; 26: 157–165.
- Casaux R., Bertolin M.L., Carlini A. Feeding habits of three seal species at the Danco Coast, Antarctica—a re-assessment. Polar Biology. 2011; 34: 1615–1620.
- Casaux R.J., Mazzotta A.S., Barrera-Oro E.R. Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biology. 1990; 11: 63–72.
- Croxall J.P., Prince P.A., Reid K. Dietary segregation of krill-eating South Georgia seabirds. Journal of Zoology. 1997; 242: 531–556.
- Daniels R.A. Feeding ecology of some fishes of the Antarctic Peninsula. Fishery Bulletin. 1982; 80: 575–588.
- Eastman J.T. The evolution of Antarctic fishes: questions for consideration and avenues for research. Cybium. 1995; 19: 371–389.
- Eastman J.T. The nature of the diversity of Antarctic fishes. Polar Biology. 2005; 28: 93–107.
- Forcada J. Duarte C.M. The impact of climate change on Antarctic megafauna. Impacts of global warming on polar ecosystems. 2008; Bilbao: BBVA Foundation. 83–112.
- Gon O., Heemstra P.C. Fishes of the Southern Ocean. 1990; Grahamstown, South Africa: J.L.B. Smith Institute of Ichthyology.
- Gröhsler T. Feeding habits as indicators of ecological niches: investigations of Antarctic fish conducted near Elephant Island in late autumn/winter 1986. Archive of Fishery and Marine Research. 1994; 42: 17–34.
- Høines Å.S., Bergstad O.A. Resource sharing among cod, haddock, saithe, and pollack on a herring spawning ground. Journal of Fish Biology. 1999; 55: 1233–1257.
- Høines Å.S., Bergstad O.A. Food partitioning by flatfish species on a herring spawning ground. Sarsia. 2002; 87: 19–34.
- Hureau J.-C. Biologie comparée de quelques poissons antarctiques (Nototheniidae). (Comparative biology of some Antarctic fish [Nototheniidae].) Bulletin de l′Institut Océanographique 68. 1970; Monaco: Oceanographic Institute.
- Iken K., Barrera-Oro E.R., Quartino M.L., Casaux R.J., Brey T. Grazing by the Antarctic fish Notothenia coriiceps: evidence for selective feeding on macroalgae. Antarctic Science. 1997; 9: 386–391.
- Klemetsen A. The food of the long-rough dab (Hippoglossoides platessoides limandoides Bloch) in Balsfjorden, north Norway. Sarsia. 1993; 78: 17–24.
- Kock K.-H. Antarctic fish and fisheries. 1992; Cambridge: Cambridge University Press.
- La Mesa M., Vacchi M., Castelli A., Diviacco G. Feeding ecology of two nototheniid fishes, Trematomus hansoni and Trematomus loennbergii, from Terra Nova Bay, Ross Sea. Polar Biology. 1997; 17: 62–68.
- La Mesa M., Vacchi M., Zunini Sertorio T. Feeding plasticity of Trematomus newnesi (Pisces, Nototheniidae) in Terra Nova Bay, Ross Sea, in relation to environmental conditions. Polar Biology. 2000; 23: 38–45.
- Linkowski T.B., Presler P., Zukowski C. Food habits of nototheniid fishes (Nototheniidae) in Admiralty Bay (King George Island, South Shetland Islands). Polish Polar Research. 1983; 4: 79–95.
- Linton L.R., Davies R.W., Wrona F.J. Resource utilization indices: an assessment. Journal of Animal Ecology. 1981; 50: 283–292.
- Lynnes A.S., Reid K., Croxall J.P., Trathan P.N. Conflict or co-existence? Foraging distribution and competition for prey between Adélie and chinstrap penguins. Marine Biology. 2002; 141: 1165–1174.
- Moreno C.A., Bahamonde N. Nichos alimentarios y competencia por alimento entre Notothenia coriiceps neglecta Nybelin y Notothenia rossii marmorata Fischer en Shetland del Sur, Antarctica. (Alimentary niches and competition for food between Notothenia coriiceps neglecta Nybelin and Notothenia rossii marmorata Fischer at the South Shetland Islands, Antarctica.). Serie Científica del Instituto Antártico Chileno. 1975; 3: 45–62.
- Odum E.P. Fundamentals of ecology. 1971; Philadelphia: W.B. Saunders Company.
- Permitin Y., Tarverdiyeva M. The feeding of fish of the family Nototheniidae and Chaenichthyidae of the South Orkney Islands. Biologiya Morya. 1978; 4: 75–81.
- Rakusa-Suszczewski S., Piasek A. Size, feeding and action of proteolytic enzymes in the Antarctic fish of the Trematomus genus (Nototheniidae), Bulletin of the Polish Academy of Sciences. Biological Scientific Series. 1973; 21: 139–144.
- Richardson M.G. The dietary composition of some Antarctic fish. British Antarctic Survey Bulletin. 1975; 41/42: 113–120.
- Schwarzbach W. Die fischfauna des östlichen und südlichen Weddellmeeres: geographische verbreitung, nahrung und trophische stellung der fischarten. (The demersal fish fauna of the eastern and southern Weddell Sea: geographical distribution, feeding of fishes and their trophic position in the food web.) Berichte zur Polarforschung 54. 1988; Bremerhaven: Alfred Wegener Institute.
- Siegel V., Kawaguchi S., Ward P., Litvinov F., Sushin V., Loeb V., Watkins J. Krill demography and large-scale distribution in the southwest Atlantic during January/February 2000. Deep-Sea Research Part II. 2004; 51: 1253–1273.
- Targett T.E. Trophic ecology and structure of coastal Antarctic fish communities. Marine Ecology Progress Series. 1981; 4: 243–263.
- Tyler A.V. Food resource division among northern, marine demersal fishes. Fisheries Research Board of Canada. 1972; 29: 997–1003.
- Vacchi M., La Mesa M., Castelli A. Diet of two coastal nototheniid fish from Terra Nova Bay, Ross Sea. Antarctic Science. 1994; 6: 61–65.
- Wilson R.P. Resource partitioning and niche hyper-volume overlap in free-living pygoscelid penguins. Functional Ecology. 2010; 24: 646–657.
- Xavier J.C., Louzao M., Thorp S.E., Ward P., Hill C., Roberts D., Croxall J.P., Phillips R.A. Seasonal changes in the diet and feeding behaviour of a top predator indicate a flexible response to deteriorating oceanographic conditions. Marine Biology. 2013; 160: 1597–1696.