Abstract
Most birds preen their feathers with an oily excrete from the uropygial (preen) gland. This oily excrete contains persistent organic pollutants (POPs), which make the preen gland a potential route of depuration of POPs in birds. Black-legged kittiwakes (Rissa tridactyla) were studied during two periods of high energy demand: incubation and chick-rearing. A rather high concentration of POPs in preen gland tissue indicates that the preen gland secrete is an excretory pathway for POPs in kittiwakes. The similarity in the POP profile detected in this study of liver, preen gland and feathers suggests that POPs found in the feathers are excreted through the preen gland. The finding also indicates that excretion of POPs through the preen gland is compound unspecific. This qualitative study should be followed up by a new quantitative study to determine the importance of excretion of POPs through the preen gland.
To access the supplementary material for this article, please see the supplementary files under Article Tools, online.
Seabirds and mammals mainly obtain POPs from their diet (Borgå et al. Citation2005). The persistence of these substances results in bioaccumulation in the organisms, as well as biomagnification within the food chain (Borgå et al. Citation2004). Despite the distance to considerable sources, organisms in the Arctic are found to be influenced by POPs (Letcher et al. Citation2010). These are mainly introduced to the Arctic through long-range transport including the atmosphere, oceans, ice packs and large rivers (Barri et al. Citation1992; MacDonald & Bewers Citation1996; Burkow & Kallenborn Citation2000), and also by migrating species that transport POPs back and forth from their winter habitats (Fort et al. Citation2014). In the Arctic, the highest POP concentrations are observed in apex predators such as polar bear (Ursus maritimus) and glaucous gull (Larus hyperboreus; Skaare et al. Citation2000; Fisk et al. Citation2001). The surface feeding piscivorous black-legged kittiwake (Rissa tridactyla; hereafter called kittiwake) does not have as high concentration of POPs as the top predators, but still accumulates considerable amounts of POPs (Borgå et al. Citation2001).
The major routes of excretion of POPs in mammals and birds are through faeces and urine (Walker et al. Citation2006). Other major routes of excretions are eggs for birds (e.g., Verreault et al. Citation2006), lactation for mammals (e.g., Wolkers et al. Citation2004) and secretion glands, for instance, for otter (e.g., van den Brink & Jansman Citation2006). The contaminants may also diffuse through the gut wall and metabolites are excreted together with the bile. Metabolism of POPs mainly occurs in the liver, where enzymes like cytochrome P450 catalyse the process (Guengerich Citation1991).
According to the persistence and slow metabolism and thereby accumulating nature of POPs one might expect elevating concentrations with increasing age in marine organisms. Accordingly, POPs accumulate with age in polar bears, especially in males (Bernhoft et al. Citation1997), despite their rather high capacity to metabolize certain POPs (Skaare et al. Citation2000; Kucklick et al. Citation2002; Verreault, Gabrielsen et al. Citation2005). However, a new study of POPs in humans showed that the age regression to POPs is not related to the age itself, but to birth cohorts of individuals (Nøst et al. Citation2013; Nøst et al. Citation2014). This has not, to our knowledge, been shown in wild mammals or birds. As mammalian females transfer POPs to their offspring through placenta and milk (Bernhoft et al. Citation1997; Bytingsvik et al. Citation2012), the regression to age is weaker for females. Similarly, female seabirds transfer POPs to their offspring through egg-yolk (Verreault et al. Citation2006). The deposit of contaminants in their eggs allows them to lower their body burden of POPs compared to males. The number and concentration of POPs found in seabirds are large, indicating a low capacity to metabolize POPs (Henriksen et al. Citation2000; Verreault, Gabrielsen et al. Citation2005). Paradoxically, in long-lived bird species, the POP concentrations are not documented to increase with age in individuals above the age of sexual maturation (Anderson & Hickey Citation1976; Mora et al. Citation1993; Henriksen Citation1995; Bustnes et al. Citation2003). Studies of kittiwakes (Robinson et al. Citation1967) and Brünnich's guillemots (Uria lomvia; Donaldson et al. Citation1997) found that age accumulation was restricted to the period between chicks or first-year juveniles and adult birds. Henriksen (Citation1995) found stable PCB concentrations in kittiwakes from two years of age, while van den Brink et al. (Citation1998) found no age-dependent accumulation in Adélie penguins (Pygoscelis adeliae) and southern fulmars (Fulmarus glacialoides). Glaucous gulls seem to reach an age-related equilibrium before the age of first breeding (Bustnes et al. Citation2003). Furthermore, American herring gulls (Larus smithsonianus) from the Great Lakes attained the theoretical DDT and PCB equilibrium eight months after fledging (Anderson & Hickey Citation1976). These findings indicate that an additional pathway of POP excretion, other than via the liver, is important in seabirds. Besides the large focus on metabolic degradation of persistent contaminants in the liver (Verreault et al. Citation2010), our knowledge of alternative excretion pathways of contaminants in birds is still limited, with the exception of the transfer of POPs to eggs (Gabrielsen et al. Citation1995; Helgason et al. Citation2008).
The preen gland (uropygial gland) and its oil are found to contain relatively high concentrations of organic pollutants (Ingebrigtsen et al. Citation1981; Ingebrigtsen et al. Citation1984; van den Brink et al. Citation1998). It is hypothesized that it could contribute to the excretion of POPs and prohibits their accumulation over time (Ingebrigtsen et al. Citation1981; Frank et al. Citation1983; Ingebrigtsen et al. Citation1984; van den Brink Citation1997; Yamashita et al. Citation2007; Jaspers et al. Citation2008). The preen gland is present in most birds and is largest in aquatic birds. It is a sebaceous gland appearing as a prominent swelling, dorsally at the base of the tail feathers (Stevens Citation1996; Kent Citation2001; Yamashita et al. Citation2007). An oily secretion is excreted from the gland and transferred to the birds’ feathers by preening, resulting in a waterproof plumage (Stevens Citation1996; Kent Citation2001; Yamashita et al. Citation2007). In gulls (Laridae) the daily excretion amount is estimated at about 600 mg of preen solution (Jacob Citation1976). Another excretion route for pollutants can be through moulting feathers.
Feathers have been suggested as a suitable matrix for non-destructive monitoring of POPs in terrestrial birds (Jaspers, Voorspoels et al. Citation2007; Jaspers et al. Citation2008; Jaspers et al. Citation2009; Jaspers et al. Citation2011; Jaspers et al. Citation2013). The plumage of seabirds and other aquatic bird species is regularly flushed by water. Water can thereby wash off external contaminants and preen oil, but at the same time, contaminants transported in the water can be attached to the feathers. Few studies on feather as a biomonitoring matrix for aquatic birds exist and the few available data show mostly non-significant correlations between POPs of feather and internal organs (Jaspers, Voorspoels et al. Citation2007).
The aims of the current study were to examine the ability of kittiwakes to excrete POPs through the preen gland and to gain further insight in seasonal changes in POP concentrations in seabirds during the energy-demanding breeding period. In addition, we tested the suitability of feathers as an appropriate non-destructive matrix for measuring contaminant levels. To do so, we investigated the levels and composition of contaminants in internal organs and feathers of kittiwakes. More specifically, we predicted that (1) the POPs from feathers are of internal origin; (2) the excretion of POPs through the preen gland is not congener specific; and (3) the oil (fat) content of the preen gland is lowered during energy-demanding periods, leading to increased concentration of lipid-soluble POPs in the chick-rearing period.
Materials and methods
Adult kittiwakes were sampled in 2009 in Kongsfjorden, Svalbard, during incubation and chick-rearing. Kittiwakes expend more energy during chick-rearing than during incubation (Gabrielsen Citation2009). A total of 24 breeding kittiwakes were sacrificed and livers, preen glands and feathers sampled. Half of the kittiwakes were sampled during the incubation period and the other half during the second week of the chick-rearing period. To minimize disturbance to nesting pairs, and to have a balanced sex ratio, only pairs were collected. For each individual, biometric measurements were recorded before the birds were killed by neck dislocation. The sexes were determined by gonad inspection. Samples of liver, preen gland and feather were collected and stored frozen at −20°C until analysis. The sampling was in accordance with the current regulation of the Norwegian Animal Welfare Act and permission to collect kittiwakes was provided by the Governor of Svalbard (200900103-17).
Sample preparation and analysis of POPs were conducted at the Norwegian Institute for Air Research in Tromsø. Liver, preen gland and feather samples were analysed for PCBs (i.e., PCB-99, -101, -105, -118, -123, -128, -138, -141, -149, -153, -156, -157, -167, -170, -180, -183, -187, -189 and -194), 1,1-bis-(4-chlorophenyl)-2,2-dichloroethene (p,p′-DDE), hexachlorocyclohexanes (α-HCH, β-HCH and γ-HCH), chlordanes (trans-chlordane, cis-chlordane, oxy-chlordane, trans-nonachlor and cis-nonachlor), mirex, HCB, heptachlor and heptachlor epoxide. The compounds of interest were extracted from 2.0 g of liver and 1.0 g of preen gland using cold column extraction, described by Herzke et al. (Citation2003). Lipid content of liver and preen gland samples was gravimetrically determined. The feathers were washed with ultrapure MilliQ-water (MilliQ Advantage A10 Ultrapure Water Purification System) to remove dust and particles, and dried at room temperature for at least two days. Approximately 500 mg body feathers were cut in pieces, put in a beaker and extracted three times with 50 ml cyclohexane:acetone (3:1) for 15 min in an ultrasonic bath (Branson 5510). The extract was filtered through a cotton-filled pipette. Lipids and other matrix were removed by gel-permeation chromatography using a dual pre-packed Envirogel system (Bio-Beads S-X3 resins) at a flow rate of 5 mL/min dichloromethane. The fraction between 75 and 106 mL was collected and concentrated to 0.5 mL (Turbovap) while changing the solvent to cyclohexane followed by a florisil clean-up. An additional gel-permeation chromatography step was necessary in order to purify the extracts from leakage from a thermoplastic elastomer (see the Supplementary file).
Organohalogenated pesticides, DDE and PCBs were analysed by gas chromatography with mass-spectrometry in selected ion monitoring mode. An Agilent 7890A GC with split/splitless injector coupled to an Agilent 5975 C mass selective detector was used with helium as carrier gas and methane as reagent gas in negative chemical ionization mode (Vieweg et al. Citation2012). For details on targeted m/z, see the Supplementary file.
Quality assurance of the analyses was performed by including laboratory blanks and two certified standard reference materials used in sequence (PCBs and pesticides: SRM 1588b cod liver oil and SRM 1945 in whale blubber; both from the National Institute of Standards and Technology, Gaithersburg, MD, USA). LOD was defined as three times signal to noise for the analysed matrix or blank value. The LODs ranged from 0.02 to 81.7 ng/g lipid weight (lipid wt) in feathers, from 0.011 to 163 ng/g lipid wt in preen glands and from 0.1 to 30.9 ng/g lipid wt in livers. Because of blank contamination, the LODs for HCB were relatively high compared to previous analyses (Angot Citation2009) with 30.5 ng/g lipid wt for liver, 326 ng/g lipid wt for preen gland and 30.5 ng/g lipid wt for feathers.
A total of 12 liver samples and 12 preen gland samples were chosen for lipid class characterization. The samples were randomly chosen, but with equal numbers from incubation and chick-rearing and with equal number of females and males. The analyses were performed at Unilab Analyse AS in Tromsø, Norway. Neutral and polar lipid classes were separated and identified on a monolithic silica column using high-performance liquid chromatography coupled to an evaporative light-scattering detector (Graeve & Janssen Citation2009).
The lipid classes analysed were cholesteryl ester, wax ester, triacylglycerol, fatty alcohol, cholesterol, diacylglycerol, free fatty acid, monoacylglycerol, galactocerebroside, cardiolipin, phosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol, phosphatidic acid, phosphatidylserine, phosphatidylcholine, sphingomyelin and lysophosphatidylcholine.
The concentration mean for a compound was calculated only if more than 60% of the samples had a concentration above the LOD (Verreault, Muir et al. Citation2005). Only these compounds were considered in the statistical analyses. For the compounds included in statistical analyses, values below the LOD were given a value LOD/2 to avoid missing values in the statistical analyses (Bernhoft et al. Citation1997). The significance level was set at <0.05 and all statistics were performed with the statistical software R, version 2.10.1 (R Core Team Citation2015). Adjustment for multiple comparison was not performed as this can lead to more errors of interpretation when the data under evaluation is actual observation on nature and not random numbers (Rothman Citation1990).
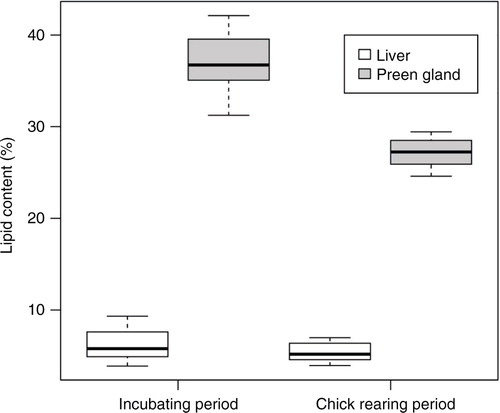
The lipid percentage data were arcsine-square root transformed to meet the assumptions of constant variation and normal distribution. Prior to analysis, one gross outlier (69% fat in one preen gland, compared to the other samples with a mean [SD] of 27 [1.7]% for period two, i.e., chick-rearing) was removed from the lipid data set. The outlier was suspected to be a result of an analytical failure. Since the lipid content of liver and preen gland are significantly different, the lipid normalized POP concentrations were used for statistical calculation. The concentrations were natural log transformed in order to reduce variance heterogeneity and obtain approximately normal distribution.
The experimental design, with three different samples taken from each individual kittiwake and the sampling of pairs, creates a dependency among samples. To correct for the random effect, a linear mixed effect model was applied in analyses where tissue type, sampling period and sex were included as variables. The fitted linear mixed effect model followed by contrast analyses to reveal differences in the POP concentrations. Furthermore, two-way ANOVA followed by Tukey's multiple comparisons test for unplanned comparisons (Tukey honest significant difference test) examined differences between sexes and sampling periods in each compound within each tissue type.
A PCA visualized the POP profiles in liver, preen gland and feather, as well as the pattern of POPs in relation to lipid classes. Redundancy analysis with ANOVA was performed to reveal significant impact of the predictor variable—tissue type—on the distribution of POPs and lipid classes.
Results
The mean body mass of the kittiwakes was 398 g (n=12) during the incubation period. It was significantly lower (361 g, n=12) during the early chick-rearing period. Females were significantly lighter than the males (sex; F=46, P<0.001, period; F=15, P<0.001). The β-HCH, HCB, heptachlor, cis-chlordane, oxy-chlordane, trans-nonachlor, cis-nonachlor, mirex, p,p′-DDE, PCB (penta-PCBs: -99, -105, -118; hexa-PCBs: -128, -138, -141, -149, -153, -156, -157, -167; hepta-PCBs: -170, -180, -183, -187, -189; and the octa-PCB: -194) compounds were detected above the LODs in more than 60% of the liver and preen samples (Supplementary Tables S1, S2). The most abundant contaminant group was ΣPCBs, making up 78–80% of the ΣPOPs in feathers, liver and preen gland (). In the current study, the only significant difference in POP concentrations for sexes appeared for mirex, where both liver and preen gland concentrations were higher for males (t=2.9, P<0.01 for both organs).
Table 1 The mean, ±SD, median and data range of POP concentrations (ng/g wet weight (wt)) in feathers, livers and preen glands of adult kittiwakes from Ny-Ålesund, Svalbard. Compounds detected below the LOD in more than 60% of the samples are reported as not detected (nd).
The POP concentrations were always lowest in feathers (). Because of the high lipid content in preen gland tissue (34%±9.3), the mean wet weight (wet wt) concentration of POPs in kittiwakes was found, as expected, to be significantly higher in preen gland samples than in livers (). Correcting for lipids, the POP concentrations in preens and livers approach each other (, Supplementary Table S2). During incubation, higher concentrations of lipid wt HCB (t=3.7, P<0.001) and lower lipid wt concentration of β-HCH, oxy-chlordane, p,p′-DDE and penta-PCBs was found in livers than in preen glands (t=−4.9, −2.4, −2.9, −2.1 and P=<0.001, 0.02,<0.01, 0.04, respectively). Later in the breeding season, the lower lipid wt concentrations of β-HCH (t=2.4, p=0.02) and higher HCB (t=4.4, P<0.001) still holds, while the significant difference for oxy-chlordane, p,p′-DDE and penta-PCBs disappeared (t<1.5, P>0.15). However, the hexa-PCBs, hepta-PCBs and PCB-194 (t=2.9, 5.0, 5.6 and P=<0.01,<0.001,<0.001, respectively) were significantly higher in livers than in preen at chick-rearing.
Fig. 1 Concentration of sum 19 PCBs in female and male kittiwake tissues. Feather (ng/g wet weight [wt]), liver and preen gland (ng/g lipid wt) from the incubation period (1) and the chick-rearing period (2).
![Fig. 1 Concentration of sum 19 PCBs in female and male kittiwake tissues. Feather (ng/g wet weight [wt]), liver and preen gland (ng/g lipid wt) from the incubation period (1) and the chick-rearing period (2).](/cms/asset/d72d933f-0759-4e4d-96d3-805880e263e8/zpor_a_11821478_f0001_ob.jpg)
The β-HCH, HCB, oxy-chlordane, mirex, penta-PCB, hexa-PCB, hepta-PCB and PCB-194 concentrations increased (t>2.5, P<0.02) between the two periods in both livers and preen glands ( illustrates ΣPCBs). Likewise, concentrations of p,p′-DDE (t=2.2, P=0.04) in livers increased from the incubation period to the chick-rearing period.
The concentrations of oxy-chlordane and p,p′-DDE (t=−2.5, −2.6 and P=0.02, 0.02, respectively) in kittiwakes’ feathers decreased from the incubation period to the chick-rearing period. Furthermore, all other contaminant concentrations of feathers, except PCB-194, decreased from the incubation period to the chick-rearing period, but these results were not significant (P>0.07).
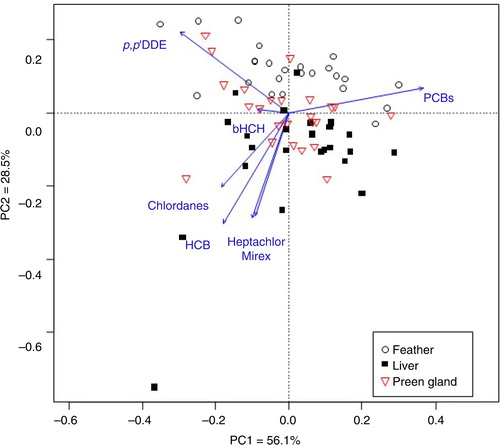
The present study found some significant, but small, differences in POP profiles between feathers, livers and preen glands (). Despite the small magnitude of these differences, our study highlights that the POP profile of feathers is more similar to the POP profile of preen glands than the profile of liver samples (). The PCA plot () illustrates that liver, preen gland and feather separates along the second axis and that the cluster of feather samples is slightly more similar to preen glands than livers. However, the variation in POP patterns explained by this predictor is very small (less than 5%). Furthermore, the fingerprint composition of POPs changes a bit from the incubation period to the chick-rearing period. The percentage portion of HCB, trans-nonachlor, cis-nonachlor, p,p′-DDE and PCB-128 decreased in liver (F>4.5, P<0.05), while PCB-153, -167, -170, -180, -187 and -194 increased (F>5, P<0.04) from incubation to the chick-rearing period. The change in preen was much less, with a decrease of trans-nonachlor and cis-nonachlor (F>4.3, P<0.02) only. The percentage portion of HCB, trans-nonachlor, cis-nonachlor, p,p′-DDE and PCB-128 decrease (F>4.5, P<0.05), while PCB-153, -167, -180, -183, -189 and -194 (F>6.4, P<0.02) increased from incubation to the chick-rearing period in feather.
Fig. 2 The POP profiles of feathers, preen glands and livers from kittiwakes. The 95% confidence intervals are indicated with error bars.
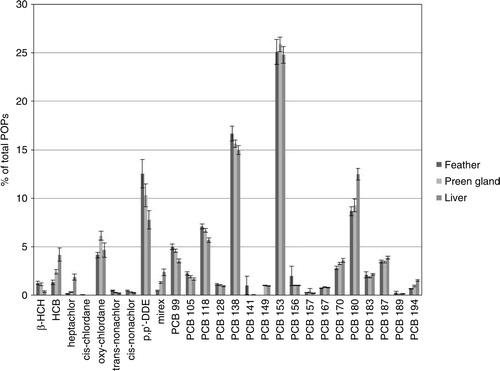
Fig. 3 Indirect ordination analysis from a PCA presenting the POPs (β-HCH, HCB, heptachlor, Σchlordanes, p,p′-DDE and ΣPCBs) profile in kittiwake feathers, livers and preen glands. The arrows point in the direction of increasing mean contribution of POP load. Percent variability explained by the two first principal components (PC1 and PC2) is given.
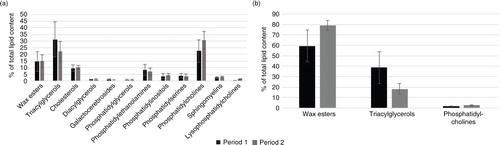
The seasonality contributed significantly to a lower lipid content in preen glands (t=9.80, df=21, P<0.001), but not in livers (t=1.41, df=21, P=0.17) (). The lipid classes, wax esters, triacylglycerols, cholesterols, diacylglycerols, galactocerebrosides, phosphatidylglycerols, phosphatidylethanolamines, phosphatidylinositols, phosphatidylserines, phosphatidylcholines, sphingomyelins and lysophosphatidylcholines, were detected in one or more of the analysed samples (). The four most important lipid classes were found to be wax esters (15% and 70% in liver and preen gland, respectively), triacylglycerols (27% and 28%), cholesterols (10% and 0%) and phosphatidylcholines (27% and 2%; ). The amount of triacylglycerols decreased, whereas the phosphatidylcholines increased from the incubation period to the chick-rearing period in both liver and preen gland (). Wax esters remained stable in liver, whereas they increased from the incubation period to the chick-rearing period in preen gland ().
Discussion
The reported concentrations of contaminants in kittiwakes from Kongsfjorden are similar to previous concentrations measured in kittiwake livers from the Barents Sea (Henriksen et al. Citation1996; Borgå et al. Citation2001), except for ΣPCBs and DDE, which were only half of the earlier reported concentrations (Henriksen et al. Citation1996; Borgå et al. Citation2001). For kittiwake feathers and preen glands, there are no POPs data available for comparison. Jaspers et al. (Citation2009) reported 220 ng/g mean wet wt ΣPCBs from tail feathers of herring gull (Larus argentatus) from Belgium, three and a half time the concentration found in the present study (). West Greenland white-tailed eagles (Haliaeetus albicilla) showed a tenfold increase of ΣPCBs from tail feather to body feather with a mean of 420 ng/g wet wt for the body feathers (Jaspers et al. Citation2011). The results from the present study are within the lower range of the values for tail feathers (). Furthermore, the study of Jaspers et al. (Citation2011) clearly showed that different feathers from the same bird varied significantly in contaminant concentrations, but as in the current study, the profile of POPs did not differ between tissue and feather types, except for the primary feather number 2.
Female seabirds transfer contaminants to their offspring through egg-yolk (Gabrielsen et al. Citation1995; Verreault et al. Citation2006; Helgason et al. Citation2008), and thereby reduce their body burden of POPs compared to male seabirds. While previous studies reported differences between the sexes (Gabrielsen et al. Citation1995), others did not (Sagerup, Savinov et al. Citation2009), leading to a non-consistent pattern regarding gender differences for contaminant concentrations in seabirds. In the current study, the low sample size (n=2×12) may have failed to detect significant differences between the sexes. Moreover, the variations in contaminant concentrations are high (), thereby masking apparent higher levels in the males (). The lipid content of the preen gland was found to be similar between female and male kittiwakes, which may indicate that both sexes would benefit equally from excretion of POPs through the preen gland.
High concentrations of POPs in preen gland tissue have been reported earlier (Ingebrigtsen et al. Citation1981; Frank et al. Citation1983; Ingebrigtsen et al. Citation1984) and is the basis for the hypothesis of significant excretion of POPs through the preen gland. The high concentrations of contaminants measured in the preen gland along with the finding of the same contamination on kittiwakes’ feathers, and a POP profile in feathers being similar to that of the preen gland (), indicate that the preen gland may be seen as an organ for excretion of POPs in kittiwakes (Frank et al. Citation1983).
Since birds preen their plumage with oil from the preen gland, the detection of POPs in the feathers might indicate excretion of POPs through the preen gland. The presence of POPs in the kittiwake feathers (; ) is in accordance with earlier studies on common loons (Gavia immer; Frank et al. Citation1983), common magpies (Pica pica; Jaspers et al. Citation2008) and white-tailed eagle (Jaspers et al. Citation2011) and indicate an excretion of POPs through the preen gland (Frank et al. Citation1983; Jaspers et al. Citation2008). However, the POPs in feathers may have other alternative sources of origin. First, the compounds might be remains from the growth period. As the feather stops to grow, the blood vessels atrophy and circulating to the feather ends (Burger & Gochfeld Citation1992). The cyclohexane/acetone treatment used for extraction of POPs in feathers might have extracted POPs from inside the feathers tissue. However, Jaspers et al. (Citation2008) found lower concentrations of POPs in acetone-washed feather and concluded that the main source of POPs in feathers came from the preen gland. Second, while the bird preens its feathers, dirt on the feathers is mixed and trapped in the preen oil on the feathers. Lastly, a deposition of airborne pollution has been reported for 5 of 13 heavy metals in four birds of prey (Dauwe et al. Citation2003). However, this phenomenon was excluded for organic compounds in the common buzzards (Buteo buteo; Jaspers, Covaci et al. Citation2007). The present study of kittiwakes shows some significant, but small, differences in POP profiles between feathers, livers and preen glands (, ). The PCA plot and the barplot together visualize that these differences are small and that the preen gland and feather profiles are most closely related, thereby supporting the prediction that POPs on kittiwakes’ feathers is mainly of internal origin. Additionally, the fact that POP concentrations change from the incubation period to the chick-rearing period, when no moulting occur, is a further indication that the main source of POPs on feathers is the preen gland.
The significant decrease in body mass (9%) from the incubation period to the chick-rearing period is mainly due to the greater energy demands of attending to chicks (Bech et al. Citation2002). This is in accordance with mass loss generally found in breeding birds (Moreno Citation1989), including kittiwakes (Henriksen et al. Citation1996; Langseth et al. Citation2001; Moe et al. Citation2002). During chick-rearing, kittiwake liver mass decreases significantly more than mass lost from the other organs or from the body overall (Langseth et al. Citation2001). This study shows that the lipid content of liver lipids remained stable, whereas preen gland lipid content decreased. This is consistent with observation made in glaucous gulls, where the total liver lipid contents of severely emaciated birds were comparable to the ones found in healthy gulls (Sagerup, Helgason et al. Citation2009).
The increase in POP concentrations from the incubation period to the chick-rearing period is in accordance with earlier findings in liver and blood of kittiwakes (Henriksen et al. Citation1996; Nordstad et al. Citation2012). During the breeding season the kittiwakes’ energy demands increase and shift from self-maintenance to chick care (Moe et al. Citation2002) and their field metabolic rate increases from the incubation period to the chick-rearing period (Fyhn et al. Citation2001). Body mass in birds decrease as body lipids are used in reproduction (Moe et al. Citation2002). The lipophilic contaminants are thereby redistributed and concentrated in the smaller amount of body lipids (Henriksen et al. Citation1996).
Whereas contaminant concentrations increased in liver and preen gland samples, feathers showed the opposite trend, with contaminant concentrations decreasing from the incubation period to the chick-rearing period (). In contrast to the present study, Frank et al. (Citation1983) reported higher contamination levels in feathers from emaciated common loons. However, these loons are not directly comparable to the kittiwakes we studied as the loons were sampled as carcasses; some were partially decomposed and the causes of death unknown.
Feathers are not connected to the birds’ circulatory system, and the only POPs that might change concentrations in relation to the seasonal factor are those contributed from external sources (Jaspers et al. Citation2008). The trend for decreasing contamination in feathers during the chick-rearing period may indicate that the energy reserves are too scarce for kittiwakes to sustain the same preen oil production. A general body mass decrease from incubation to chick-rearing and corresponding changes in body composition (Langseth et al. Citation2001; Bech et al. Citation2002) supports this suggestion. As reported in the present study, the POPs may be more concentrated in the preen gland during the chick-rearing period, but because of fat deficiency, the production of preen oil might be lower. A second explanation for the lower or stable contaminant concentrations found on kittiwake feathers may relate to the parents’ need to provide continuously food to the chicks (Bech et al. Citation2002). This entails increased exposure to seawater, which could be washing off contamination from the feathers. In addition, increased flight activity might reduce the time available for preening.
From the present study it is not possible to predict the quantitative amount of POPs that are excreted by the preen gland. Frank et al. (Citation1983) assumed the amount of POPs excreted via the preen gland to be minor, but Jacob (Citation1976) reported a daily excretion of 600 mg preen oil for birds in the Laridae family. The amount is, of course, questionable as species of the Laridae family range in body mass from ca. 100 to above 2000 g (del Hoyo et al. Citation1996). How much preen oil kittiwakes excrete is unknown, but, assuming that the small kittiwake (ca. 400 g) excretes less than Jacob's suggested 600 mg, a back of the envelope calculation illustrates that excretion through the preen gland could be important. The total body burden of POPs was approximately 40% (per gram) of the liver concentration (Verreault et al. Citation2007). The present average body burden of POPs would then be approximately 100000 ng. A low excretion rate of preen oil (30 mg) would therefore excrete about 10% of the estimated body burden during a year. However, if the excretion is 150 mg per day (25% of Jacob's estimate), 50% of the body burden is gone during a year. These calculations were made with high uncertainty but they illustrate that the amount of POPs excreted by the preen gland should not be neglected.
Unfortunately, lipid composition could not be analysed in the sampled feathers as the amount of lipid was too small. The liver was found to exhibit a greater diversity in lipid class composition, while the water repellent wax esters dominated the preen gland samples (). The solubility of POP compounds might be unequal for different lipid classes. However, the present study found all POP compounds present in both livers and preen glands. Some minor differences were detected in POP profiles (). This may indicate that in these organs, the specific POP compounds are mainly dissolved in the lipid pool as a whole, and are not associated to a certain lipid class (Gabrielsen et al. Citation1995). The excretion of these lipid-soluble compounds through the preen gland seems therefore not congener specific.
Conclusion
Our study supports the earlier finding that POPs found on bird feathers are mainly of internal origin and are excreted through the preen gland. We further suggest that the excretion of lipid-soluble POP compounds is unspecific. The lipid content of the preen gland is lowered during the energy-demanding chick-rearing period. This may indicate that kittiwakes allocate energy from the preen gland to another body function when rearing chicks. The concentrations of POPs in feathers were much lower (2–50 times in this study) than in liver and preen gland. In addition, time spent on water, reduced time for preening or such factors as volatilization may influence POP concentrations of body feathers in kittiwakes. More studies on feather types and seasonal variations are needed before a conclusion can be made about using feathers as a matrix for monitoring POPs in kittiwakes. The present study was not able to quantify the excretion of POPs from the preen gland. However, the preen oil in this study contained a high concentration of POPs, which indicates that a daily excretion of the contaminants occurs via the preen gland. Further work is recommended to perform quantitative studies of the preen gland's importance for POP excretion in seabirds.
Supplementary Material
Download PDF (262.5 KB)Acknowledgements
This study was financed by the Norwegian Polar Institute, the Norwegian Institute for Air Research, the Fram Centre’s flagship research programme, Hazardous Substances, and the University of Bergen. We would like to thank Ida Egge Johnsen, Gry Gasbjerg and Tore Nordstad for valuable help in the field. We are also grateful to Sophie Bourgeon and two anonymous reviewers whose comments significantly improved the manuscript.
Notes
To access the supplementary material for this article, please see the supplementary files under Article Tools, online.
References
- Anderson D.W., Hickey J.J. Dynamics of storage of organochlorine pollutants in herring gulls. Environmental Pollution. 1976; 10: 183–200.
- Angot H. Spatial differences in organochlorine pesticide concentrations in the benthic community in Svalbard. 2009; Master's thesis, National Graduate School of Chemistry of Montpellier.
- Barri L.A., Gregor D., Hargrave B., Lake R., Muir D.C.G., Shearer R., Tracey B., Bidleman T. Arctic contaminants: sources, occurrence and pathways. Science of the Total Environment. 1992; 122: 1–74.
- Bech C., Langseth I., Moe B., Fyhn M., Gabrielsen G.W. The energy economy of the Arctic-breeding kittiwake (Rissa tridactyla): a review. Comparative Biochemistry and Physiology Part A. 2002; 133: 765–776.
- Bernhoft A., Wiig Ø., Skaare J.U. Organochlorines in polar bears (Ursus maritimus) at Svalbard. Environmental Pollution. 1997; 95: 159–175.
- Borgå K., Fisk A.T., Hoekstra P.F., Muir D.C.G. Biological and chemical factors of importance in the bioaccumulation and trophic transfer of persistent organochlorine contaminants in Arctic marine food webs. Environmental Toxicology and Chemistry. 2004; 23: 2367–2385.
- Borgå K., Gabrielsen G.W., Skaare J.U. Biomagnification of organochlorines along a Barents Sea food chain. Environmental Pollution. 2001; 113: 187–198.
- Borgå K., Wolkers H., Skaare J.U., Hop H., Muir D.C.G., Gabrielsen G.W. Bioaccumulation of PCBs in Arctic seabirds: influence of dietary exposure and congener biotransformation. Environmental Pollution. 2005; 134: 397–409.
- Burger J., Gochfeld M. Trace-element distribution in growing feathers—additional excretion in feather sheaths. Archives of Environmental Contamination and Toxicology. 1992; 23: 105–108.
- Burkow I.C., Kallenborn R. Sources and transport of persistent pollutants to the Arctic. Toxicology Letters. 2000; 112: 87–92.
- Bustnes J.O., Bakken V., Skaare J.U., Erikstad K.E. Age and accumulation of persistent organochlorines: a study of Arctic-breeding glaucous gulls (Larus hyperboreus). Environmental Toxicology and Chemistry. 2003; 22: 2173–2179.
- Bytingsvik J., Lie E., Aars J., Derocher A.E., Wiig O., Jenssen B.M. PCBs and OH-PCBs in polar bear mother and cub pairs: a comparative study based on plasma levels in 1998 and 2008. Science of the Total Environment. 2012; 417: 117–128.
- Dauwe T., Bervoets L., Pinxten R., Blust R., Eens M. Variation of heavy metals within and among feathers of birds of prey: effects of molt and external contamination. Environmental Pollution. 2003; 124: 429–436.
- del Hoyo J., Elliott A., Sargatal J., Cabot J. Handbook of the birds of the world. 1996; Barcelona: Lynx Edicions.
- Donaldson G.M., Braune B.M., Gaston A.J., Noble D.G. Organochlorine and heavy metal residues in breast muscle of known-age thick-milled murres (Uria lomvia) from the Canadian Arctic. Archives of Environmental Contamination and Toxicology. 1997; 33: 430–435.
- Fisk A.T., Hobson K.A., Norstrom R.J. Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the northwater polynya marine food web. Environmental Science & Technology. 2001; 35: 732–738.
- Fort J., Robertson G.J., Grémillet D., Traisnel G., Bustamante P. Spatial ecotoxicology: migratory Arctic seabirds are exposed to mercury contamination while overwintering in the northwest Atlantic. Environmental Science & Technology. 2014; 48: 11560–11567.
- Frank R., Lumsden H., Barr J.F., Braun H.E. Residues of organochlorine insecticides, industrial-chemicals, and mercury in eggs and in tissues taken from healthy and emaciated common loons, Ontario, Canada, 1968–1980. Archives of Environmental Contamination and Toxicology. 1983; 12: 641–654.
- Fyhn M., Gabrielsen G.W., Nordoy E.S., Moe B., Langseth I., Bech C. Individual variation in field metabolic rate of kittiwakes (Rissa tridactyla) during the chick-rearing period. Physiological and Biochemical Zoology. 2001; 74: 343–355.
- Gabrielsen G.W. Sakshaug E. Seabirds in the Barents Sea. Ecosystem Barents Sea. 2009; Trondheim: Tapir Academic Press. 415–452.
- Gabrielsen G.W., Skaare J.U., Polder A., Bakken V. Chlorinated hydrocarbon in glaucous gulls (Larus hyperboreus) in the southern part of Svalbard. Science of the Total Environment. 1995; 160/161: 337–346.
- Graeve M., Janssen D. Improved separation and quantification of neutral and polar lipid classes by HPLC-ELSD using a monolithic silica phase: application to exceptional marine lipids. Journal of Chromatography B. 2009; 877: 1815–1819.
- Guengerich F.P. Reactions and significance of cytochrome-P-450 enzymes. Journal of Biological Chemistry. 1991; 266: 10019–10022.
- Helgason L.B., Barrett R.T., Lie E., Polder A., Skaare J.U., Gabrielsen G.W. Levels and temporal trends (1983–2003) of persistent organic pollutants (POPs) and mercury (Hg) in seabird eggs from northern Norway. Environmental Pollution. 2008; 155: 190–198.
- Henriksen E.O. Levels and congener pattern of PCBs in kittiwakes, Rissa tridactyla, in relation to mobilization of body-lipids associated with reproduction. 1995; Master's thesis, University of Tromsø.
- Henriksen E.O., Gabrielsen G.W., Skaare J.U. Levels and congeners pattern of polychlorinated biphenyls in kittiwakes (Rissa tridactyla), in relation to mobilization of body-lipids associated with reproduction. Environmental Pollution. 1996; 92: 27–37.
- Henriksen E.O., Gabrielsen G.W., Trudeau S., Wolkers H., Sagerup K., Skaare J.U. Organochlorines and possible biochemical effects in glaucous gulls (Larus hyperboreus) from Bjørnøya, the Barents Sea. Archives of Environmental Contamination and Toxicology. 2000; 38: 234–243.
- Herzke D., Gabrielsen G.W., Evenset A., Burkow I.C. Polychlorinated camphenes (toxaphenes), polybrominated diphenylethers and other halogenated organic pollutants in glaucous gull (Larus hyperboreus) from Svalbard and Bjørnøya (Bear Island). Environmental Pollution. 2003; 121: 293–300.
- Ingebrigtsen K., Brevik E.M., Nafstad I. Distribution and elimination of hexachlorbenzene (HCB) after single oral exposure in Japanese quail (Coturnix coturnix japonica). Journal of Toxicology and Environmental Health Part A. 1981; 8: 845–856.
- Ingebrigtsen K., Skaare J.U., Teigen S.W. Organochlorine residues in two Norwegian puffin (Fratercula arctica) colonies. Journal of Toxicology and Environmental Health Part A. 1984; 14: 813–828.
- Jacob J. Kolattkudy P.E. Bird waxes. Chemistry and biochemistry of natural waxes. 1976; Amsterdam: Elsevier. 93–146.
- Jaspers V.L.B., Covaci A., Deleu P., Eens M. Concentrations in bird feathers reflect regional contamination with organic pollutants. Science of the Total Environment. 2009; 407: 1447–1451.
- Jaspers V.L.B., Covaci A., Deleu P., Neels H., Eens M. Preen oil as the main source of external contamination with organic pollutants onto feathers of the common magpie (Pica pica). Environment International. 2008; 34: 741–748.
- Jaspers V.L.B., Covaci A., Van den Steen E., Eens M. Is external contamination with organic pollutants important for concentrations measured in bird feathers?. Environment International. 2007; 33: 766–772.
- Jaspers V.L.B., Herzke D., Eulaers I., Gillespie B.W., Eens M. Perfluoroalkyl substances in soft tissues and tail feathers of Belgian barn owls (Tyto alba) using statistical methods for left-censored data to handle non-detects. Environment International. 2013; 52: 9–16.
- Jaspers V.L.B., Rodriguez F.S., Boertmann D., Sonne C., Dietz R., Rasmussen L.M., Eens M., Covaci A. Body feathers as a potential new biomonitoring tool in raptors: a study on organohalogenated contaminants in different feather types and preen oil of west Greenland white-tailed eagles (Haliaeetus albicilla). Environment International. 2011; 37: 1349–1356.
- Jaspers V.L.B., Voorspoels S., Covaci A., Lepoint G., Eens M. Evaluation of the usefulness of bird feathers as a non-destructive biomonitoring tool for organic pollutants: a comparative and meta-analytical approach. Environment International. 2007; 33: 328–337.
- Kent G.C. Comparative anatomy of the vertebrates. 2001; Boston, MA: McGraw Hill.
- Kucklick J.R., Struntz W.D.J., Becker P.R., York G.W., O'Hara T.M., Bohonowych J.E. Persistent organochlorine pollutants in ringed seals and polar bears collected from northern Alaska. Science of the Total Environment. 2002; 287: 45–59.
- Langseth I., Moe B., Bech C. Reduction in body mass and basal metabolic rate in breeding female black-legged kittiwakes Rissa tridactyla: an adaptation to reduce maintenance costs. Atlantic Seabirds. 2001; 3: 165–178.
- Letcher R.J., Bustnes J.O., Dietz R., Jenssen B.M., Jorgensen E.H., Sonne C., Verreault J., Vijayan M.M., Gabrielsen G.W. Exposure and effects assessment of persistent organohalogen contaminants in Arctic wildlife and fish. Science of the Total Environment. 2010; 408: 2995–3043.
- MacDonald R.W., Bewers J.M. Contaminants in the Arctic marine environment: priorities for protection. ICES Journal of Marine Science. 1996; 53: 537–563.
- Moe B., Langseth I., Fyhn M., Gabrielsen G.W., Bech C. Changes in body condition in breeding kittiwakes Rissa tridactyla . Journal of Avian Biology. 2002; 33: 225–234.
- Mora M., Auman H., Ludwig J., Giesy J., Verbrugge D., Ludwig M. Polychlorinated biphenyls and chlorinated insecticides in plasma of Caspian terns: relationships with age, productivity, and colony site tenacity in the Great Lakes. Archives of Environmental Contamination and Toxicology. 1993; 24: 320–331.
- Moreno J. Strategies of mass change in breeding birds. Biological Journal of the Linnean Society. 1989; 37: 297–310.
- Nordstad T., Moe B., Bustnes J.O., Bech C., Chastel O., Goutte A., Sagerup K., Trouvé C., Herzke D., Gabrielsen G.W. Relationships between POPs and baseline corticosterone levels in black-legged kittiwakes (Rissa tridactyla) across their breeding cycle. Environmental Pollution. 2012; 164: 219–226.
- Nøst T.H., Breivik K., Fuskevag O.M., Nieboer E., Odland J.O., Sandanger T.M. Persistent organic pollutants in Norwegian men from 1979 to 2007: intraindividual changes, age-period-cohort effects, and model predictions. Environmental Health Perspectives. 2013; 121: 1292–1298.
- Nøst T.H., Vestergren R., Berg V., Nieboer E., Odland J.Ø., Sandanger T.M. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environment International. 2014; 67: 43–53.
- R Core Team. R: a language and environment for statistical computing. 2015; Vienna, Austria: R Foundation for Statistical Computing.
- Robinson J., Richards A., Crabtree A.N., Coulson J.C., Potts G.R. Organochlorine residues in marine organisms. Nature. 1967; 214: 1307–1311.
- Rothman K.J. No adjustments are needed for multiple comparisons. Epidemiology. 1990; 1: 43–46.
- Sagerup K., Helgason L.B., Polder A., Strøm H., Josefsen T.D., Skåre J.U., Gabrielsen G.W. Persistent organic pollutants and mercury in dead and dying glaucous gulls (Larus hyperboreus) at Bjørnøya (Svalbard). Science of the Total Environment. 2009; 407: 6009–6016.
- Sagerup K., Savinov V., Savinova T., Kuklin V., Muir D.C.G., Gabrielsen G.W. Persistent organic pollutants, heavy metals and parasites in the glaucous gull (Larus hyperboreus) on Spitsbergen. Environmental Pollution. 2009; 157: 2282–2290.
- Skaare J.U., Bernhoft A., Derocher A.E., Gabrielsen G.W., Goksoyr A., Henriksen E.O., Larsen H.J.S., Lie E., Wiig Ø. Organochlorines in top predators at Svalbard—occurrence, levels and effects. Toxicology Letters. 2000; 112: 103–109.
- Stevens L. Avian biochemistry and molecular biology. 1996; Cambridge: Cambridge University Press.
- van den Brink N. Battaglia B. Preengland oil and blood samples: non-destructive methods for monitoring organochlorine levels in Antarctic top predators. Antarctic communities: species, structure and survival. 1997; Cambridge: Cambridge University Press. 413–416.
- van den Brink N.W., Jansman H.A. Applicability of spraints for monitoring organic contaminants in free-ranging otters (Lutra lutra). Environmental Toxicology and Chemistry. 2006; 25: 2821–2826.
- van den Brink N.W., van Franeker J.A., de Ruiter Dijkman E.M. Fluctuating concentrations of organochlorine pollutants during a breeding season in two Antarctic seabirds: Adelie penguin and southern fulmar. Environmental Toxicology and Chemistry. 1998; 17: 702–709.
- Verreault J., Gabrielsen G.W., Bustnes J.O. The Svalbard glaucous gull as bioindicator species in the European Arctic: insight from 35 years of contaminants research. Reviews of Environment Contamination and Toxicology. 2010; 205: 77–116.
- Verreault J., Gabrielsen G.W., Chu S.G., Muir D.C.G., Andersen M., Hamaed A., Letcher R.J. Flame retardants and methoxylated and hydroxylated polybrominated diphenyl ethers in two Norwegian Arctic top predators: glaucous gulls and polar bears. Environmental Science & Technology. 2005; 39: 6021–6028.
- Verreault J., Muir D.C.G., Norstrom R.J., Stirling I., Fisk A.T., Gabrielsen G.W., Derocher A.E., Evans T.J., Dietz R., Sonne C., Sandala G.M., Gebbink W., Riget F.F., Born E.W., Taylor M.K., Nagy J., Letcher R.J. Chlorinated hydrocarbon contaminants and metabolites in polar bears (Ursus maritimus) from Alaska, Canada, east Greenland, and Svalbard: 1996–2002. Science of the Total Environment. 2005; 351: 369–390.
- Verreault J., Shahmiri S., Gabrielsen G.W., Letcher R.J. Organohalogen and metabolically-derived contaminants and associations with whole body constituents in Norwegian Arctic glaucous gulls. Environment International. 2007; 33: 823–830.
- Verreault J., Villa R.A., Gabrielsen G.W., Skaare J.U., Letcher R.J. Maternal transfer of organohalogen contaminants and metabolites to eggs of Arctic-breeding glaucous gulls. Environmental Pollution. 2006; 144: 1053–1060.
- Vieweg I., Hop H., Brey T., Huber S., Ambrose J., Locke V., Gabrielsen G.W. Persistent organic pollutants in four bivalve species from Svalbard waters. Environmental Pollution. 2012; 161: 134–142.
- Walker C.H., Hopkin S.P., Sibly R.M., Peakall D.B. Principles of Ecotoxicology. 2006; Boca Raton, FL: Taylor & Francis.
- Wolkers H., Lydersen C., Kovacs K.M. Accumulation and lactational transfer of PCBs and pesticides in harbor seals (Phoca vitulina) from Svalbard, Norway. Science of the Total Environment. 2004; 319: 137–146.
- Yamashita R., Takada H., Murakami M., Fukuwaka M.A., Watanuki Y. Evaluation of noninvasive approach for monitoring PCB pollution of seabirds using preen gland oil. Environmental Science & Technology. 2007; 41: 4901–4906.