Abstract
Sponges are important components of high-latitude benthic communities, but their diversity and abundance in algal-dominated rocky reefs has been underestimated because of the difficulty of in situ identification. Further, the influence of canopy-forming algae on sponge richness has been poorly studied in southern high-latitude rocky reefs compared to other latitudes. Here, we quantified taxon richness of sponges in algae-dominated rocky reefs at three sites in the western Antarctic Peninsula (62–64° S) and two sites in the Magellan region (53° S). We found higher sponge richness at sites in Antarctica (15) than in Magallanes (8), with Antarctic sponge richness higher than that reported for Arctic algal beds and similar to that reported for temperate regions. Estimated sponge richness at our Antarctic sites highlights diverse sponge assemblages (16–26 taxa) between 5 and 20 m that are typically dominated by macroalgae. Our results suggest that sponge assemblages associated with canopy-forming macroalgae on southern high-latitude reefs are more diverse than previously thought.
Despite the abundance and diversity of sponges generally being considered to be negatively correlated with macroalgae (Bell & Barnes Citation2000; Cárdenas et al. Citation2012), some studies have reported positive associations between canopy-forming algae and sponges (Wright et al. Citation1997; Ávila et al. Citation2010; Cárdenas et al. Citation2016). This suggests that some sponge species might benefit from the presence of canopy-forming algae that provide sponges with protection within their holdfasts or alter the abiotic and biotic environment in ways that favour sponges, for example, by reducing ambient light and/or competition (Cárdenas et al. Citation2016). However, the associations between canopy-forming algae and sponge richness and diversity, and how these relationships change across latitudinal gradients are not well understood.
Shallow-water rocky reefs in the western Antarctic Peninsula are characterized by the presence of algae-dominated benthic communities (Zamorano Citation1983; Amsler et al. Citation1995; Valdivia et al. Citation2015). In general, macroalgae dominate the substrate from 5 to 25 m depth, with sponges and other filter-feeding organisms dominating diversity and abundance at depths greater than ca. 25 m (Zamorano Citation1983; Barnes Citation1995a, Citationb). Despite sponges being important members of Antarctic benthic communities (McClintock et al. Citation2005; Janussen & Downey Citation2014), the diversity and abundance of sponges are likely to have been underestimated in many surveys and studies of shallow-water benthic communities as they are considered taxonomically challenging and are hard to identify in the field (Bell & Barnes Citation2001; Ávila et al. Citation2015). This has led to a general view that sponges are depauperate on Antarctic Peninsular algae-dominated reefs in shallow water (<25 m) since they have not been recorded in most studies or have been treated as one species/group (e.g., Sakurai et al. Citation1996; Valdivia et al. Citation2015). Therefore, only limited information is available on the diversity and abundance of subcanopy sponges, and data is only available for a few sites in King George Island (South Shetland Islands) and Wilkes Land in East Antarctica (Clark et al. Citation2011; Newcombe & Cárdenas Citation2011). The sub-Antarctic part of the Magellan region hosts dense beds of brown algae (Macrocystis pyrifera and Lessonia spp.); however, these communities are arguably less well studied than their Antarctic equivalents.
The impacts of climate change have the potential to have dramatic effects on shallow-water benthic communities, as significant changes in light regimes associated with sea-ice cover may produce shifts from algae- to invertebrate-dominated communities or vice versa (Clark et al. Citation2013; Clark et al. Citation2015). Determining the consequences of stable-state shifts from algal to invertebrate-dominated communities on sponge diversity and abundance requires an understanding of current spatial and temporal patterns in shallow-water sponge assemblages and their relationship with canopy-forming algae. These insights may be further strengthened by contrasting the relationship between canopy-forming algae and sponges between areas with different abiotic conditions (e.g., along latitudinal gradients).
The aim of this study was to quantify the taxon richness of sponges associated with canopy-forming algae in King George Island and Doumer Island, Antarctic Peninsula and the Magellan Strait. We also aimed to compare observed and estimated sponge richness from southern high-latitude algal beds with those reported from other latitudes.
Materials and methods
Algae-dominated rocky reefs were opportunistically sampled at three sites on the Antarctic Peninsula—Collins and Fildes bays (South Shetland Islands) and South Bay (Doumer Island)—and two sites in the Magellan region—Fuerte Bulnes and Puerto del Hambre (see for descriptions of dominant macroalgal types). At each site, a series of 0.5 m2 quadrats were haphazardly placed and photographed. In Antarctica, photoquadrats were sampled from 10 to 20 m depth, whereas in the Magellan region, photoquadrats in Lessonia beds were taken at 1 m depth, and photoquadrats in Macrocystis pyrifera beds were taken at 5 m depth. Sponges were either identified to the lowest possible taxonomic level from photoquadrats, using the image analysis software CPCe version 3.5 (Kohler & Gill Citation2006) or tissue samples, following the procedure described by Hajdu et al. (Citation2011). We also used photoquadrats to quantify the direct (i.e., those using holdfasts as substratum) and indirect (i.e., those attached to the rock surface) associations of sponges with canopy-forming algae.
Table 1 The location of each of our study sites on the western Antarctic Peninsula and the Magellan Strait and the dominant canopy-forming algae present.
We estimated taxon richness and occurrence of sponges for each site, with occurrence calculated as the percentage of the total number of quadrats sampled at each site in which a taxon was found. Variation in taxon richness between sites was calculated using the non-parametric estimator Chao2=Sobs+(Q1)2/2(Q2), where Sobs is the number of species observed in the total data set, Q1 is number of species found at only one sample and Q2 species found in two samples. Chao2 was calculated using EstimateS version 9.1 (Colwell Citation2013) and provides robust estimates of true species richness even for a low number of samples (Gotelli & Colwell Citation2010).
We also collected 10–15 holdfasts of Himantothallus grandifolius and Desmarestia spp. in Antarctica, and Lessonia spp. and M. pyrifera in the Magellan region. We used Spearman's rank correlations to test for correlations between holdfast volume (determined by water displacement) and the number of epithetic sponge individuals.
Results and discussion
We recorded a total of 42 Demospongiae and 1 Calcarea taxa (). In Antarctica, observed taxon richness ranged between 15 taxa in Collins Bay and 10 taxa in Fildes and South bays (a). In the Magellan region, the observed taxon richness ranged between two taxa in Macrocystis pyrifera beds at Puerto del Hambre and eight taxa in Lessonia and M. pyrifera beds at Bulnes (a). Lower sponge richness within M. pyrifera beds at Puerto del Hambre may be explained by the substrate being mainly unstable boulders (Newcombe & Cárdenas Citation2011), whereas lower taxon richness within shallow Lessonia beds may be due to increased wave action and ultraviolet radiation, both of which can be detrimental for many sponge species (Wilkinson & Vacelet Citation1979; Roberts et al. Citation2006).
Fig. 1 The taxon richness of sponges associated with canopy-forming algae as a function of the number of quadrats sampled at sites in Antarctica (circles) and Magallanes (triangles). (a) Observed sponge richness and (b) estimated sponge richness by using Chao2 estimate. Plotted values of Chao2 are based on 100 randomizations of sample order. Note that the scale on the Y axis differs between panels.
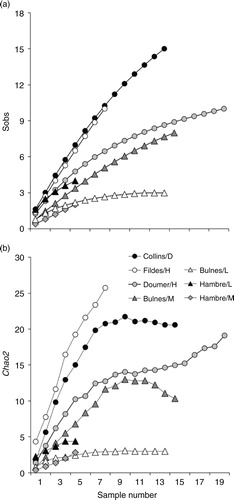
Table 2 Sponges associated with canopy-forming algae at sites in Antarctica and Magallanes. Asterisks indicate direct associations between sponge taxa and algal holdfasts. Operational taxonomic unit is abbreviated to OTU.
The Chao2 index estimated higher sponge richness for Antarctic than Magellan sites (b). Although the accumulation curves suggest Antarctic sponge richness was highest at Fildes Bay (26 taxa), sponge richness at Collins Bay (21 taxa) and Doumer Island (16 taxa) did not reach an asymptote, suggesting substantial undiscovered sponge biodiversity at these locations (b). Magellan sites had estimated taxon richness of 10 and three taxa in M. pyrifera beds at Fuerte Bulnes and Puerto del Hambre, respectively (b), and four and three taxa were in Lessonia beds in Hambre and Bulnes, respectively.
The sponges Dendrilla antarctica and Cribochalina sp. were the most frequent taxa in Collins and Fildes Bays, whereas Mycale (Oxymycale) acerata was the most common species at Doumer Island (). In Magallanes, the encrusting species Scopalina sp. was the most common species in shallow-water Lessonia beds. Massive sponges such as Mycale (Aegogropila) magellanica, Tedania sp. and the encrusting Phorbas cf. shackeltoni were the most common species in M. pyrifera beds at Fuerte Bulnes ().
Of the 43 sponge taxa recorded, only two were directly associated with holdfasts in Antarctica, compared to five in Magallanes (); however, none of these taxa exclusively associated with holdfasts and they were more commonly found on rock surfaces underneath the canopy. In Antarctica, the number of sponge individuals was positively correlated with the volume of Desmarestia spp. holdfasts, with sponges typically colonizing large holdfasts, 200–900 cm3 in size (Spearman rank=0.805, P=0.015). Sponges were associated with Desmarestia in 28.6% of the holdfast analysed (). Conversely, sponges were not epithetic on Himantothallus grandifolius holdfasts, which may be explained by the small volume and morphology of holdfasts or by chemical defences, as it has been previously demonstrated (Amsler et al. Citation2005). In the Magellan Strait, sponges were positively correlated with Lessonia spp. holdfast in the shallows (Spearman rank=0.809, P=0.009). However, there was only a low correlation with holdfasts of M. pyrifera and no relationship with holdfast volume (Spearman rank=0.174, P=0.607).
With a few exceptions (e.g., Wright et al. Citation1997; Cárdenas et al. Citation2016), most studies from other latitudes have focused on sponges directly associated with holdfasts (e.g., Smith et al. Citation1996). Further, previous research on Lessonia spp. and M. pyrifera in northern and central Chile (21 sites from 20° to 40° S) has either not reported sponges (Ojeda & Santelices Citation1984; Villouta & Santelices Citation1984) or has reported them as a group (Vásquez & Santelices Citation1984), although López-Gappa et al. (Citation1982) did report sponges in very low abundance on the Atlantic coast of Patagonia (47° S). Our results begin to address this existing knowledge gap by demonstrating that in high-latitude algal beds sponges form not only direct associations with holdfasts, but also indirect associations. Our results also suggest the existence of richer sponge assemblages than previously described for Antarctic and sub-Antarctic latitudes (see Dayton et al. Citation1974; Zamorano Citation1983; Ojeda & Santelices Citation1984; Barnes & Brockington Citation2003; Bowden Citation2005; McClintock et al. Citation2005; Clark et al. Citation2011). We observed sponge richness in the western Antarctic Peninsula to be higher than that previously reported in: (i) East Antarctica (seven taxa) by Clark et al. (Citation2011), (ii) Arctic macroalgal beds (two–three taxa) by Lippert et al. (Citation2001) and Wlodarska-Kowalczuk et al. (Citation2009) and (iii) mixed kelp beds of Ecklonia radiata and M. pyrifera (7 taxa) on the south coast of Wellington, New Zealand, by Cárdenas (unpubl. data). Sponge richness reported by our study is comparable or higher than temperate kelp beds of Sargassum spp. (12 taxa) in Baja California, Mexico (Ávila et al. Citation2010).
Results from our study suggest that canopy-forming macroalgae may play an important role in structuring sponge assemblages on Antarctic shallow rocky reefs. It is possible that canopy-formers may help maintain sponge richness in highly disturbed areas in the western Antarctic Peninsula. Further manipulative studies, however, may provide more information that will clarify if canopy plays a positive effect on sponge species. In contrast, the role of canopy-formers may play a different role as the impact of fast ice or ice-scour is absent in the study area (Cárdenas & Montiel Citation2015). Instead, protection from waves and high irradiance may be more relevant, especially for sponges in the shallows.
In recent years, there has been a growing amount of research examining impacts of global climate change and ocean acidification on sponges and their relationship with canopy-forming algae (e.g., Bell et al. Citation2015; Cárdenas et al. Citation2016); however, little is known about potential impacts of predicted changes in water temperature, pH and turbidity on sponges and their relationships with macroalgae (but see Duckworth & Peterson Citation2012; Schoenrock et al. Citation2015). Baseline information, such as that presented here, combined with experimental research will further our understanding of the drivers of current diversity patterns and potential impacts of climate change and ocean acidification on high-latitude sponges and their relationships with other members of shallow-water benthic communities.
Acknowledgements
We thank the Chilean Antarctic Institute personnel and volunteers at Professor Julio Escudero and Yelcho research stations for field and logistical support, and the Brazilian National Council for Scientific and Technological Development, Brazil. We also thank J.B. McClintock and an anonymous reviewer for their constructive comments. This research was partially funded by projects INACH:T2-08, FIC-R 2008 (Gobierno Regional de Magallanes y Antártica Chilena), FAPERJ (Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Brasil) and CONICYT/FONDECYT/INACH/INICIACION/#11150129.
References
- Amsler C.D., Iken K., McClintock J.B., Amsler M.O., Peters K.J., Hubbard J.H., Furrow F.B., Baker B.J. Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Marine Ecology Progress Series. 2005; 294: 141–159.
- Amsler C.D., Rowley R.J., Laur D.R., Quentin L.B., Ross R.M. Vertical distribution of Antarctic Peninsular macroalgae: cover, biomass and species composition. Phycologia. 1995; 34: 424–430.
- Ávila E., Ávila-Garcia A.K., Cruz-Barraza J.A. Temporal and small-scale spatial variations in abundance and biomass of seagrass-dwelling sponges in a tropical estuarine system. Marine Ecology. 2015; 36: 623–636.
- Ávila E., Blancas-Gallangos N.I., Riosmena-Rodríguez R., Paul-Chávez L. Sponges associated with Sargassum spp. (Phaeophyceae: Fucales) from the south-western Gulf of California. Journal of the Marine Biological Association of the United Kingdom. 2010; 90: 193–202.
- Barnes D.K.A. Sublittoral epifaunal communities at Signy Island, Antarctica. I. The ice foot zone. Marine Biology. 1995a; 121: 555–563.
- Barnes D.K.A. Sublittoral epifaunal communities at Signy Island, Antarctica. II. Below the ice foot zone. Marine Biology. 1995b; 121: 565–572.
- Barnes D.K.A., Brockington S. Zoobenthic biodiversity, biomass and abundance at Adelaide Island, Antarctica. Marine Ecology Progress Series. 2003; 249: 145–155.
- Bell J.J., Barnes D.K.A. A sponge diversity centre within a marine “island”. Hydrobiologia. 2000; 440: 55–64.
- Bell J.J., Barnes D.K.A. Sponge morphological diversity: a qualitative predictor of species diversity?. Aquatic Conservation: Marine and Freshwater Ecosystems. 2001; 11: 109–121.
- Bell J.J., McGrath E., Biggerstaff A., Bates T., Cárdenas C.A., Bennett H. Global conservation status of sponges. Conservation Biology. 2015; 29: 42–53.
- Bowden D.A. Quantitative characterization of shallow marine benthic assemblages at Ryder Bay, Adelaide Island, Antarctica. Marine Biology. 2005; 146: 1235–1249.
- Cárdenas C.A., Davy S.K., Bell J.J. Correlations between algal abundance, environmental variables and sponge distribution patterns on Southern Hemisphere temperate rocky reefs. Aquatic Biology. 2012; 16: 229–239.
- Cárdenas C.A., Davy S.K., Bell J.J. Influence of canopy-forming algae on temperate sponge assemblages. Journal of the Marine Biological Association of the United Kingdom. 2016; 96: 351–362.
- Cárdenas C.A., Montiel A. The influence of depth and substrate inclination on sessile assemblages in sub-Antarctic rocky reefs (Magellan region). Polar Biology. 2015; 38: 1631–1644.
- Clark G.F., Marzinelli E.M., Fogwill C.J., Turney C.S.M., Johnston E.L. Effects of sea-ice cover on marine benthic communities: a natural experiment in Commonwealth Bay, East Antarctica. Polar Biology. 2015; 38: 1213–1222.
- Clark G.F., Stark J.S., Johnston E.L., Runcie J.W., Goldsworthy P.M., Raymond B., Riddle M.J. Light-driven tipping points in polar ecosystems. Global Change Biology. 2013; 19: 3749–3761.
- Clark G.F., Stark J.S., Perrett L.A., Hill N.A., Johnston E.L. Algal canopy as a proxy for the disturbance history of understorey communities in East Antarctica. Polar Biology. 2011; 34: 781–790.
- Colwell R.K. EstimateS: statistical estimation of species richness and shared species from samples. Version 9.1. User's guide and application. 2013. Accessed on the internet at http://purl.oclc.org/estimates.
- Dayton P.K., Robilliard G.A., Paine R.T., Dayton L.B. Biological accommodation in the benthic community at McMurdo Sound, Antarctica. Ecological Monographs. 1974; 44: 105–128.
- Duckworth A.R., Peterson B.J. Effects of seawater temperature and pH on the boring rates of the sponge Cliona celata in scallop shells. Marine Biology. 2012; 160: 27–35.
- Gotelli N.J., Colwell R.K. Magurran A.E., McGill B.J. Estimating species richness. Frontiers in measuring biodiversity. 2010; New York: Oxford University Press. 39–54.
- Hajdu E., Peixinho S., Fernandez J.C.C. Esponjas Marinhas da Bahia. Guia de campo e laboratório: Sermograf, Rio de Janeiro: Museu Nacional, Serie livros 45. (Marine sponges of Bahia. Field and laboratory guide. Monograph Series 45.). 2011; Rio de Janeiro: National Museum of Brazil.
- Janussen D., Downey R.V. Koubbi P. Chapter 5.5. Porifera. Biogeographic atlas of the Southern Ocean. 2014; Cambridge: Scientific Committee on Antarctic Research. 94–102.
- Kohler K.E., Gill S.M. Coral Point Count with Excel extensions (CPCe): a Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Computers and Geosciences. 2006; 32: 1259–1269.
- Lippert H., Iken K., Rachor E., Wiencke C. Macrofauna associated with macroalgae in the Kongsforden (Spitsbergen). Polar Biology. 2001; 24: 512–522.
- López-Gappa J.J., Romanello E.E., Hernández D.A. Observaciones sobre la macrofauna y flora asociadas a los grampones de Macrocystis pyrifera (L.) C. Ag. en la Ría Deseado (Santa Cruz, Argentina). (Observations on macrofauna and flora associated with holdfasts of Macrocystis pyrifera (L.) C. Ag in Ría Deseado [Santa Cruz, Argentina].). Ecosur. 1982; 9: 67–106.
- McClintock J.B., Amsler C.D., Baker B.J., van Soest R.W.M. Ecology of Antarctic marine sponges: an overview. Integrative and Comparative Biology. 2005; 45: 359–368.
- Newcombe E.M., Cárdenas C.A. Rocky reef benthic assemblages in the Magellan Strait and the South Shetland Islands (Antarctica). Revista de Biología Marina y Oceanografía. 2011; 46: 177–188.
- Ojeda F.P., Santelices B. Invertebrate communities in holdfasts of the kelp Macrocystis pyrifera from southern Chile. Marine Ecology Progress Series. 1984; 16: 65–73.
- Roberts D.E., Cummins S.P., Davis A.R., Chapman M.G. Structure and dynamics of sponge-dominated assemblages on exposed and sheltered temperate reefs. Marine Ecology Progress Series. 2006; 321: 19–30.
- Sakurai H., Sato T., Arai H., Takasaki A., Tada S., Hori H., Kimpara I., Matsuyama T., Kodama M. Habitats of fish and epibenthic invertebrates in Fildes Bay, King George Island, Antarctica. Proceedings of the NIPR Symposium on Polar Biology. 1996; 9: 231–242.
- Schoenrock K.M., Schram J.B., Amsler C.D., McClintock J.B., Angus R.A. Climate change impacts on overstory Desmarestia spp. from the western Antarctic Peninsula. Marine Biology. 2015; 162: 377–389.
- Smith S.D.A., Simpson R.D., Cairns S.C. The macrofaunal community of Ecklonia radiata holdfasts: description of the faunal assemblage and variation associated with differences in holdfast. Australian Journal of Ecology. 1996; 21: 81–95.
- Valdivia N., Díaz M.J., Garrido I., Gómez I. Consistent richness-biomass relationship across environmental gradients in a marine macroalgal-dominated subtidal community on the western Antarctic Peninsula. PloS One. 2015; 10: 0138582. http://dx.doi.org/10.1371/journal.pone.0138582.
- Vásquez J., Santelices B. Comunidades de macroinvertebrados en discos adhesivos de Lessonia nigrescens Bory (Phaeophyta) en Chile central (Macroinvertebrates communities in holdfasts of Lessonia nigrescens Bory [Phaeophyta] in central Chile.). Revista Chilena de Historia Natural. 1984; 57: 131–154.
- Villouta E., Santelices B. Estructura de la comunidad submareal de Lessonia (Phaeophyta, Laminariales) en Chile norte y central. (Community structure of the subtidal Lessonia [Phaeophyta, Laminariales] in north and central Chile.). Revista Chilena de Historia Natural. 1984; 57: 111–122.
- Wilkinson C.R., Vacelet J. Transplantation of marine sponges to different conditions of light and current. Journal of Experimental Marine Biology and Ecology. 1979; 37: 91–104.
- Wlłodarska-Kowalczuk M., Kukliński P., Ronowicz M., Legeżyńska J., Gromisz S. Assessing species richness of macrofauna associated with macroalgae in Arctic kelp forests (Hornsund, Svalbard). Polar Biology. 2009; 32: 897–905.
- Wright J.T., Benkendorff K., Davis A.R. Habitat associated differences in temperate sponge assemblages: the importance of chemical defence. Journal of Experimental Marine Biology and Ecology. 1997; 213: 199–213.
- Zamorano J.H. Zonación y biomasa de la macrofauna bentónica en Bahía South, Archipiélago de Palmer, Antártica. (Zonation and biomass of the benthic macrofauna in South Bay, Palmer Archipelago, Antarctica.). INACH Serie Científica. 1983; 30: 27–38.
