Abstract
Background
Modulation of sexual desires is, in some cases, necessary to avoid inappropriate or illegal sexual behavior (downregulation of sexual desire) or to engage with a romantic partner (upregulation of sexual desire). Some have suggested that those who have difficulty downregulating their sexual desires be diagnosed as having a sexual ‘addiction’. This diagnosis is thought to be associated with sexual urges that feel out of control, high-frequency sexual behavior, consequences due to those behaviors, and poor ability to reduce those behaviors. However, such symptoms also may be better understood as a non-pathological variation of high sexual desire. Hypersexuals are thought to be relatively sexual reward sensitized, but also to have high exposure to visual sexual stimuli. Thus, the direction of neural responsivity to sexual stimuli expected was unclear. If these individuals exhibit habituation, their P300 amplitude to sexual stimuli should be diminished; if they merely have high sexual desire, their P300 amplitude to sexual stimuli should be increased. Neural responsivity to sexual stimuli in a sample of hypersexuals could differentiate these two competing explanations of symptoms.
Methods
Fifty-two (13 female) individuals who self-identified as having problems regulating their viewing of visual sexual stimuli viewed emotional (pleasant sexual, pleasant-non-sexual, neutral, and unpleasant) photographs while electroencephalography was collected.
Results
Larger P300 amplitude differences to pleasant sexual stimuli, relative to neutral stimuli, was negatively related to measures of sexual desire, but not related to measures of hypersexuality.
Conclusion
Implications for understanding hypersexuality as high desire, rather than disordered, are discussed.
Sexual desires must be consistently regulated to manage sexual behaviors. Downregulation may occur to avoid sexual partners who are illegal, as with pedophilia, or inappropriate, as with infidelity. Upregulation may occur to engage with a romantic partner or increase stimulation to facilitate a genital response sufficient for intercourse. Under the influence of alcohol, it is the sexual arousal, rather than the amount of alcohol consumed, that best predicts intentions to engage in risky sexual behaviors (George et al., Citation2009; Prause, Staley, & Finn, Citation2011). To date, success in developing treatments to help regulate sexual desires have been sparse, ineffective, or drastic. For example, hypoactive sexual desire disorder lacks any strong treatment for upregulation (Heiman, Citation2002). For downregulation, pharmacological treatments have been used to treat a variety of sexual urges directed inappropriately (Briken, Hill, & Berner, Citation2003), but these carry a risk for serious, not infrequent, side effects (Krueger, Hembree, & Hill, Citation2006). Sexual ‘addiction’ may represent a unique case of regulation failure, where the problem is not that sexual desire is directed toward a problematic target, but the level of the sexual desire itself is thought to be central to the problem.
Sexual regulation problems appear in those reporting higher levels of sexual desire (Winters, Christoff, & Gorzalka, Citation2010). Once called ‘excessive sexual desire disorder’ (Marshall & Marshall, Citation2001), these problem behaviors have been spun into many labels thought to describe a constellation of disease symptoms (e.g. ‘hypersexuality’). It is unclear, however, whether the additional features of the proposed disease (Kafka, Citation2010) add any explanatory power. For comparison, consider excessive television viewing in children. More hours viewing television is associated with many health problems (Boulos, Vikre, Oppenheimer, Chang, & Kranarek, Citation2012), which feeds back to increase television viewing (Fuller-Tyszkiewicz, Skouteris, Hardy, & Halse, Citation2012). Several treatments have been created that effectively decrease television viewing time (e.g. Robinson, Citation1999). Treatments focus on reducing the number of hours viewing television behaviorally without a disease overlay such as ‘television addiction’ and are effective. This suggests a similar approach might be appropriate for high sexual desire if the proposed disease model does not add explanatory power beyond merely high sexual desire.
In this study, brain responses were monitored using electroencephalography to visual emotional, including sexual, stimuli in a group who reported problems regulating their viewing of visual sexual stimuli (VSS). Event-related potentials (ERPs) were used to assess whether differences in responses to the sexual stimuli were related to sexual desire levels, hypersexual levels, both separately, or neither.
Processing sexual stimuli, presumably, differs in hypersexuals from non-hypersexuals, but the literature is non-specific about the neurological features of hypersexuality. This makes clear predictions difficult. Work in this area is rarely experimental (Mundry et al., Citation2011). In fact, the current authors are not aware of any other research using ERPs to investigate hypersexuality to date. Hypersexuals do not appear to have any gross executive control abnormalities measured with neuropsychological tests (Reid, Garos, & Carpenter, Citation2011), but may have more subtle attention deficits or specific attentional deficits to sexual stimuli. A small sample of hypersexual men did make more errors of both commission and omission on a Go/No-Go task (Miner, Raymond, Mueller, Lloyd, & Lim, Citation2009). Much has been written about hypersexual's use of VSS to regulate their mood. In fact, it is suggested as a core feature of the disorder (Kafka, Citation2010) and an important component of treatment (Adams & Robinson, Citation2001). Hypersexuals do not report more negative affect at baseline (Prause, Staley, & Fong, Citation2013), though, suggesting the affective dysregulation commonly described clinically in hypersexuality might occur at some other stage of processing sexual stimuli. Some suggest that hypersexuals engage in sexual behaviors when they are in a negative mood (Bancroft, Carnes, & Janssen, Citation2005; Bancroft & Vukadinovic, Citation2004). This is, of course, in stark contrast to major depressive disorder where low sexual desire co-occurs with negative mood, although the complicating suppression of sex drive by antidepressants also may play a role (Kennedy & Rizvi, Citation2009).
In addition to ‘hypersexuality’, some have described this symptom cluster as sexual ‘addiction’ or sexual ‘impulsivity’. Although an exhaustive review of studies relating brain reactivity to impulsivity or addictions is beyond the scope of this study, studies of ERP components in impulsivity and addictions could guide predictions for hypersexuality. Impulsivity has been associated with P300 decrements to novel stimuli (Justus, Finn, & Steinmetz, Citation2001; Russo, De Pascalis, Varriale, & Barratt, Citation2008). The direction of the relationship between trait impulsivity and ERP components is not documented consistently (Carrillo-de-la-Peña & Barratt, Citation1993), and controlling for factors like general intelligence has eliminated the relationship (Russo et al., Citation2008). Addiction-specific stimulus processing has been associated with increased late ERP components (i.e.>300 ms). For example, late ERP components elicited by drug-specific cues in those who have a drug problem are increased in cocaine (Van de Laar, Licht, Franken, & Hendriks, Citation2004), heroin (Franken, Stam, Hendriks, & Van den Brink, Citation2003), and cigarette (Warren & McDonough, Citation1999) users. Similar to these studies, affective stimuli have been used in comparison to population-specific stimuli (e.g. drug cues). For example, a study of cocaine users included both drug and affective cues with multiple control groups to identify enhanced processing of drug cues (Dunning et al., Citation2011). Cocaine users in that study also exhibited decreased amplitude in late ERP components to pleasant and unpleasant pictures relative to controls.
ERPs, as measured in this study, would help delineate the individual (and potentially overlapping) contributions of sexual impulsivity and sexual addition on hypersexuality. One ERP component, the P300, has been studied in relation to sexual desire levels. Amplitudes of the P300 elicited by auditory stimuli during erotic films are reduced more in women with higher sexual desire than women with lower sexual desire (Vardi et al., Citation2009), and this reduction is comparable to other highly arousing negative stimuli (Carvalho, Leite, Galdo-Álvarez, & Gonçalves, Citation2011). Presumably, the sexual stimuli have greater attentional capture for individuals with high sexual desire, which is reflected in reduced P300 amplitude to external auditory stimuli. Auditory-evoked P300 amplitudes during a sexual stimulus are different from P300 amplitudes evoked by the onset of the sexual stimulus itself. As compared to the auditory P300 collected during the sexual films, P300 stimulus-locked sexual images are higher in amplitude and similar to other high-arousal, negatively valenced stimuli (Cuthbert, Schupp, Bradley, McManis, & Lang, Citation1998). These findings are consistent, as both indicate greater attention allocated to processing sexual stimuli at the expense of processing competing (e.g. auditory) stimuli.
Several interpretations of P300 modulation to sexual stimuli in relation to sexual desire levels are possible. Those with higher sexual desire were generally slower to identify a target when it appeared in the area of a sexual image in a competing attention dot probe task (Prause, Janssen, & Hetrick, Citation2008), although this was not replicated in clinical groups for low sexual desire (Brauer et al., Citation2012). Also, those who are more sexually excitable miss more target stimuli in Go/No-Go tasks (Macapagal, Janssen, Fridberg, Finn, & Heiman, Citation2011). Finally, women with lower sexual desire fixate more on contextual than sexual parts of erotic scenes than controls (Lykins, Meana, & Minimi, Citation2011). These attentional effects affected by sexual desire level also are malleable within-person to exogenous testosterone (Van der Made et al., Citation2009). Given related research on attention and sexual desire, those with high sexual desire could exhibit greater P300 to sexual stimuli than neutral stimuli, due to greater affective salience. Alternatively, sexual stimuli could elicit lower P300 than neutral stimuli in those with high sexual desire, due to stronger attentional capture by the stimulus.
Predicting the relationship of P300 amplitude elicited by sexual stimuli and sexual desire levels is more complex. Those with higher sexual desire appear to allocate more attention to sexual stimuli than those with lower sexual desire (Carvalho et al., Citation2011; Macapagal et al., Citation2011; Prause et al., Citation2008; Vardi et al., Citation2009). Usually more salient, or emotional, stimuli elicit greater P300 amplitude (Cuthbert et al., Citation1998). However, P300 amplitudes elicited by population-specific stimuli habituate more rapidly for that population (e.g. those engaged in extreme sports presented with novel stimuli) than controls (Fjell et al., Citation2007). Therefore, individuals with high sexual desire could exhibit large P300 amplitude difference between sexual stimuli and neutral stimuli due to salience and emotional content of the stimuli. Alternatively, little or no P300 amplitude difference could be measured due to habituation to VSS.
This study was designed to determine whether sexual desire level alone, or a cluster of symptoms unique to hypersexuality, predict neural reactivity to sexual stimuli. Specifically, questionnaire measures of sexual desire and hypersexuality were used to predict ERP amplitudes to a series of emotional, including sexual pictures. The ability of the hypersexual measures to predict variance in the mean amplitude of the P300 beyond the variance predicted by sexual desire alone was tested. Given that this is the first time ERPs were recorded in hypersexuals, and literature on addiction (higher P300) and impulsivity (lower P300) suggest opposite predictions, the direction of the hypersexual effect was specified mainly on theoretical grounds. Consistent with the limited research using ERPs to examine sexual cue reactivity in relation to sexual desire, and consistent with broader emotion research, we predicted that those with higher sexual desire would exhibit higher P300 amplitudes to sexual stimuli than to neutral stimuli.
Methods
Participants
Fifty-two participants included men (N =39) and women (N =13) ranging in age from 18 to 39, (M age=24.35, SD age =4.92), with normal or corrected-to-normal vision and hearing. Most (N =45) reported being heterosexual and either single (N =11) or in a monogamous intimate relationship (N =28). The majority (N =47) had sexual intercourse at least once per month in the last year with an average of 3.31 different partners (see ). All but one participant reported viewing visual erotica in the past month.
Table 1 Demographic characteristics of sample (N =52)
Initial plans called for patients in treatment for sexual addiction to be recruited, but the local Institutional Review Board prohibited this recruitment on the grounds that exposing such volunteers to VSS could potentiate a relapse. Instead, participants were recruited from the Pocatello, Idaho community by online advertisements requesting people who were experiencing problems regulating their viewing of sexual images. This recruitment strategy appears to have successfully recruited participants with scores comparable to those labeled as ‘patients’ with hypersexual problems (see ). For example, in a large convenience sample the average Sexual Compulsivity Scale (SCS) scores for women M (SD) = 20.4 (7.2) and men M (SD) = 24.3 (8.0) seeking treatment for sexual compulsivity (Winters et al., Citation2010) were comparable to the current sample scores for women M (SD)=21.08 (4.63) and men M (SD)=22.31 (6.05), whereas the female M (SD) = 14.2 (4.2) and male M (SD) = 16.5(4.9) non-treatment-seekers in Winter and colleague's study scored lower. Despite reporting these problems, evidence shows participants did not experience any immediate problems or discomfort as a result of participating in this protocol (Prause et al., Citation2013). Participants gave informed consent before participating and received payment (or course credit, if a student and preferred). This study was approved by Idaho State University's institutional review board.
Table 2 Trait measures
Stimuli
Two hundred and twenty-five color pictures were selected from two standardized sets of stimuli commonly used in psychological research (Lang, Bradley, & Cuthbert, Citation1999; Spiering, Everaerd, & Laan, Citation2004) to represent pleasant (N =75; e.g. skydiving), neutral (N =75; e.g. portrait), and unpleasant (N =75; e.g. mutilated body) categories. The pleasant stimuli were matched on their level of general arousal with the most arousing unpleasant stimuli (the neutral stimuli will evoke lower levels of arousal). Every stimulus included a person. About half of the pleasant stimuli (N =38) were sexual stimuli depicting one man and one woman interacting with varying levels of sexual activities (e.g. caressing, vaginal intercourse; Spiering et al., Citation2004). These images were specifically selected for this study and this unique participant sample. We selected these stimuli instead of more images from the commonly used sexual images of the International Affective Picture System (IAPS; Lang et al., Citation1999). IAPS-type ‘sexual’ images have been shown to be processed as ‘romantic’, not ‘sexual’ (Spiering et al., Citation2004). Although some have noted similar processes between more romantic versus more sexual stimuli within the IAPS (Sabatinelli, Bradley, Lang, Costa, & Versace, Citation2007), it seemed appropriate to ensure the sexual images were sufficiently explicit to be consistently understood as sexual in a study focused on sexual motivation. Also, men and women were shown the same sexual images. This avoided a confound usually overlooked when men and women are shown sexual images of the opposite gender. Heterosexual women report lower sexual motivation in response to photographs of nude males than nude females (Chivers, Seto, & Blanchard, Citation2007); therefore, showing participants opposite-sex nudes would have resulted in an unbalanced stimulus set for men and women. This stimulus set included four conditions: unpleasant, neutral, pleasant-non-sexual (Lang et al., Citation1999), and pleasant–sexual (depicting explicit sexual activities; Spiering et al., Citation2004). Each trial consisted of a fixation cross displayed for 120 ms, followed on the next screen refresh by the onset of the photograph. The photograph remained on for 1,000 ms, after which an ‘*’ appeared for 1,000 ms. Two blocks of stimuli were presented with a break after the first half of the trials (113 trials), or about 4 min. The two-block task duration was about 8 min.
Questionnaires
Each participant completed four questionnaires: Sexual History Form, Sexual Desire Inventory (SDI), SCS, and Cognitive and Behavioral Outcomes of Sexual Behavior Questionnaire (CBOSB). These trait measures are summarized in .
Sexual History Form
The Sexual History Form collects general demographic and sexual behaviors. Demographic questions included age, education, and relationship status. Sexual behavior information questions included number of lifetime sexual partners, number of lifetime sexual intercourse partners, masturbation frequency, worry about sexual problems, and orgasm consistency (percent times reached orgasm when masturbating by any method, percent times reached orgasm when engaged in sexual activity with a partner). The sexual history form included questions about demographic and sexual history derived from the National AIDS Behavior Survey (Binson & Catania, Citation1998).
Sexual Desire Inventory
The SDI measures levels of sexual desire using two scales composed of seven items each (Spector, Carey, & Steinberg, Citation1996). The first, the Solitary Sexual Desire scale, measures an individual's desire for autoerotic sexual activity. The second, the Dyadic Desire scale, measures an individual's desire for sexual activity with a partner. The dyadic subscale is commonly used as an index of trait sexual desire level (Giargiari, Mahaffey, Craighead, & Hutchison, Citation2005; Prause et al., Citation2008). Both are investigated, because the only published study investigating P300 and sexual desire used a measure that did not differentiate solitary and dyadic sexual scales, which makes distinguishing the direction of sexual desire difficult (Vardi et al., Citation2006). However, solitary sexual behaviors are consistently underreported (e.g. Halpern, Udry, Suchindran, & Campbell, Citation2000), so it is likely that similar influences may lead to biased reporting of desire for same. The SDI has been psychometrically supported in studies assessing sexual desire (King & Allgeier, Citation2000; Spector & Fremeth, Citation1996). Test–retest reliability was calculated at r =0.76 over a 1-month period (Spector et al., Citation1996). SDI scores have been used as an index of trait sexual desire level (Giargiari et al., Citation2005).
Sexual Compulsivity Scale
The SCS measures compulsive traits linked to difficulty resisting sexual behaviors despite exposure to risk (Kalichman & Rampa, Citation1995). The scale consists of 10 statements related to compulsive thoughts, preoccupations, and behaviors associated with sexual activity (e.g. ‘My sexual appetite has gotten in the way of my relationships’, ‘My sexual thoughts and behaviors are causing problems in my life’). Respondents are asked to rate each statement on a 4-point scale ranging from ‘not at all like me’ to ‘very much like me’. The scale has been shown to be internally consistent for men and women with Cronbach's α for each equaling 0.76 and 0.81, respectively (Reece, Plate, & Daughtry, Citation2001). The SCS has been administered to members of high-risk groups for HIV infection as well as college students (Dodge, Reece, Cole, & Sandfort, Citation2004). Each SCS item is scored on a 4-point scale ranging from 1 (never applies to me) to 4 (always applies to me), with a minimum score of 10 and a maximum score of 40. A total score is computed from the sum of all items.
Cognitive and Behavioral Outcomes of Sexual Behavior Questionnaire
The CBOSB assesses the extent to which an individual is concerned about the possible consequences resulting from their sexual behaviors (McBride, Reece, & Sanders, Citation2007). The consequences fall within six domains: financial, legal, physical, psychological, spiritual, and social consequences. Each domain is further divided into two distinct cognitive and behavioral outcome scales, which separate the respondent's extent of worry from the actual, experienced consequences. Rated on a 4-point scale ranging from ‘never’ to ‘always’, the cognitive outcomes scale consists of 20 items concerning the extent the respondent worried about his/her sexual activities in the past year resulted in negative outcomes. Rated on a binary scale of ‘yes’ or ‘no’, the behavioral outcomes scale consists of 16 items concerning the extent the respondent experienced negative outcomes as a result of his/her sexual activities in the past year. The CBOSB is not widely used, but is the only psychometrically tested instrument that quantifies actual experienced impairment in separable domains.
Pornography Consumption Effects Scale
The Pornography Consumption Effects Scale (PCES) assesses the self-perceived effects of pornography use on an individual's sexual behavior, attitudes, and perceptions of the opposite gender (Hald & Malamuth, Citation2008). The measure contains 47 items divided into positive (27 items; e.g. ‘Has taught you something new about your sexual desires?’) and negative (20 items; e.g. ‘Has made you less satisfied with your life?’) effects from viewing pornography. Respondents are asked to indicate the extent they experience various effects on a 7-point Likert scale ranging from 1 to 7 to indicate ‘not at all’ to ‘an extremely large extent’, respectively. The scale has been shown to be internally consistent for positive (Cronbach's α=0.91) and negative (Cronbach's α=0.82) effect dimensions. Scores are the average of items in the subscale (positive, negative) of interest.
Procedure
After providing informed consent, participants completed questionnaires on a secure computer in a private testing room. Online administration increases reporting of socially undesirable behaviors (Locke & Gilbert, Citation1995). Then, ERPs were collected in a sound-attenuated, private space, within an internal room containing no windows or doors to external hallways. After placement of the electrodes, participants were seated in a comfortable, reclined exam table with a back-and-foot support approximately 130 cm away from the computer monitor. Participants viewed sexual stimuli presented as a series of picture stimuli and films (presentation order was counterbalanced). EEG data recorded during the films will be presented elsewhere. Stimuli were presented in a pseudorandomized order one at a time (1,000 ms each) using DMDX (Forster & Forster, Citation2003) on a 1,280×1,024 monitor with 75-Hz refresh and 32-bit color depth. Participants were instructed to attend to each stimulus and were given a break halfway through the task.
Electroencephalographic recordings
Electrophysiological data were recorded with Neuroscan Acquire software 4.4 in conjunction with a 40-channel NuAmps amplifier (Neuroscan, Inc., El Paso). A 40-channel cap (NuAmp QuickCap, Compumedics) collected EEG activity using sintered Ag–AgCl electrodes placed in accordance with the 10–20 International System (Jasper, Citation1958). All signals were digitized at 1,000 Hz during data collection. EEG activity was recorded using linked ear lobes as a reference. Horizontal electrooculogram was measured with electrodes placed infra-orbital and supra-orbital to the middle of the right eye and vertical EOG was measured with electrodes placed at the outer canthis of each eye. All impedances were kept below 10 kΩ.
ERP data reduction
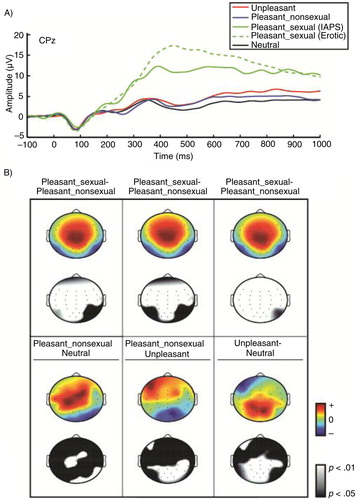
Pre-processing included downsampling to 256 Hz, bandpass filtering between 0.1 and 55 Hz, and eye-blink removal. Bad channels were identified as having activity four standard deviations away from the mean (on average, fewer than one electrode per participant met this criterion, 0.71 electrodes per participant). These channels were replaced using spline interpolation in the EEGlab software (Delorme & Makeig, Citation2004). Eye-blink removal was accomplished using an independent component analysis (ICA) technique. The ICA utility in the EEGLab software was used to derive components then, using and in-house template matching algorithm (Jung et al., Citation2000), blink components were identified and removed from the data. ERP epochs were defined in relation to onset of each stimulus from −1,000 ms pre- to 2,000 ms post-stimulus with a baseline correction of 110 ms preceding the stimulus. An ERP was averaged across each stimulus type (unpleasant, neutral, pleasant–non-sexual and pleasant–sexual). Within each trial, individual electrodes in which activity exceeded±100 µV were omitted from analysis. Applying these criteria, 9.04% electrode trials were excluded. The P300 component was defined by the positive deflection between 250 and 650 ms post-stimulus onset. This window was selected on the basis of visual inspection and consistent with previous research investigating P300 response (e.g. Hajcak, MacNamara, & Olvet, Citation2010). The mean amplitude across nine electrodes (C3, Cz, C4, CP3, CPz, CP4, P3, Pz, and P4) was calculated for analysis.Footnote Other electrode sites appear in topographical plots to show that the main effects were not unique to this average across locations (see ).
Fig. 1 (a) Stimulus-locked ERP waveforms (amplitudes in µV, time in ms) plotted at CPz to each unpleasant (red line), pleasant–nonsexual (blue line), pleasant–sexual (IAPS only, green line), pleasant–sexual (erotic images only, green dashed line), and neutral (black line). For illustration, the two types of pleasant–sexual stimuli are each plotted though they are averaged together for statistical comparisons. (b) Topographical difference (color heads) and statistical (black and white heads) head maps are presented. Difference and statistical head maps are plotted for each simple-effect between experimental conditions for the P300 component window.
Data analysis
Four stimuli (neutral, unpleasant, pleasant–non-sexual, and pleasant–sexual) were entered in repeated-measures ANOVA calculated for mean P300 ERP amplitude measured across nine electrodes selected for analysis. When significant violations of sphericity were detected, Greenhouse–Geisser-corrected effects and epsilon (ɛ) were reported (as recommended by Jennings & Wood, Citation1976; Picton et al., Citation2000). Simple-effect t-tests were calculated to clarify significant main effects. Difference scores between neutral and the unpleasant, pleasant–non-sexual, and pleasant–sexual conditions were calculated and used as the dependent variable with the self-report questionnaire measures (Solitary, Dyadic, PCES Total, SCS Total, CBOSB Behavioral, and CBOSB Cognitive) as the independent variables in regression analyses. Specifically, to directly test the unique influence of sexual desire over and above measures of hypersexuality on P300 amplitude elicited by sexual and non-sexual stimuli, stepwise regressions were performed. Correlations between the ERP measures and the self-report measures were also computed. Effects that did not reach statistical significance, defined as p >0.05, are not discussed.
Results
Event-related potentials
Manipulation check: P300 and stimulus type
In the analysis of the P300 component, the main effect of stimulus type was significant, F(3,153) = 183.60, M Se=91.13, p<0.001, ɛ=0.54 (see ). Simple-effect t-tests revealed that P300 mean amplitude in the neutral condition (M =2.54 µV) was less positive than the unpleasant (M =3.68 µV), t(51) = 4.11, p<0.001), the pleasant–sexual (M =11.79 µV), t(51) = 15.57, p<0.001), and the pleasant–non-sexual (M =3.00 µV), t(51) = 1.75, p =0.086) conditions. Also, the P300 mean amplitude for the pleasant–sexual condition (M =11.79 µV) was more positive than the unpleasant (M =3.72 µV), t(51) = 14.05, p<0.001), and pleasant–non-sexual conditions (M =3.00 µV), t(51) = 14.81, p<0.001). Given that this replicated expected, previous findings, the next planned test was conducted.
Hypothesis test: P300 regression on sexual desire and hypersexual measures
To assess the relationship between P300 amplitude in response to emotional, particularly sexual, images and the measures of sexual desire and hypersexuality, stepwise regressions were calculated.Footnote Condition (unpleasant, pleasant–non-sexual, and pleasant–sexual) differences from neutral were calculated and used as dependent variables with the self-report measures as independent variables. Regressions were calculated for each of the three difference scores. The pleasant–sexual difference from neutral proved significant, R 2=0.11, F(1,51) = 6.28, p =0.015, with the dyadic measure as the only unique predictor in the model, p =0.015.
Exploratory: P300 correlations with questionnaires
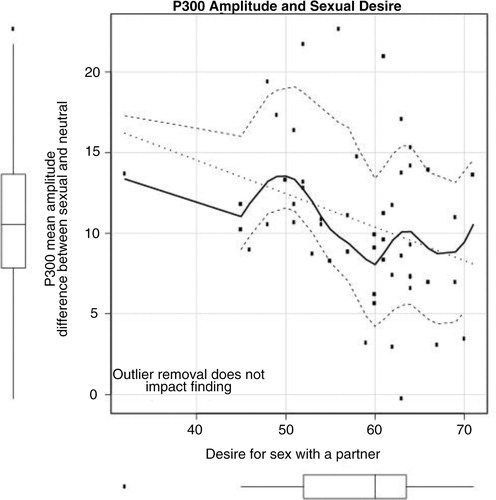
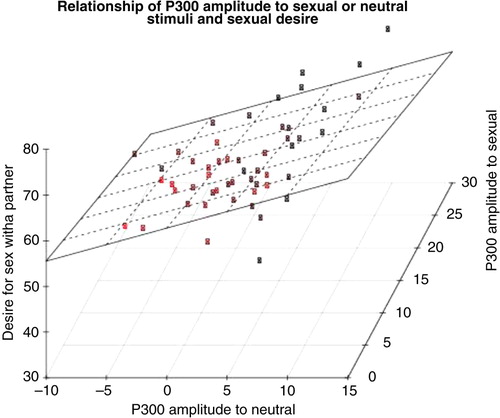
Pearson's correlations were calculated among the mean amplitudes measured in the P300 window and the self-report questionnaire data. The only correlation reaching significance was the difference score calculated between neutral and pleasant–sexual conditions in the P300 window with the desire for sex with a partner measure, r(52) = − 0.332, p =0.016. The relationship between P300 amplitude difference score and sexual desire is portrayed in , with P300 to the neutral and sexual stimuli shown separately in .
Discussion
To the authors’ knowledge, this represents the first examination of neural reactivity to sexual stimuli in those complaining of problems regulating their viewing of VSS. While the Institutional Review Board did not permit recruitment of patients seeking treatment, those recruited proved just as severe as those who do seek treatment on measures of hypersexuality. A strong, within-participant control approach was used as these individuals varied in their level of hypersexuality. ERPs were recorded to emotional, including sexual, images – an approach consistent with other research to cues in sex addiction (Brand et al., Citation2011). This allowed for a direct test of the unique contribution of hypersexuality to neural reactivity elicited by sexual stimuli beyond sexual desire level. The mean amplitude of the P300 to sexual stimuli relative to neutral stimuli was negatively related to sexual desire level. Of the three assessments of hypersexuality examined, none were significantly related to P300 amplitude in response to sexual images. This is consistent with a previous study using questionnaires (Winters et al., Citation2010). This represents the first functional, neurophysiological study of hypersexuality in a large sample of individuals who varied in their level of ‘hypersexuality’.
The P300 is well known and often used to measure neural reactivity to emotional, sometimes sexual, visual stimuli. A drawback to indexing a large, slow ERP component is the inherent nature of overlapping cognitive processes that underlie such a component. In this report, the P300 could be, and most likely is, indexing multiple ongoing cognitive processes. For example, the VSS presented are probably not novel to these participants given the high amount of VSS they report viewing. They might have strong, automatic, appetitive responses to sexual stimuli, which could be masked by low novelty suppressing the P300. Compare, for example, the interaction/suppression of working memory load and anxiety on the late positive potential (LPP) amplitude to negative versus neutral pictures (MacNamara, Ferri, & Hajcak, Citation2011). A promising avenue to test some of these P300 interpretation issues would be to collect a control sample of volunteers (Prause, Steele, Staley, Sabatinelli, Hajcak,& Fong, Citation2013). For example, this would provide a larger variance in VSS consumption to allow direct testing of the importance of VSS novelty. Although this study specifically used pretested, more explicit sexual stimuli than are typical in neuroscience research, even more novel sexual stimuli might overcome habituation/suppression effects due to low stimulus novelty.
Another possibility is that the P300 is not the best place to identify relationships with sexually motivating stimuli. The slightly later LPP appears more strongly linked to motivation. The earliest ERP components are thought to be more sensitive to sensory components of stimuli (Olofsson, Nordin, Sequeira, & Polich, Citation2008), whereas later ERP components appear more sensitive to motivational processes (Bradley, Hamby, Löw, & Lang, Citation2007; Schupp et al., Citation2000). Specifically, later components tend to increase to the presentation of both pleasant and unpleasant images (De Cesarei, Codispoti, Schupp, & Stegagno, Citation2006). Amongst these later ERP components, different topographies support making meaningful distinctions in their interpretation (Makeig et al., Citation1999). Sexual images produce remarkable increases in ERP amplitude, but especially in late (500–750 ms) windows, as compared to high-arousal sports photographs in both men and women (Van Lankveld & Smulders, Citation2008). As the LPP could provide confirmation or refuting evidence, it is currently being explored in a separate analysis (Prause, Steele, Staley, Sabatinelli, Hajcak, & Fong, Citation2013).
The contrived sample and laboratory setting may have implications for interpreting the results. Those who reported problems regulating their viewing of VSS were recruited not only because it is the most common problem behavior reported in clinics (Reid et al., Citation2012), but also because these stimuli are very accessible to laboratory testing. Others patients cite visiting prostitutes, infidelity, or other partnered behaviors as a primary problem. In addition, participants were instructed not to stimulate themselves during testing. Considering most people report masturbating when viewing erotica (Reid et al., Citation2012), this study could only be described as studying ‘cues’ rather than ‘consumption’ or ‘experience’. This is consistent with drug cue research, and similarly might be thought of as reflecting anticipation, activation of memories/associations, or activating regulatory processes since the cue cannot be consumed. Although the current sample was just as severe as a treatment-seeking sample (see ‘Participants’ section), they may have differed from treatment seeking ‘sex addicts’ in other ways. For example, volunteers for sexual psychophysiological studies tend to hold less conservative sexual values (Wiederman, Citation1999), so these participants might have a different level of shame than is typical in those seeking treatment (Reid, Citation2010). Shame proneness has been shown to interact with another ERP component, the error-related negativity (Tops, Boksem, Wester, Lorist, & Meijman, Citation2006). As ever, these results warrant replication with different participants and protocols more focused on external validity.
Given that high sexual drive is supposed to be a primary problem in sexual addiction (see review above), it was unexpected that these common measures of sexual addiction were not related to neural responsivity, while sexual desire itself was. One of the frequent critiques of sexual addictions is that it pathologizes normative, socially unaccepted, sexual behaviors (Levine & Troiden, Citation1988). These data appear consistent with that perspective. Essentially, the sex addiction measures used do not add any predictive utility for the P300 beyond that already captured by the sexual desire level. Examining just the questionnaires, the desire for sex with a partner scale was significantly correlated with the SCS, r(52) = 0.519, p <0.001, but it was not related to the CBOSB behavioral r(52) = − 0.121, p =0.393 or cognitive, r(52) = 0.248, p =0.077, or the PCES, r(52) = 0.036, p =0.798, scales. One interpretation is that the sex addiction scales are sensitive to social expectations and violations, such as shame and regret, and that these processes are not captured well by P300 variance. Another possibility is that these scales do not adequately capture the sexual addiction construct. Although several scales were analyzed in this study to increase the likelihood of identifying a scale that would be related to P300 variance, more scales exist (e.g. Reid et al., Citation2011) that might better include the proposed core feature of high sexual drive. Finally, the effect of sexual addiction on P300 may be so small that the present sample was not large enough to detect it. As this is the first functional neurological study of VSS-P, no effect size estimates were available to run power analyses prior to data collection. However, a post-hoc power analysis was calculated with GPower (Faul, Erdfelder, Lang, & Buchner, Citation2007). The current sample and analyses were sufficient to detect an effect size as small as f 2=0.2. This effect size is commonly considered small and meets the common power convention of 0.8 (see Cohen, Citation1988). In general, the sample was relatively large for an EEG study, represented a wide range of scores on measures of sexual problems, and was sufficient to detect the relationship with sexual desire level.
In conclusion, the first measures of neural reactivity to visual sexual and non-sexual stimuli in a sample reporting problems regulating their viewing of similar stimuli fail to provide support for models of pathological hypersexuality, as measured by questionnaires. Specifically, differences in the P300 window between sexual and neutral stimuli were predicted by sexual desire, but not by any (of three) measures of hypersexuality. If sexual desire most strongly predicts neural responses to sexual stimuli, management of sexual desire, without necessarily addressing some of the proposed concomitants of hypersexuality, might be an effective method for reducing distressing sexual feelings or behaviors. As a first study of neurophysiological responses in those complaining of difficulty regulating their viewing of VSS, studies to replicate and extend these findings are needed.
Conflict of interest and funding
Research funded by the Graduate Student Research and Scholarship Committee at Idaho State University to Cameron Staley.
Notes
The direct effect was consistent across sites. An average is presented to reduce the number of tests and simplify presentation of the results. Also see for topographic plots.
The specific theoretical question was whether hypersexual measures accounted for variance beyond sexual desire level alone. Thus, a two-step, hierarchical regression forcing dyadic sexual desire in the first step as predictor and hypersexual measures entered together in a second step was initially conducted. The pattern of results remained the same as what is reported in the stepwise regression. Given the possible critique that one hypersexual measure might have outperformed others, stepwise results are presented. In addition, forcing entry of hypersexual measures before the sexual desire measure still resulted in only sexual desire significantly predicting P300 amplitude.
References
- Adams K. M, Robinson D. W. Shame reduction, affect regulation, and sexual boundary development: Essential building blocks of sexual addiction treatment. Sexual Addiction & Compulsivity. 2001; 8(1): 23–44.
- Bancroft J, Carnes L, Janssen E. Unprotected anal intercourse in HIV-positive and HIV-negative gay men: The relevance of sexual arousability, mood, sensation seeking, and erectile problems. Archives of Sexual Behavior. 2005; 34(3): 299–305.
- Bancroft J, Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. Journal of Sex Research. 2004; 41(3): 225–234.
- Binson D, Catania J. A. Respondents’ understanding of the words used in sexual behavior questions. Public Opinion Quarterly. 1998; 62(2): 190–208.
- Boulos R, Vikre E. K, Oppenheimer S, Chang H, Kranarek R. B. ObesiTV: How television is influencing the obesity epidemic. Physiology & Behavior. 2012; 107(1): 146–153.
- Bradley M. M, Hamby S, Löw A, Lang P. J. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007; 44(3): 364–373.
- Brand M, Laier C, Pawlikowski M, Schächtle U, Schöler T, Altstötter-Gleich C. Watching pornographic pictures on the internet: Role of sexual arousal ratings and psychological–psychiatric symptoms for using internet sex sites excessively. Cyberpsychology, Behavior, and Social Networking. 2011; 14(6): 371–377.
- Brauer M, Leeuwen M, Janssen E, Newhouse S. K, Heiman J. R, Laan E. Attentional and affective processing of sexual stimuli in women with hypoactive sexual desire disorder. Archives of Sexual Behavior. 2012; 41(4): 891–905.
- Briken P, Hill A, Berner W. Pharmacotherapy of paraphilias with long-acting agonists of luteinizing hormone-releasing hormone: A systematic review. Journal of Clinical Psychiatry. 2003; 64(8): 890–897.
- Carrillo-de-la-Peña M. T, Barratt E. S. Impulsivity and ERP augmenting/reducing. Personality and Individual Differences. 1993; 15(1): 25–32.
- Carvalho S, Leite J, Galdo-Álvarez S, Gonçalves Ó. F. Psychophysiological correlates of sexually and non-sexually motivated attention to film clips in a workload task. PLoS One. 2011; 6(12): e29530.
- Chivers M. L, Seto M. C, Blanchard R. Gender and sexual orientation differences in sexual response to sexual activities versus gender of actors in sexual films. Journal of Personality and Social Psychology. 2007; 93(6): 1108–1121.
- Cohen J. D. Statistical power analysis for the behavioral sciences (2nd ed). 1988; New York, NY: Lawrence Erlbaum Associates.
- Cuthbert B. N, Schupp H. T, Bradley M, McManis M, Lang P. J. Probing affective pictures: Attended startle and tone probes. Psychophysiology. 1998; 35(3): 344–347.
- De Cesarei A, Codispoti M, Schupp H. T, Stegagno L. Selectively attending to natural scenes after alcohol consumption: An ERP analysis. Biological Psychology. 2006; 72(1): 35–45.
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004; 134(1): 9–21.
- Dodge B, Reece M, Cole S. L, Sandfort T. G. M. Sexual compulsivity among herterosexual college students. Journal of Sex Research. 2004; 41(4): 343–350.
- Dunning J. P, Parvaz M. A, Hajcak G, Maloney T, Alia-Klein N, Woicik P. A, etal. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users—An ERP study. European Journal of Neuroscience. 2011; 33(9): 1716–1723.
- Faul F, Erdfelder E, Lang A.-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007; 39(2): 175–191.
- Fjell A. M, Aker M, Bang K. H, Bardal J, Frogner H, Gangås O. S, etal. Habituation of P3a and P3b brain potentials in men engaged in extreme sports. Biological Psychology. 2007; 75(1): 87–94.
- Forster K. I, Forster J. C. DMDX: A windows display program with millisecond accuracy. Behavioral Research Methods, Instruments, Computers. 2003; 35(1): 116–124.
- Franken I. H. A, Stam C. J, Hendriks V. M, Van den Brink W. Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology. 2003; 170(2): 205–212.
- Fuller-Tyszkiewicz M, Skouteris H, Hardy L. L, Halse C. The associations between TV viewing, food intake, and BMI: A prospective analysis of data from the longitudinal study of Australian children. Appetite. 2012; 59(3): 945–948.
- George W. H, Davis K. C, Norris J, Heiman J. R, Stoner S. A, Schacht R. L, etal. Indirect effects of acute alcohol intoxication on sexual risk-taking: The roles of subjective and physiological sexual arousal. Archives of Sexual Behavior. 2009; 38(5): 538–550.
- Giargiari T. D, Mahaffey A. L, Craighead W. E, Hutchison K. E. Appetitive responses to sexual stimuli are attenuated in individuals with low levels of sexual desire. Archives of Sexual Behavior. 2005; 34(5): 547–556.
- Hajcak G, MacNamara A, Olvet D. M. Event-related potentials, emotion, and emotion regulation: An integrative reviwe. Developmental Neuropsychology. 2010; 35(2): 129–155.
- Hald G. M, Malamuth N. M. Self-perceived effects of pornography consumption. Archives of Sexual Behavior. 2008; 37(4): 614–625.
- Halpern C. J. T, Udry J. R, Suchindran C, Campbell B. Adolescent males’ willingness to report masturbation. Journal of Sex Research. 2000; 37(4): 327–332.
- Heiman J. R. Sexual dysfunction: Overview of prevalence, etiological factors, and treatments. Journal of Sex Research. 2002; 39(1): 73–78.
- Jasper H. H. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958; 10: 371–375.
- Jennings J. R, Wood C. C. The ε-adjustment procedure for repeated-measures analysis of variance. Psychophysiology. 1976; 13: 277–278.
- Jung T.-P, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski T. J. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000; 111: 1745–1758.
- Justus A. N, Finn P. R, Steinmetz J. E. P300, disinhibited personality, and early-onset alcohol problems. Alcoholism: Clinical & Experimental Research. 2001; 25(10): 1457–1466.
- Kafka M. P. Hypersexual disorder: A proposed diagnosis for DSM-V. Archives of Sexual Behavior. 2010; 39(2): 377–400.
- Kalichman S, Rampa D. Sexual sensation seeking and sexual compulsivity scales: Reliability, validity, and perdicting HIV risk behavior. Journal of Personality Assessment. 1995; 65(3): 586–601.
- Kennedy S. H, Rizvi S. Sexual dysfunction, depression, and the impact of antidepressants. Journal of clinical psychopharmacology. 2009; 29(2): 157–164.
- King B. E, Allgeier E. R. The sexual desire inventory as a measure of sexual motivation in college students. Psychological Reports. 2000; 86(1): 347–350.
- Krueger R. B, Hembree W, Hill M. Prescription of medroxyprogesterone acetate to a patient with pedophilia, resulting in Cushing's syndrome and adrenal insufficiency. Sexual Abuse. 2006; 18(2): 227–228.
- Lang P. J, Bradley M. M, Cuthbert B. N. The International Affective Picture System (IAPS): Technical manual and affective ratings. 1999
- Levine M. P, Troiden R. R. The myth of sexual compulsivity. Journal of Sex Research. 1988; 25(3): 347–363.
- Locke S. D, Gilbert B. O. Method of psychological assessment, self-disclosure, and experimental differences: A study of computer, questionnaire, and interview assessment formats. Journal of Social Behavior & Personality. 1995; 10(1): 255–263.
- Lykins A. D, Meana M, Minimi J. Visual attention to erotic images in women reporting pain with intercourse. Journal of Sex Research. 2011; 48(1): 43–52.
- Macapagal K. R, Janssen E, Fridberg D. J, Finn P. R, Heiman J. R. The effects of impulsivity, sexual arousability, and abstract intellectual ability on men's and women's go/no-go task performance. Archives of Sexual Behavior. 2011; 40(5): 995–1006.
- MacNamara A, Ferri J, Hajcak G. Working memory load reduces late positive potential and this effect is attenuated with increased anxiety. Cognitive. Affective & Behavioral Neuroscience. 2011; 11(3): 321–331.
- Makeig S, Westerfield M, Jung T.-P, Covington J, Townsend J, Sejnowski T. J, etal. Functionally independent components of the late positive event-related potential during visual spatial attention. Journal of Neuroscience. 1999; 19(7): 2665–2680.
- Marshall L. E, Marshall W. L. Excessive sexual desire disorder among sexual offenders: The development of a research project. Sexual Addiction & Compulsivity. 2001; 8(3–4): 301–307.
- McBride K. R, Reece M, Sanders S. A. Predicting negative outcomes of sexuality using the compulsive sexual behavior inventory. International Journal of Sexual Health. 2007; 19(4): 51–62.
- Miner M. H, Raymond N, Mueller B. A, Lloyd M, Lim K. O. Preliminary investigation of the impulsive and neuroanatomical characteristics of compulsive sexual behavior. Psychiatry Research: Neuroimaging. 2009; 174(2): 146–151.
- Mundry T. E, Hodgins D. C, El-Guebaly N, Wild T. C, Colman I, Patten S. B, etal. Conceptualizing exessive behavior syndromes: A systematic review. Current Psychiatry Reviews. 2011; 7(2): 138–151.
- Olofsson J. K, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008; 77(3): 247–265.
- Picton T. W, Bentin S, Berg P, Donchin E, Hillyard S. A, Johnson R. J, etal. Guidlines for using human event-related potentials to study congnition: Recording standards and publication criteria. Psychophysiology. 2000; 37: 127–152.
- Prause N, Janssen E, Hetrick W. P. Attention and emotional responses to sexual stimuli and their relationship to sexual desire. Archives of Sexual Behavior. 2008; 37(6): 934–949.
- Prause N, Staley C, Finn P. Effects of acute ethanol consumption on sexual arousal and sexual risk taking. Archives of Sexual Behavior. 2011; 40(2): 373–384.
- Prause N, Staley C, Fong T. W. No evidence of emotion dysregulation in “hypersexuals” reporting their emotions to a sexual film. Sexual Addiction and Compulsivity. 2013; 20(1–2): 106–126.
- Prause N, Steele V. R, Staley C, Sabatinelli D, Hajcak G, Fong T. Neural evidence of underreactivity to sexual stimuli in those reporting problems regulating their viewing of visual sexual stimuli. manuscript under review. 2013
- Reece M, Plate P. L, Daughtry M. HIV prevention ans sexual compulsivity: The need for an integrated strategy of public health and mental health. Sexual Addiction & Compulsivity. 2001; 8(2): 157–167.
- Reid R. C. Differentiating emotions in a sample of men in treatment for hypersexual behavior. Journal of Social Work Practice in the Addictions. 2010; 10(2): 197–213.
- Reid R. C, Carpenter B. N, Hook J. N, Garos S, Manning J. C, Gilliland R, etal. Report of findings in a DSM-5 field trial for hypersexual disorder. Journal of Sexual Medicine. 2012; 9(11): 2868–2877.
- Reid R. C, Garos S, Carpenter B. N. Reliability, validity, and psychometric development of the hypersexual behavior inventory in an outpatient sample of men. Sexual Addiction & Compulsivity. 2011; 18(1): 30–51.
- Robinson T. N. Reducing children's television viewing to prevent obesity: A randomized controlled trial. Journal of the American Medical Association. 1999; 282(16): 1561–1567.
- Russo P. M, De Pascalis V, Varriale V, Barratt E. S. Impulsivity, intelligence, and P300 wave: An empirical study. International Journal of Psychophysiology. 2008; 69(2): 112–118.
- Sabatinelli D, Bradley M. M, Lang P. J, Costa V. D, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. Journal of Neurophysiology. 2007; 98(3): 1374–1379.
- Schupp H. T, Cuthbert B. N, Bradley M. M, Cacioppo J. T, Ito T, Lang P. J. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000; 37(2): 257–261.
- Spector I. P, Carey M, Steinberg L. The sexual desire inventory: Development, factor structure, and evidence of reliability. Journal of Sex & Martial Therapy. 1996; 22(3): 174–190.
- Spector I. P, Fremeth S. M. Sexual behaviors and attitudes of geriatric residents in long-term care facilities. Journal of Sex & Martial Therapy. 1996; 22(4): 235–246.
- Spiering M, Everaerd W, Laan E. Conscious processing of sexual information: Mechanisms of appraisal. Archives of Sexual Behavior. 2004; 33(4): 369–380.
- Tops M, Boksem M. A. S, Wester A. E, Lorist M. M, Meijman T. F. Task engagement and the relationship between the error-related negativity, agreeableness, behavior shame proneness and cortisol. Psychoneuroendocrinology. 2006; 31(7): 847–858.
- Van de Laar M. C, Licht R, Franken I. H, Hendriks V. M. Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology. 2004; 117(1–2): 121–129.
- Van der Made F, Bloemers J, Yassem W. E, Kleiverda G, Everaerd W, Van Ham D, etal. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. Journal of Sexual Medicine. 2009; 6(3): 777–790.
- Van Lankveld J. J, Smulders F. T. The effect of visual sexual content on the event-related potential. Biological Psychology. 2008; 79(2): 200–208.
- Vardi Y, Sprecher E, Gruenwald I, Yarnitsky D, Gartman I, Granovsky Y. The P300 event-related potential technique for libido assessment in women with hypoactive sexual desire disorder. Journal of Sexual Medicine. 2009; 6: 1688–1695.
- Vardi Y, Volos M, Sprecher E, Granovsky Y, Gruenwald I, Yarnitsky D. A P300 event related potential technique for assessment of sexually oriented interest. Journal of Urology. 2006; 176: 2736–2740.
- Warren C. A, McDonough B. E. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999; 110(9): 1570–1584.
- Wiederman M. Volunteer bias in sexuality research using college student participants. Journal of Sex Research. 1999; 36(1): 59–67.
- Winters J, Christoff K, Gorzalka B. B. Dysregulated sexuality and high sexual desire: Distinct constructs?. Archives of Sexual Behavior. 2010; 39(5): 1029–1043.