Abstract
We report the release of oxygen (O2) under dark conditions in aerobic soils. This unexpected process is hidden by respiration which constitutes the dominating reversal O2 flux. By using H2 18O in different soils, we confirmed that 16O18O and 18O2 released under dark soil conditions originated from added H2 18O. Water is the only large-scale source of electrons for reduction of CO2 in soils, but it has not been considered as an electron donor because of the very strong oxidation system needed. A high share of soil inorganic material seems to favor the release of O2.
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
1. Introduction
1.1. Background
Oxidation of water leading to the release of oxygen (O2) has been explicitly associated with photosynthetic organisms. This process was studied by Robert Hill more than 70 yr ago (Walker, Citation2002). No other known large-scale aerobic biological O2 production has been discovered. This is understandable since the O2-evolving complex is one of the strongest biological oxidation systems known (Wydrzynski, Citation2008). Under anaerobic conditions, O2 production has been shown by chlorate-reducing bacteria (Nilsson et al., Citation2013), and denitrification with methane as an electron donor has been associated with the release of O2 as an intermediate (Ettwig et al., Citation2010, Citation2012).
1.2. Initial observations
Previous studies indicated that a part of CO2 in the soil atmosphere was allocated into an internal soil CO2 sink in different types of soils (Fleischer and Bouse, Citation2008; Fleischer, Citation2012). The process occurred in darkness, that is, photosynthesis could not explain this within-soil reduction of CO2. The mechanism of this process remains speculative; however, the supply of NH4 + stimulated the use of CO2 as a carbon source thus indicating that nitrification was the possible chemoautotrophic process involved. Therefore, consumption of O2 was expected to increase as a result of the O2-consuming NH4 + additions, but instead it decreased. This might be interpreted as within-soil release of O2. A deeper insight into the prerequisites for these findings was required, and this study was initiated.
1.3. Hypothesis
If we suppose that a chemoautotrophic process in soils drives the reduction of CO2, we must answer an important question: Where do the electrons come from? The only source available on a large scale is water. However, the possibility that soil nitrification drives the reduction of CO2 using H2O as an electron donor does not coincide with our current knowledge. This implies that the release of O2 is masked by the predominating O2 consumption in soils. If oxidation of water, in some way analogous to the process in green plants, occurred in the soil darkness, would tests using H2 18O demonstrate a release of 18O2?
2. Materials and methods
We used sieved (2 mm) composite soil samples (ø25 mm) from 0 to 5 cm depth. The fresh litter of the O-horizon was excluded. The samples were transported at near sampling temperatures (approx. ±2°C deviation) and safely protected from light from the day of sampling until they were analysed.
For incubations with added H2 18O, we used 12-ml Exetainers containing 0.5 or 1 g soil. The Exetainers were initially open to air for 3-11 d at incubation temperature. High moisture samples took the longest time. This enabled loss of moisture later compensated for again by the additions at incubation start. Oscillating moisture is the natural situation in soils following precipitation/dry periods. In addition to being kept in the darkness, the Exetainers were also wrapped with aluminum foil as an additional safety measure.
H2 18O (97% 18O) was from Armar Chemicals in Döttingen, Switzerland. At the start of incubation, 100 or 150 µl H2 18O was added. One hundred microlitres of formalin (4%) per gram of soil did not inhibit all biological activity in one sample initially tested. Double dose (200 µl) of formalin was therefore used, in addition to one sample with 100 µl of a strong HgCl2 water solution (15 mg ml−1), which was also used as a second, parallel control that is very efficient under the prevailing aerobic conditions (Trevors, Citation1996). Microbial activity was inhibited in all samples that received formalin or HgCl2. The results are based on three incubations (the two references, and the non-poisoned sample from which microbial respiration and release of 18O2 and 16O18O was calculated).
The amount of water added was the same in the intact and inhibited samples. The Exetainers were then sealed. Samples from 2011 were incubated for longer periods (Table and 3 ) until measurement of the isotopic composition in the atmosphere of the Exetainers.
An OmniStar™ quadrupole gas mass spectrometer, Pfeiffer Vacuum, was used to determine the oxygen gas masses; 32 u, 34 u and 36 u, and the carbon dioxide mass 44 u. A sample needle attached to the gas-MS was used to penetrate the septum of the sealed Exetainer. The gas-MS was operated in multiple ion detection mode collecting data to a single file for all measurements within a dataset to ensure as stable an operation of the ion emission source, quadrupole system and detector as possible.
The data section assigned to each sample started once a visibly stable signal was achieved from the instrument, normally 1–2 min after septum penetration. For each sample, data were recorded for around 2 min (approx. 2 sec/point), whereof the last 30 data points were used to determine an average signal current and its standard deviation for respective mass of interest. Before each sample, a measurement on ambient air was used to recalibrate the system and check for the gas-MS stability. Further details on the method are found in Supplementary file.
All values recorded in the data file were corrected for the gas-MS vacuum chamber pressure fluctuations at a single data point level. The air measurement preceding each sample was used to determine the average background signal and its standard deviation over 100 data points. The average BG+3·SDBG was used as a limit of qualitative determination, and the average BG+10·SDBG was used as a limit of quantitative determination (Currie, Citation1968); see Supplementary file.
Ordinary air was used as the start atmosphere in all Exetainers. As a result, a subtraction of the preceding air measurement automatically corrects the measurement for naturally occurring species at respective mass of interest. In this case, mainly the correction of mass 36 for its 36Ar contents is useful in order not to overestimate the 18O2 production in the experiments. A new calibration was made for each sample based on the air measurement preceding it; details on the calibration procedure are found in Supplementary file. A complete calculation from measured current to tabulated O2 production rates is given for the Biskopstorp sample in Supplementary file.
Sampling sites are described in . After 2 h, the pH of 10 g soil in 25 ml of water/1 M KCl was measured.
Table 1. Characteristics of the sites studied
3. Results
During an initial study in 2009, 14 soil samples were collected from 11 Swedish and German forest, grassland and agricultural sites. Oxygen in CO2 can have a respiratory origin, but can also be the result of abiotic water–carbonic acid exchange. The CO2 molecular weights 46 and 48, as well as 44 originating from the water in the fresh soil sample, were found. Significant production of 18O2 and 18O16O was detected in 10 of the 14 soil samples ().
Table 2. Release of 18O2 and 18O16O after dark incubation of H2 18O in different soils during autumn 2009
Whether O2 released to the soil atmosphere was the result of a biological process deserves to be unequivocally delineated. We therefore used controls with inhibited biological activity during the tracer study in 2011. In the inhibited controls, no release of 18O2 or 18O16O occurred. Release of 18O2 and 18O16O was associated with uninhibited soil, but it was low in the acidic soils (). At the two sites Annaberg and Sparneck, with very acidic soils, no release of 18O2 or 18O16O was detected and soil respiration was low.
Table 3. Release of 18O2 and 18O16O, and simultaneous soil respiration estimated as decrease in 16O2 after dark incubation of H2 18O in different soils during the summer 2011
4. Discussion and conclusion
The most obvious risk of misdetection due to other elements or compounds with a mass of 34 or 36 is that of 36S, but this would require that elemental sulphur in the gas phase, which is highly unlikely. A small fraction of 36Ar occurs naturally which we corrected for, using background subtraction. Hydrochloric acid (pKa −7) is not a constituent of the soils and the release of this compound, if present in soils with pH>3, would require it to be carried over to the gas phase, which is also unlikely. The highest release of 18O2 and 18O16O occurred in soils with pH>5. 35Cl and 37Cl amounts to 76 and 24% naturally, but no hydrochloric acid could be detected at mass 38, that is, there is no HCl interference at mass 36. These deductions are further supported by those analyses that indicated nothing but background at mass 36 in some of the samples. This implies that not only was 18O2 not found, but possible misdetections of the other elements/compounds can be excluded. Moreover, masses 36 and 34, over background levels, were only detected in the uninhibited samples. If the process is solely non-biological, there should be at least traces above the background of 18O2 present in inhibited samples. Since this is not the case, 18O2 was only detected in biologically active samples, the most probable explanation is that a biological process is the source of 18O2.
Some biological processes, which can result in O2 production in the dark, are known. Most well known is the decomposition of hydrogen peroxide mostly attributed to ultraviolet radiation in aquatic environments (Pamatmat, Citation1997; Wilson et al., Citation2000). If occurring in the dark, H2 18O2 is formed from the reduction of 18O2 which, in turn, must first be released by the indicated oxidation of H2 18O. But H2 18O was added to soil that was constantly kept dark. A direct hydrogen peroxide pathway to 18O2 is not feasible under the experimental conditions described above.
A second biological process that leads to O2 production is microbial oxochlorate respiration (Nilsson et al., Citation2013), in which oxygen is produced when chlorite is decomposed. However, it is unlikely that the soil samples used in this study contain significant amounts of oxochlorates. Oxochlorates only accumulate to measureable amounts when leaching and microbial activity is low, for example, in very arid environments. Therefore, we think that this alternative can be ruled out.
Finally, it has been suggested that oxygen is produced by dismutation of nitric oxide (NO) in anaerobic methane oxidisers (Ettwig et al., Citation2010, Citation2012). These organisms appear to utilise an alternative mode of denitrification, involving disproportionation of NO into N2 and O2 instead of reduction to N2O. The O2 produced appears to be used for oxidation of reduced substrates, for example, methane. As nitrate and nitrite are present in the soil samples used, we cannot exclude the possibility that an NO dismutase is involved in oxygen production in our experiments. However, this would require that denitrification processes (including the NO dismutase) are active in the soil samples under the strictly aerobic conditions in our study.
Non-biological production of O2 that requires large amounts of energy at high pressures or at very high temperatures, including thermal decomposition of water, and electrolysis, are known technologies that can be disregarded since the incubations were carried out at 15°C and atmospheric pressure.
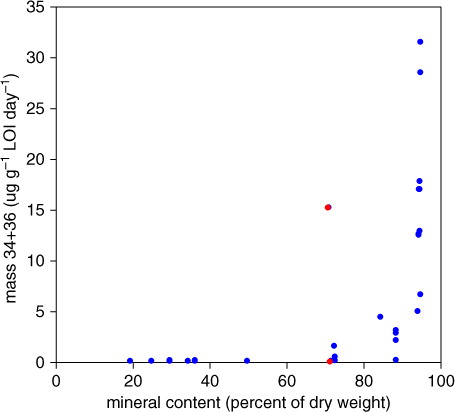
A largely unexpected biochemical pathway in forest, agricultural and grassland soils is indicated. It is masked by the reversal, dominating soil respiration concurrently consuming O2 in the soil. After 41 d of H2 18O-incubation in darkness the relation of 18O16O to 18O2 was still high in some of the samples, that is, the share of H2 16O from the original fresh sample was high. In addition to the role of pH, one clue from our results could be that the most active O2 releasing organic material was in soils with a high share of inorganic material () which, similar to sorption of organic material (Kalbitz et al., Citation2005), provides sufficient facilities for biofilm attachments to mineral surfaces. As has been reported, nitrifiers are known to develop protecting biofilms (Hagopian and Riley, Citation1998). However, recent indications of almost ubiquitous nitrification including Archaea and Bacteria (Martens-Habbena et al., Citation2009) call for a more in-depth study to identify the ecological niches with dark oxidation of water in aerobic soils.
Fig. 1 Sum of masses 34 and 36 produced in soil organic material (LOI) versus soil inorganic material. The deviating value (upper red dot) is from the forest research site Skällås 2009 (two years after P–K fertilisation). Another two years later mass 34+36 was not detected at this site (lower red dot).
Calibration
Download MS Word (34.5 KB)5. Acknowledgements
The authors thank Karsten Pedersen for advice early in the project, L.-G. Franzén for regular discussions on possible metabolic pathways, Gunnar Jacks for assistance with soil texture descriptions, Christian Ekberg for his comments and advice and four unknown referees for valuable comments. This work was supported by the Brita and Sven Rahmn Foundation.
Notes
To access the supplementary material to this article, please see Supplementary files under Article Tools online.
References
- Currie L. A . Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal. Chem. 1968; 40: 586–593.
- Ettwig K. F , Butler M. K , Le Paslier D , Pelletier E , Mangenot S , co-authors . Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature. 2010; 464: 543–548.
- Ettwig K. F , Speth D. R , Reimann J , Wu M. L , Jetten M. S. M , co-authors . Bacterial oxygen production in the dark. Front. Microbiol. 2012; 3: 1–8.
- Fleischer S . Interaction between N and C in soil has consequences for global carbon cycling. J. Resour. Ecol. 2012; 3: 16–19.
- Fleischer S , Bouse I . Nitrogen cycling drives a strong within-soil CO2-sink. Tellus B. 2008; 60: 782–786.
- Gerstberger P . Die BITÖK-Untersuchungsflächen in Fichtelgebirge und Steigerwald. 2001; Bayreuter Forum Ökologie . 90, 194 pp. ISSN 0944-4122.
- Hagopian D. S , Riley J. G . A closer look at the bacteriology of nitrification. Aquacultural Eng. 1998; 18: 223–244.
- Kalbitz K , Schwesig D , Rethemeyer J , Matzner E . Stabilization of dissolved organic matter by sorption to the mineral soil. Soil. Biol. Biochem. 2005; 37: 1319–1331.
- Klaus S , Stephan T . Nationalpark Hainich. Laubwaldpracht im Herzen Deutschlands.
- Knohl A , Schultze E-D , Kolle O , Buchmann N . Large carbon uptake by an unmanaged 250-year-old deciduous forest in central Germany. Agric. Forest Meteorol. 2003; 118: 151–167.
- Liu J , Aronsson H , Blombäck K , Persson K , Bergström L . Long-term measurements and model simulations of phosphorus leaching from a manured sandy soil. J. Soil. Water Conservat. 2012; 67: 101–110.
- Martens-Habbena W , Berube P. M , Urakawa H , de la Torre J. R , Stahl D. A . Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009; 461: 976–979.
- Nilsson T , Rova M , Smedja-Bäcklund A . Microbial metabolism of oxochlorates: a bioenergetics perspective. Biochim. Biophys. Acta. 2013; 1827: 189–197.
- Pamatmat M. M . Non-photosynthetic oxygen production and non-respiratory oxygen uptake in the dark: a theory of oxygen dynamics in plankton communities. Mar. Biol. 1997; 129: 735–746.
- Schwärzel K , Menzer A , Clausnitzer F , Spank U , Häntzschel J , co-authors . Soil water content measurements deliver reliable estimates of water fluxes: a comparative study in a beech and a spruce stand in the Tharandt forest (Saxony, Germany). Agric. Forest Meteorol. 2009; 149: 1994–2006.
- Sikström U . Pre-harvest soil acidification, liming or N fertilization did not significantly affect the survival and growth of young Norway spruce. Silva Fennica. 2005; 39: 341–349.
- Trevors J. T . Sterilization and inhibition of microbial activity in soil. J. Microbiol. Meth. 1996; 26: 53–59.
- Walker D. A . ‘And whose bright presence’ – an appreciation of Robert Hill and his reaction. Photosynth. Res. 2002; 73: 51–54.
- Wilson C. L , Hinman N. W , Sheridan R. P . Hydrogen peroxide formation and decay in iron-rich geothermal waters: the relative roles of abiotic and biotic mechanisms. Photochem. Photobiol. 2000; 71: 691–699.
- Wydrzynski T. J . Water splitting by Photosystem ll – where do we go from here?. Photosynth. Res. 2008; 98: 43–51.