Abstract
Today, the treatment for children and adolescents with attention-deficit/hyperactivity disorder (ADHD) is predominantly pharmacological. However, not all individuals respond to medication or some may experience problematic side effects. In addition, the compliance and treatment fidelity to medication is sometimes limited; thus, effective non-pharmacological treatment options are desirable. Neurocognitive training (NCT) methods such neurofeedback (NF) and working memory (WMt) have shown efficacy treating the primary symptoms of ADHD in non-blinded trials. Still, larger, comparative, blinded, pragmatic randomized controlled trials (RCTs) are needed to ensure the efficacy and effectiveness of these methods, and to identify an optimal training variant. Furthermore, little is known about predictors of treatment response to NCTs, such as genetic variants. In this article, we present the protocol of a pragmatic RCT for three NCT methods: slow cortical potential (SCP) training and live z-score (LZS) training (two NF variants), and working memory training (WMt). These are evaluated against each other and a waiting list control/treatment as usual group. In a clinical outpatient setting, 200 children and adolescents with ADHD aged 9–17 years with common comorbidities are randomized to either one of the treatment groups or the waiting list control group (n=50/group). The treatment groups (SCP/LZS/WMt) receive a total of 25 highly frequent training sessions (5/week for 5 weeks). A comprehensive assessment comprising ADHD core symptoms, psychopathology, neuropsychology, neurophysiology, quality of life, and health-related measures are collected pre- and post-treatment and at a 6-month follow-up. Primary outcomes are blinded teacher and unblinded parent ratings and self-ratings on the Conners 3 for ADHD. We expect that participants receiving NCT will exhibit improved core ADHD symptomatology compared with waiting list controls. Moreover, we hypothesize that the type of NCT (i.e. SCP, LZS, WMt) and participant characteristics (e.g. genetic predisposition, age, IQ, gender, verbal skills, and comorbidity) will predict patterns of treatment effects on the various outcomes.
Attention-deficit/hyperactivity disorder (ADHD) is a heterogenic, common neurodevelopmental condition affecting an estimated 5% of school-age children (Citation1–Citation3). The exact biological pathways leading to ADHD are still unknown, despite it being one of the most-studied psychiatric disorders (Citation4). Twin studies have consistently indicated that ADHD is highly heritable. Recent studies have suggested that the underlying genetic architecture of ADHD comprises both rare and common variants but to different extent among individual cases (Citation5). The genetic factors identified so far cluster in specific biological pathways including synaptic transmission, catecholamine metabolic processes, G-protein signaling pathways, and cell migration (Citation6). ADHD has also a significant genetic overlap with other psychiatric disorders including autism spectrum disorder and schizophrenia (Citation7).
ADHD causes significant impairments in several areas of life, and increases the risk for mental illness later in life. ADHD is associated with deterioration of school performance, elevated risk of accidents and substance misuse, teenage pregnancy, bullying, social isolation, family conflicts, anxiety, hopelessness, and depression (Citation8, Citation9). Many of the core symptoms persist into adulthood (Citation10–Citation12). Long-term follow-up studies show that individuals with ADHD more often fail to finish education, have difficulties keeping employments, have an increased risk for criminal behavior, take more long-term sick leave, and have problems with handling finances and their households. Psychiatric comorbidity is estimated to occur in about 80% of individuals with ADHD (Citation13–Citation15).
International and regional guidelines recommend a multimodal treatment approach in ADHD by combining psychosocial and educational interventions with medication. Nevertheless, the most available and commonly used intervention is drug therapy, particularly methylphenidate and atomoxetine treatment that have both yielded positive short-term effects on inattention, impulsivity, and hyperactivity (Citation16). These medications act primarily on symptoms, (i.e. they effectively suppress symptoms), but currently it seems unlikely that they have any curative effects (Citation17, Citation18). Moreover, medication might cause unwanted side effects (Citation19). About 20–30% of children and adolescents with ADHD do not respond to drug therapy, and even among those who do respond there is need for additional treatments, especially long-term improvement in symptomatology and functional outcomes. Finally, the compliance and treatment fidelity to medication is sometimes compromised (Citation20, Citation21), and therefore, demonstrates why alternative or complementary effective non-pharmacological treatment options are desirable.
Neurocognitive training
Neurocognitive training (NCT) methods like neurofeedback (NF) and working memory training (WMt) are non-invasive methods which in recent years have received increased attention (Citation22, Citation23). NF influences the brain's electrical activity through operant/classical learning and thereby enhances an individual's ability for self-regulation, that is, to flexibly adapt brain activity to more effectively meet the changing demands of the environment (Citation24). WMt focuses on improving executive attentional functions through challenging exercises using computerized software. While several different variants of NF options are available, the current mainstream methods are slow cortical potential training (SCP) and frequency training (mainly theta/beta ratio training). Live z-score (LZS), a variant of frequency training, has become increasingly popular among private practitioners, although larger randomized controlled trials (RCTs) evaluating its efficacy are lacking. In LZS, the participant is training a broad array of brain functions using the electroencephalogram (EEG), both based on power (absolute and relative) and connectivity (coherence, asymmetry, and phase difference) (Citation25). In most studies so far, NF has consisted of 2–3 sessions per week (Citation26–Citation30), while WMt mostly consisted of daily training sessions (Citation31). Previous controlled trials have found moderate efficacy for NF and WMt regarding the improvement of core symptoms of ADHD, impulsivity, hyperactivity, and inattention with few side effects (only fatigue after initial training) (Citation27, Citation28) (Citation32, Citation33). Nevertheless, although NF and WMt have demonstrated robust preliminary evidence, questions still remain regarding the nature of their effects, sustainability, and practicability. For instance, recent meta-analytic studies indicate limited effects on ADHD symptoms when focusing on blinded assessment measures (Citation23, Citation34). In addition, owing to a shortage of comparative studies, it is unclear which NCT method is potentially superior to the others regarding core ADHD symptomatology and other intervention outcomes.
Training outcomes
Typically, the treatment effects of NCT in ADHD are determined by using change of core symptoms in ADHD symptomatology, that is, inattention, impulsivity, and hyperactivity, when applying the Conners’ Parent/Teacher Rating Scale or similar rating scales to operationalize problem behavior (Citation23). Furthermore, different types of neuropsychological tests are frequently included in NCT trials, such as the Continuous Performance Test, Digit Span, or the Stroop Test (Citation34). All of these tests operationalize elements of cognition that have been found to be impaired in ADHD, such as attention, working memory, inhibition, and other executive functions. More recently, the use of biomarkers in the form of physiological measures has become popular to obtain objective indicators of NCT efficacy. For instance, event-related potentials (ERPs) and the power of resting state EEG have been shown to respond to NF (Citation35) and WMt (Citation36). Furthermore, it has been shown that it is possible to differentiate learners from non-learners on the basis of stronger baseline contingent negative variation (Citation32) and that pretreatment levels of these neurophysiological markers have been related to the clinical outcome of NCT (Citation37, Citation38). Therefore, finding reliable objective neurophysiological biomarkers relevant to NCT might help to predict the probability of successful intervention and guide individualized training. The link between genetic variants and treatment response has been studied in ADHD regarding pharmacological treatments with modest results (Citation39). Similar approaches have not been performed yet for any NCT treatment of ADHD. Recently, this area of intervention research (also referred to as ‘therapy genetics’) has been used in a handful of studies in obsessive compulsive disorder, posttraumatic stress disorder, anxiety disorders, and depression. Although studies have included moderately sized samples of (ranging from N=66–200 individuals), heuristic and promising findings have occurred (Citation40). It has also been suggested that the best predictors of response may come from the etiological genetic variants instead of the common variants (Citation40, Citation41).
Study protocol rationale
Having learned from and building on previous NCT intervention research outlined above, in this article, we describe the protocol of a comparative, pragmatic RCT of two types of NF (plus treatment as usual, TAU), WMt (plus TAU), and a waiting list control group (TAU only). The study protocol seeks to combine the following methodological novelties and strengths: 1) comparative design (SCP vs. LZS vs. WMt vs. TAU only), 2) a relatively large sized sample of patients with ADHD (N=200, 4×n=50), 3) blinded ratings of primary outcomes (teacher report), 4) well-defined inclusion and exclusions criteria that tolerate common neuropsychiatric comorbidities, 5) psychometrically sound outcome measures, 6) multiple informants (participants, parents, and teachers), 7) a naturalistic clinical setting to calculate the added value of NCTs in addition to TAU (pragmatic study), and 9) an intense NCT training approach (5/week for 5 weeks). The protocol also aims to investigate NCT treatment mechanisms in terms of personalized medicine, by analyzing the moderating and mediating effects of particular participant characteristics (e.g. genetic predisposition, age, IQ, gender, verbal skills, and comorbidity) on treatment outcomes.
Methods
Ethics
The study protocol is approved by the Ethical Review Board in Stockholm (Dnr.2013/739-31, amendment: Dnr.2013/1729-32). The trial is registered with ClinicalTrials.gov (NCT01841151). Written informed consent is given by the legal guardian/s, and the participating child/ adolescent prior to participation.
Design
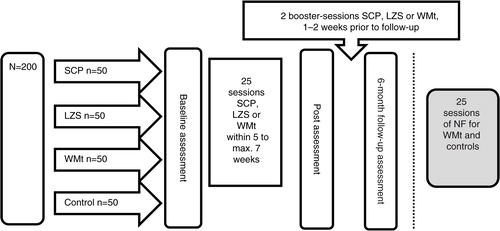
The current study is a single-center, comparative, pragmatic RCT. Two hundred participants with a primary diagnosis ADHD are randomized to one of three different active training groups (SCP, LZS, WMt) along with TAU or waiting list control group receiving standard clinical services (TAU only) [4×n=50]. Randomized participants are assessed at baseline, at post-intervention (after 5–7 weeks of training), and at 6-month follow-up (see ). All participants have to be free from psychoactive medication for 48 h prior to the neurocognitive assessments. While all participants receive standard care by different obligatory pediatric, child, and adolescent psychiatric or habilitation services, the active NCT groups are exclusively conducted at BUP-KIND, a specialized child and adolescent psychiatric outpatient unit of the division of Child and Adolescent Psychiatry (BUP), Stockholm County Council. The NCT is carried-out with a high frequency and consists of five sessions per week for 5 weeks for a total of 25 sessions. Missed sessions (e.g. due to illness or public holidays, etc.) are added at the end of the training period, with the total training period not exceeding 7 weeks. Two booster sessions of SCP, LZS, and WMt are added 1–2 weeks prior to follow-up assessments. All training is conducted individually assisted by a trainer. Owing to ethical considerations, participants randomized to the TAU waiting list control group are offered WMt or NF subsequently.
Participants and recruitment
The N=200 children and adolescents with primary clinical diagnosis of ADHD (ICD-10: F90.0; DSM-IV-TR: 314.00, 314.01) aged 9–17 years are recruited by referrals from obligatory health care providers for children and adolescents within Stockholm County, primarily BUP, Habilitation and Health (ADHD-Center), and several pediatric outpatient units (BUM). In addition, self-referrals are included; information about the study are posted on Facebook via interest organizations, and spread through other media (e.g. flyers at conferences, homepage of Center of Neurodevelopmental Disorders at Karolinska Institutet (KIND)). Health care within the Stockholm County is currently still based on the ICD-10/DSM-IV-TR manuals (not DSM-5, but awaiting ICD-11), why they were also applied in this trial. Parents of participants that are interested in entering the study attend a meeting where oral information on the project is provided and individual questions are answered. After the meeting, parents receive information brochures and consent forms. Once written consent is received, inclusion and exclusion criteria (see ) are reviewed based on previous clinical assessments and medical records. Importantly, to ensure external clinical validity of the study results, common neurodevelopmental comorbidity such as autism spectrum disorder, learning disabilities, and communication and motor disorders are tolerated. Thereafter, included participants are randomized via a computerized randomizer (www.random.org) to one of the four study groups.
Table 1 Inclusion and exclusion criteria
Interventions
SCP training
SCPs are bioelectrical activity in the brain, a form of ERPs locked in time. They are characterized by negative and positive shifts lasting from 300 ms to several seconds (Citation42). The negative shifts are believed to reflect the brain's state of increased cortical excitability, while positive shifts reflect inhibition and reduced excitability. During SCP the participant trains to create these shifts (negative or positive) consciously or intentionally, thereby enhancing the ability to shift between excitability and inhibition. In our study, the TheraPrax-QEEG® system (neuroConn GmbH, Ilmenau, Germany) is used for the SCP training. The participant sits in front of a computer screen and is asked to use his brain activity to either move an object up on the screen for a negative shift or down for a positive shift. The training segments last in total 8 s (2 s for baseline, 6 s of training). One session consists of four blocks, with 36 trials each. Positive and negative shifts are trained in random order at a 1:1 ratio. Transfer trials are also trained. Here the participant has to imagine positive or negative shifts according to the guidelines presented on the screen. During the first week, 20% of the trials are transfer trials, which then are increased to 40% for the second week and to 50% in the remaining 3 weeks. Vertical and horizontal eye movements in addition to eye blinks are recorded and computed before every session and used for an online correction during training. For trials containing artifacts that could not be corrected, the trial is aborted and restarted. At the 15th session, the participant receives a so-called transfer-card, which consists of a picture of a bird used in the SCP. The participant is asked to look at the card and to simulate the mental state that was experienced during the SCP session. This is to be done daily and preferably in connection with education and homework in order to improve the generalization of the training effects to daily life settings. The compliance for the generalization training is recorded at each session. Also, a token system is used to motivate the participants to complete the training. A total of five tokens can be earned for each session, based on punctuality of arrival time, successful completion of the session, and holding still during the training (in order to minimize artifacts). At the end of the training period, the participant receives a voucher corresponding to the number of tokens earned. Vouchers can be exchanged for concrete gifts/rewards, such as cinema tickets.
LZS training
Compared to SCP, LZS is thought to train a person and his or her brain activity towards ‘normality’ by computing, viewing, and processing z-scores representing a normative database in real time and giving feedback based on the current individuals EEG in relation to the normative database. This is achieved by joint time–frequency analysis (Citation43). The software instantly measures different aspects of EEG, such as relative and absolute amplitude and connectivity measures, and compares them to the normative z-scores. The Atlantis II® (Brainmaster Technologies, Inc., Bedford, OH, USA) is used in this study with a standard laptop PC for the LZS training. The software computes a total of 90 targets (60 concerning power, and 30 concerning connectivity) (see ). Feedback is provided based on the z-score deviations. The training protocol has been designed in collaboration with Tom Collura, founder of Brainmaster (www.brainmaster.com). It consists of two channels in two 20-min sections (first half: C3 and C4; second half: Fz and Cz), 40 min in total. Feedback for the first 5–20 min is provided by a Flashgame, where the participant generates ‘brain-cells in a jar’ on a computer screen. The less the participant's brain activity deviates from the z-score norm values, the quicker the jar fills up. At the first session, the participant plays the game for 20 min, which then it is lowered to 10 min and finally 5 min per session. For the remaining time (20–35 min), feedback is provided via movies with a dimmer, with positive feedback leading to a brighter screen and better movie visibility. At the 15th session, the participant receives a transfer-card, which consists of a picture of the ‘brain-cell-game’. Similarly to the SCP, to achieve generalization, the participant is asked to look at the card and to imagine mental state experienced during the LZS session, preferably at school or while doing homework. As for SCP, the trainee can acquire tokens for training punctuality, compliance, and cooperation.
Working memory training
WMt usually consists of several computer-based visuospatial and verbal memory tasks, which challenge different aspects of the working memory. In the current study, we use Minneslek Flex (www.flexprogram.org), a Swedish training tool that is widely used across the country in school settings. There are two versions that mainly differ in their thematic content, while following the same procedure and strategy in each version. Owing to their high comparability, the participant may choose which of the two to use. Each session consists of six different modules with 12 trials each, and each module trains for either visuospatial or verbal memory functions. The difficulty level is adjusted automatically based on the individual performance. Comparable to SCP and LZS, at the 15th session, the participant receives a transfer-card, showing a picture of one of the exercises/modules of the WMt for generalization training. Also, token economy is applied for training punctuality, compliance, and cooperation during the training.
TAU only/waiting list control group
All participating children and adolescents continued TAU as this was not an exclusion criterion for this pragmatic trial. The waiting list control-group participants received ongoing TAU only. Standard care mainly consists of medication, but also individual cognitive behavior therapy, psychoeducation for parents, and dietary supplements and/or restrictions. Ongoing TAU of each participant is monitored and registered during the study. The participants are asked to maintain TAU during the trial participation, and not to terminate or start any other treatment. Control group participants are offered NCT training of their choice equivalent to the active NCT groups, subsequent to the study's follow-up assessments.
Assessments and measures
Participants will be assessed at baseline (pre), directly after the training (post), as well as 6-month after finalizing the training (follow-up). Participants with ongoing psychoactive drug medication have a 48 h wash-out period prior to the pre-, post- and follow-up assessments. The primary outcome in the form of core ADHD symptoms are measured using the Conners 3 parent, teacher, and self-rating scales (Citation44). Moreover, the assessment consists of a cognitive test battery, neurophysiological measures, rating scales for executive functions, quality of life (all secondary outcomes), questionnaires for sleep, diet and exercise, and parent stress (baseline only or exploratory outcomes). provides a summary the single measures across pre-, post- and follow-up assessments.
Table 2 Baseline, post- and follow-up assessments
Primary outcome measures
The Conners 3 (Citation44) is an updated version of the Conners’ Rating Scales-Revised, and one of the most widely used scales internationally to assess ADHD symptoms in research and practice. It includes parent, teacher, and youth/self-rating versions, that are composed of seven subscales (Executive Functioning, Learning problems, Aggression, Peer Relations, Family Relations, Hyperactivity/Impulsivity, and Inattention). In this study, the Swedish adaptation of the full-length Conners 3 consisting of 99–115 items (depending on the informant) is used. The internal consistency of the Swedish version is good (α>0.82), and the test–retest reliability is good to excellent for the parent (r tt=0.73–0.95), teacher (r tt=0.73–0.83), and self-rating (r tt=0.63–0.81) version (Citation45).
Secondary outcome measures
The Conners’ Continuous Performance Test-II (CPT-II) (Citation46) is a task-oriented computerized Continuous Performance Test. It measures inattentiveness, impulsivity, sustained attention, and vigilance. Twelve measures are combined for an ADHD-index. Tapping (Citation47) is a computerized time, spatial amplitude, and frequency critical motor control task. Every 1,200 ms, a tone is presented, and the participant has to tap at the same pace by pressing the right mouse button. After 15 cued trials, the participant is asked to continue tapping at the previously cued rate for 41 uncued trials. Within-subject standard deviation is calculated for the variability in tapping. In the Duration Discrimination task (Citation48) for time perception, two unfilled intervals (target and comparison) defined by two brief tones (50 ms; 1,000 Hz) before and after the intervals are presented to the participant. The task is to discriminate between longer and shorter intervals. The participant responds by pressing the left button if they experience the first tone as longer and right button if they think the second tone is longer. The trial intervals are separated by 800 ms, and inter-trial interval is 1,000 ms. The target interval shifts randomly from first to second place, and the longer is adjusted up or down in 10 ms increments depending on the accuracy of response. Time anticipation (Citation47) is a computerized task to access time perception, memory, and cognitive impulsivity combined. In the test, the participant has to beam oxygen to a spaceship to save the crew. As soon as the ship becomes visible, the participant has to press a button. After 10 trials the ship remains invisible, and the participant has to estimate when the invisible ship appears, as it always appears at the same time. Feedback is given in both visible as invisible trials. In the first block the response rate is every 400 ms and on the second block at 2,000 ms. Classical verbal working memory functions are assessed using the subtests Digit Span and Letter-Number Sequencing from the Wechsler Intelligence for Children-IV (WISC-IV) or the Wechsler Adult Intelligence Scales (WAIS-IV) (Citation49, Citation50) are administered as measurements for verbal working memory capacity. For visual–spatial working memory, the subtest Spatial Span from the WISC-IV Integrated (Citation51) or WAIS-IV as Neuropsychological Instrument (Citation52) are used. In addition, working memory is assessed with the Find the phone task, which is a computerized test where the participant has to find the ringing telephone, among several distractors. The task is to avoid selecting the phones that have already been answered. The number of times an already answered phone is selected is used as a measure of deficits in the working memory. The task is similar to the spatial working memory task of the Cambridge Neuropsychological Test Automated Battery (Citation53).
The Behavior Rating Inventory of Executive Function (Citation54, Citation55) is an 86-item questionnaire for parents and teachers, consisting of eight scales that form a Behavioral Regulation Index and a Metacognition Index. The KIDSCREEN-27 (Citation56) is a self-report questionnaire consisting of 27 items applicable to children aged 8–18 years about perceived quality of life that has shown robust psychometric properties (Citation57).
Quantitative EEG (qEEG) is measured through 19 electrodes placed according to the international 10–20 system using a recording cap (www.easycap.brainproducts.com/). The measurements are collected during eyes-closed and eyes-open resting states for 3–5 min, depending on the cleanness of the recordings (e.g. artifacts due to movement). The TheraPrax-QEEG® system (neuroConn GmbH, Ilmenau, Germany) is used for the data collection and the NeuroGuide® software (www.appliedneuroscience.com/) for the analysis of the absolute and relative power of delta, theta, alpha, and beta frequencies. Event-related potentials (ERPs) are neuronal processes underlying measurable overt behavior such as speed and accuracy of processing information. ERPs provide a direct measure of the brain's covert activity and timing, specifically preparatory and inhibitory processes. Two tasks are conducted concerning ERPs: The CPT-OX, which is a cued Continuous Performance Task, with 400 letters presented briefly (150 ms) every 1.65 s in a pseudorandom sequence at the center of a computer screen. The participant is asked to press a button when the target letter ‘X’ is preceded by the cue letter ‘O’ (go condition, ‘O’ followed by different letter than X, no-go condition=CPT-OX). The attentional and preparatory brain processes are assessed by Contingent Negative Variation (CNV), which is commonly considered to reflect cognitive and attentional preparation and the P300 which is viewed as an index of neurophysiological response inhibition (or no-go) (Citation58). Average reaction time, number of omissions/commissions and mean amplitude of CNV and P300 is assessed using a Flankers test, which is a reaction time task, where the participant has to respond as quickly as possible, while avoiding errors. The participant is required to indicate the direction of a target stimulus in an array of three stimuli. Error-related negativity (ERN) (Citation59) is recorded when participants make errors in the flanker task. ERN presents as a negative detection approximately 50–100 ms following the erroneous response. It is thought to reflect error-related brain activity namely individual's ability to monitor behavior. Average reaction time and number of errors are assessed.
Complementary/exploratory measures
Parenting stress is examined using The Swedish Parenthood Stress Questionnaire (SPSQ) (Citation60). The SPSQ is derived from the Parenting Stress Index (Citation61) and consists of 34 items. The sleep patterns of the participants are documented by parents or by the participating adolescents themselves using a simple sleep diary, where they indicate bedtime, wake-up time, perceived sleep quality, number of night-time awakenings, use of sleep medication, total hours of sleep, and need for sleep during the day. The sleep diary is completed three times for 1 week in the following time periods: week before baseline, the week after training, and the week before follow-up assessment. Nutrition and physical activity are explored via the Swedish National Food Administration's published Diet and Exercise Questionnaire, which is completed by parents, which has been slightly modified for the with children.
Therapy genetics
Saliva samples for DNA extraction are collected from each of the consenting participants using the Oragene DNA OG500 kit (DNA Genotek). Genome-wide methods such as SNP microarrays and next-generation sequencing will be used for variant discovery and characterization of the genomic background of the participants. Our aim is to identify genetic variants and biological pathways that could predict the NCT treatment outcomes.
Sample size estimation and statistical analyses
We expect that participants receiving NCT will exhibit improved core ADHD symptomatology and secondary and exploratory outcomes compared with waiting list controls. Moreover, we hypothesize differing effects of type of NCT (SCP, LZS, WMt) based on the participant characteristics (e.g. genetic predisposition, age, IQ, gender, verbal skills, and comorbidity) will predict patterns of treatment effects on the various outcomes. The sample size calculation refers to the three primary outcome endpoints: change in Conners 3 total scores and subscales for parent, self-report, and blind teacher ratings between baseline and follow-up assessment, in the Intention To Treat (ITT) sample. MANOVA for repeated measures (three measurement points, within–between subjects’ interactions, post-hoc tests) will be used for the statistical analysis for the RCT study. Based on available evidence about NCT efficacy medium effects for the primary outcomes are expected. With N=200 (n=50 SCP vs. n=50 LZS vs. n=50 WMtr vs. n=50 TAU only controls) and alpha=5%, the power (1-beta) is >99% for medium effects (G Power 3.1.7). All data provided for the participants will be included in the analyses. Data will be tested for normality and homogeneity of variance. To verify that the treatment group and control groups are comparable for continuous and categorical demographic variables at pretreatment, a series of independent-samples t-tests and chi-square tests will be conducted. As for primary outcomes, analyses for secondary outcomes will be conducted according to ITT principles. In order to analyze for the significance of potential factors predicting outcomes (age, IQ, language abilities, gender, comorbidity, genetic variants) in the active NCTs training groups, these are analyzed using a logistic regression to explain dichotomized (by median-split) Conners 3 outcomes as the dependent variable.
Discussion
There is a need for effective, feasible clinical interventions for children and adolescents with ADHD in addition to drug treatment. A substantial minority of cases do not respond or respond only marginally to drug treatment and others refuse medication treatment, owing to side effects or a general unwillingness to take drugs. NCT are non-invasive methods that have demonstrated good preliminary evidence in several international studies in reducing the core symptoms of ADHD in children and adolescents. However, more evidence is needed. Therefore, we have initiated a large comparative RCT study to investigate the efficacy and effectiveness of WMt and two varieties of NF for children and adolescents with ADHD in naturalistic clinical setting for the first time in Scandinavia. We plan to examine with the pragmatic RCT design 1) how a more intensive training frequency affects ADHD core symptom, 2) which of the NCT variants is superior to the others, and 3) whether NCT adds value to existing clinical practice.
The results from this study will help to create tools and recommendations for ‘individualized therapy’ through moderator analyses including neurophysiological as well as genetic markers to predict treatment response. These markers can be used to better tailor treatment plans for the affected individuals to achieve the best health gain and minimizing invaluable action. No previous studies have shown gene×training interactions in ADHD. As costs for genome sequencing are rapidly decreasing, we expect that personalized genomics will be an essential part of medicine and clinical decision making in ADHD in the future. Therefore, it is crucial to combine genetic analyses with treatment response data. The study is the first of its kind in Sweden and abroad.
Conflict of interest and funding
The authors declare that there is no conflict of interest regarding the planned study or the publication of this paper. This study is supported by the Stockholm County Council and the Swedish Research Council in cooperation with all Swedish County Councils. Sven Bölte is supported by the Swedish Research Council (grant no. 523-2009-7054). Kristiina Tammimies is supported by Swedish Foundation of Strategic Research (grant no. ICA14-0028).
References
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014; 43: 434–42.
- De Schipper E, Mahdi S, Coghill D, de Vries PJ, Gau SS-F, Granlund M, etal. Towards an ICF core set for ADHD: a worldwide expert survey on ability and disability. Eur Child Adolesc Psychiatry. 2015; 24 : 1509–21.
- De Schipper E, Lundequist A, Wilteus AL, Coghill D, de Vries PJ, Granlund M, etal. A comprehensive scoping review of ability and disability in ADHD using the International Classification of Functioning, Disability and Health-Children and Youth Version (ICF-CY). Eur Child Adolesc Psychiatry. 2015; 24 : 859–72.
- Thapar A, Cooper M, Eyre O, Langley K. Practitioner review: what have we learnt about the causes of ADHD?. J Child Psychol Psychiatry. 2013; 54: 3–16.
- Martin J, O'Donovan MC, Thapar A, Langley K, Williams N. The relationship between common and rare genetic variants in ADHD. Translational Psychiatry. 2015; 5 : 10–13.
- Hawi Z, Cummins TDR, Tong J, Johnson B, Lau R, Samarrai W, etal. The molecular genetic architecture of attention deficit hyperactivity disorder. Mol Psychiatry. 2015; 20: 289–97.
- Doherty JL, Owen MJ. Genomic insights into the overlap between psychiatric disorders: implications for research and clinical practice. Genome Med. 2014; 6: 29.
- Harpin VA. The effect of ADHD on the life of an individual, their family, and community from preschool to adult life. Arch Dis Child. 2005; 90(Suppl 1): i2–7.
- Barkley RA. Major life activity and health outcomes associated with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002; 63(Suppl. 12): 10–15.
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010; 177: 299–304.
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med. 2006; 36: 159–65.
- Geissler J, Lesch K-P. A lifetime of attention-deficit/hyperactivity disorder: diagnostic challenges, treatment and neurobiological mechanisms. Expert Rev Neurother. 2011; 10 : 1467–84.
- Barkley RA, Brown TE. Unrecognized attention-deficit/hyperactivity disorder in adults presenting with other psychiatric disorders. CNS Spectr. 2008; 13: 977–84.
- Sobanski E, Brüggemann D, Alm B, Kern S, Deschner M, Schubert T, etal. Psychiatric comorbidity and functional impairment in a clinically referred sample of adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci. 2007; 257: 371–7.
- Joelsson P, Chudal R, Gyllenberg D, Kesti A-K, Hinkka-Yli-Salomäki S, Virtanen J-P, etal. Demographic characteristics and psychiatric comorbidity of children and adolescents diagnosed with ADHD in specialized healthcare. Child Psychiatry Hum Dev. 2015. Sept 23: Online.
- Swanson J, Arnold LE, Kraemer H, Hechtman L, Molina B, Hinshaw S, etal. Evidence, interpretation, and qualification from multiple reports of long-term outcomes in the Multimodal Treatment Study of children with ADHD (MTA): Part II: supporting details. J Atten Disord. 2008; 12: 15–43.
- Hinshaw SP, Arnold LE. Attention-deficit hyperactivity disorder, multimodal treatment, and longitudinal outcome: evidence, paradox, and challenge. Wiley Interdiscip Rev Cogn Sci. 2015; 6: 39–52.
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, etal. The MTA at 8 years: prospective follow-up of children treated for combined-type ADHD in a multisite study. J Am Acad Child Adolesc Psychiatry. 2009; 48: 484–500.
- Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, etal. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007; 46: 1015–27.
- Barner JC, Khoza S, Oladapo A. ADHD medication use, adherence, persistence and cost among Texas Medicaid children. Curr Med Res Opin. 2011; 27(Suppl 2): 13–22.
- Safren SA, Duran P, Yovel I, Perlman CA, Sprich S. Medication adherence in psychopharmacologically treated adults with ADHD. J Atten Disord. 2007; 10: 257–60.
- Hodgson K, Hutchinson AD, Denson L. Nonpharmacological treatments for ADHD: a meta-analytic review. J Atten Disord. 2014; 18 : 275–282.
- Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, etal. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013; 170: 275–89.
- Arns M, Heinrich H, Strehl U. Evaluation of neurofeedback in ADHD: the long and winding road. Biol Psychol. 2014; 95: 108–15.
- Collura TF. Specifying and developing references for live Z-score neurofeedback. NeuroConnections. 2014; Spring : 26–39.
- Gevensleben H, Rothenberger A, Moll GH, Heinrich H. Neurofeedback in children with ADHD: validation and challenges. Expert Rev Neurother. 2012; 12: 447–60.
- Mayer K, Wyckoff SN, Strehl U. One size fits all? Slow cortical potentials neurofeedback: a review. J Atten Disord. 2012; 17: 393–409.
- Meisel V, Servera M, Garcia-Banda G, Cardo E, Moreno I. Reprint of ‘Neurofeedback and standard pharmacological intervention in ADHD: a randomized controlled trial with six-month follow-up.’. Biol Psychol. 2014; 95: 116–25.
- Steiner NJ, Frenette EC, Rene KM, Brennan RT, Perrin EC. In-school neurofeedback training for ADHD: sustained improvements from a randomized control trial. Pediatrics. 2014; 133: 483–92.
- Takahashi J, Yasumura A, Nakagawa E, Inagaki M. Changes in negative and positive EEG shifts during slow cortical potential training in children with attention-deficit/hyperactivity disorder: a preliminary investigation. Neuroreport. 2014; 25: 618–24.
- Melby-Lervåg M, Hulme C. Is working memory training effective? A meta-analytic review. Dev Psychol. 2013; 49 : 270–291.
- Gevensleben H, Holl B, Albrecht B, Schlamp D, Kratz O, Studer P, etal. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur Child Adolesc Psychiatry. 2010; 19: 715–24.
- Duric NS, Assmus J, Gundersen DI, Elgen IB. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry. 2012; 12: 107.
- Cortese S, Ferrin M, Brandeis D, Buitelaar J, Daley D, Dittmann RW, etal. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2014; 54: 164–74.
- Heinrich H, Gevensleben H, Freisleder FJ, Moll GH, Rothenberger A. Training of slow cortical potentials in attention-deficit/hyperactivity disorder: evidence for positive behavioral and neurophysiological effects. Biol Psychiatry. 2004; 55: 772–5.
- Langer N, von Bastian CC, Wirz H, Oberauer K, Jäncke L. The effects of working memory training on functional brain network efficiency. Cortex. 2013; 49: 2424–38.
- Gevensleben H, Holl B, Albrecht B, Schlamp D, Kratz O, Studer P, etal. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int J Psychophysiol. 2009; 74: 149–57.
- Wangler S, Gevensleben H, Albrecht B, Studer P, Rothenberger A, Moll GH, etal. Neurofeedback in children with ADHD: specific event-related potential findings of a randomized controlled trial. Clin Neurophysiol. 2011; 122: 942–50.
- Bruxel EM, Akutagava-Martins GC, Salatino-Oliveira A, Contini V, Kieling C, Hutz MH, etal. ADHD pharmacogenetics across the life cycle: new findings and perspectives. Am J Med Genet Part B Neuropsychiatr Genet. 2014; 165: 263–82.
- Lester KJ, Eley TC. Therapygenetics: using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biol Mood Anxiety Disord. 2013; 3: 4.
- Uher R. The implications of gene-environment interactions in depression: will cause inform cure?. Mol Psychiatry. 2008; 13: 1070–8.
- Birbaumer N. Slow cortical potentials: plasticity, operant control, and behavioral effects. Neuroscience. 1999; 5: 74–8.
- Collura TF, Guan J, Tarrant J, Bailey J, Starr F. EEG biofeedback case studies using live Z-score training and a normative database. J Neurother. 2010; 14: 22–46.
- Conners CK. Conners. 2008; North Tonawanda, NY: Multi-Health Syst. 3rd ed.
- Gallant S, Conners CK, Rzepa S, Pitkanen J, Marocco M, Sitarenios G. Psychometric Properties of the Conners 3rd Edition. Poster presented at the annual meeting of the American Psychological Association . 2007. San Francisco; August.
- Conners CK, Staff MHS, Connelly V, Campbell S, MacLean M, Barnes J. Conners’ Continuous Performance Test II (CPT II V. 5). Multi-Health Syst Inc. 2000; 29: 175–96.
- Toplak ME, Tannock R. Tapping and anticipation performance in attention deficit hyperactivity disorder. Percept Mot Skills. 2005; 100(3 Pt 1): 659–75.
- Toplak ME, Rucklidge JJ, Hetherington R, John SCF, Tannock R. Time perception deficits in attention-deficit/ hyperactivity disorder and comorbid reading difficulties in child and adolescent samples. J Child Psychol Psychiatry. 2003; 44: 888–903.
- Wechsler D. The Wechsler intelligence scale for children – fourth edition. 2003; Stockholm, Sweden: Pearson assessment. Swedish manual of the WISC-IV.
- Benson N, Hulac DM, Kranzler JH. Independent examination of the Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV): what does the WAIS-IV measure?. Psychol Assess. 2010; 22: 121–30.
- Wechsler D, Kaplan E, Fein D, Kramer J, Morris R, Delis D, etal. Wechsler intelligence scale for children-fourth edition-integrated. 2004; San Antonio, TX: Harcourt.
- Nyman H. WAIS-III NI: WAIS-III som neuropsykologiskt instrument. 3rd ed. 2004; Stockholm, Sweden: Pearson assess. Swedish manual of the WASI-III NI.
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990; 28: 1021–34.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000; 6: 235–8.
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating of executive function. 2000; Lutz, FL: Psychol Assess Resour.
- Urzua A, Cortes E, Vega S, Prieto L, Tapia K. Psychometric properties of the KIDSCREEN-27 self-reported quality of life questionnaire in chilean adolescents. Ter Psicol. 2009; 27: 83–92.
- Robitail S, Ravens-Sieberer U, Simeoni MC, Rajmil L, Bruil J, Power M, etal. Testing the structural and cross-cultural validity of the KIDSCREEN-27 quality of life questionnaire. Qual Life Res. 2007; 16: 1335–45.
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of ADHD? Evidence from brain electrical activity. J Neural Transm. 2004; 111: 841–64.
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin Psychol Rev. 2008; 28: 1343–54.
- Östberg M, Hagekull B, Hagelin E. Stability and prediction of parenting stress. Infant Child Dev. 2007; 16: 207–23.
- Doll EJ. Parenting stress index. 2nd ed. 1989; Professional School Psychology. 307–12.