Abstract
Tropical aerosols of PM2.5 and PM10 were collected at a rural site in Morogoro, Tanzania (East Africa), and analysed for stable carbon isotopic composition (δ13C) of dicarboxylic acids (C2–C9), glyoxylic acid (ωC2) and glyoxal (Gly) using gas chromatography/isotope ratio mass spectrometer. PM2.5 samples showed that δ13C of oxalic (C2) acid are largest (mean, −18.3±1.7‰) followed by malonic (C3, −19.6±1.0‰) and succinic (C4, −21.8±2.2‰) acids, whereas those in PM10 are a little smaller: −19.9±3.1‰ (C2), −20.2±2.7‰ (C3) and −23.3±3.2‰ (C4). The δ13C of C2–C4 diacids showed a decreasing trend with an increase in carbon numbers. The higher δ13C values of oxalic acid can be explained by isotopic enrichment of 13C in the remaining C2 due to the atmospheric decomposition of oxalic acid or its precursors. δ13C of ωC2 and Gly that are precursors of oxalic acid also showed larger values (mean, −22.5‰ and −20.2‰, respectively) in PM2.5 than those (−26.7‰ and −23.7‰) in PM10. The δ13C values of ωC2 and Gly are smaller than those of C2 in both PM2.5 and PM10. On the other hand, azelaic acid (C9; mean, −28.5‰) is more depleted in 13C, which is consistent with the previous knowledge; that is, C9 is produced by the oxidation of unsaturated fatty acids emitted from terrestrial higher plants. A significant enrichment of 13C in oxalic acid together with its negative correlations with relative abundance of C2 in total diacids and ratios of water-soluble organic carbon and organic carbon further support that a photochemical degradation of oxalic acid occurs during long-range transport from source regions.
1. Introduction
Low-molecular-weight dicarboxylic acids and related compounds comprise a significant fraction of organic aerosols and can play an important role in atmospheric chemistry and radiative forcing of the Earth's climate (Saxena and Hildemann, Citation1996). Dicarboxylic acids and related polar compounds are largely produced by photochemical processes in the atmosphere (Kawamura et al., Citation1996; Fisseha et al., Citation2004), but they can also be generated from primary sources including fossil fuel combustion (Kawamura and Kaplan, Citation1987) and biomass burning (Narukawa et al., Citation1999; Kundu et al., Citation2010). Photochemical oxidation and breakdown of relatively higher molecular weight diacids and other precursors are important sources of lower molecular weight diacids in the atmosphere (Kawamura and Yasui, Citation2005). The removal and mixing processes can affect the ambient concentrations of these compounds during long-range atmospheric transport (Mochida et al., Citation2003).
Different approaches have been applied to understand the sources of atmospheric aerosols as well as their long-range transport (Kawamura et al., Citation2000; Schmidt et al., Citation2004; Wang et al., Citation2006). Compound-specific stable carbon isotope analysis of lower molecular weight organic compounds has been used to determine the extent of photochemical processing and contribution of effective mixing process of the compounds in the atmosphere using the estimated kinetic isotope effect of target compound with OH radicals (Kawamura and Watanabe, Citation2004; Wang and Kawamura, Citation2006). Compound-specific stable carbon isotope ratios that are more specific in source identifications of higher molecular weight organic compounds have been applied in many scientific fields (e.g. Lichtfouse, Citation2000; Schmidt et al., Citation2004).
Photochemical processing of aerosols in the atmosphere largely affects the composition of water-soluble matter in aerosols. Their composition is important in controlling the ability of a particle to be activated as cloud condensation nuclei (CCN) (Sun and Ariya, Citation2006; Shilling et al., Citation2007). Because diacids and related polar compounds are largely produced by photochemical oxidation, compound-specific stable carbon isotope analysis of these compounds can be a useful tool for the assessment of photochemical aging of organic aerosols (Kawamura and Watanabe, Citation2004; Wang and Kawamura, Citation2006).
Previous studies have been conducted on stable carbon isotope ratios of water-soluble organic species in atmospheric aerosols (Kawamura and Watanabe, Citation2004; Irei et al., Citation2006; Aggarwal and Kawamura, Citation2008). However, little is known about the stable isotopes in tropical aerosols (Pavuluri et al., Citation2011), and no studies have been conducted in Africa. Here, we report for the first time the stable carbon isotopic compositions of low-molecular-weight (C2–C9) diacids, glyoxylic acid (ωC2) and glyoxal (Gly) in tropical aerosols (PM2.5 and PM10) collected at a rural background site in Tanzania, East Africa, in May through August 2011. Based on δ13C data, we discuss the sources and atmospheric processing of aerosols during long-range transport to the sampling site. This study provides the first baseline data sets of compound-specific stable carbon isotope analysis of water-soluble organic aerosols from East Africa.
2. Experimental
2.1. Aerosol sampling
Aerosol sampling was carried out at Morogoro (300 000 inhabitants, ), a rural site about 200 km west of the coast of the Indian Ocean (Mkoma and Kawamura, Citation2013) where the city of Dar es Salaam, a business capital in Tanzania, locates. The PM2.5 and PM10 low-volume samplers were deployed at a University campus of Sokoine University of Agriculture (06°47′40.8′′S, 37°37′44.5′′E, altitude 504 m a.s.l). Aerosols were collected at 2.7 m above ground level using ‘Gent type’ samplers (flow rate 17.0 L min−1; Maenhaut et al., Citation1994) and pre-combusted (450°C for 4 h) quartz fibre filters (Pallflex 2500QAT-UP, 47 mm). A total of 10 samples of PM2.5 and 20 set of PM10 samples with 2 field blanks for each were collected on approximately 24 h basis in 2011 from 30 May to 13 June (for PM2.5) and 30 May to 8 August (for PM10). Before and after sampling, the filters were placed in a pre-heated glass vial with a Teflon-lined screw cap and kept at −20°C during storage. The samples were transported to the atmospheric chemistry laboratory at the Institute of Low Temperature Science, Hokkaido University (Japan) and stored in a freezer at −20°C prior to analysis. All the procedures were strictly quality-controlled to avoid any possible contamination of the samples.
2.2. Determination of δ13C of diacids, glyoxylic acid and glyoxal
Stable carbon isotopic compositions (δ13C) of low-molecular-weight dicarboxylic acids, glyoxylic acid and glyoxal relative to Pee Dee Belemnite (PDB) were measured using the method developed by Kawamura and Watanabe (Citation2004). Briefly, 2 µl of n-C13 alkane (internal standard) was added to 40 µl of a portion previously derivatised for diacid analysis (Mkoma and Kawamura, Citation2013). The δ13C of the derivatives were determined using a gas chromatograph (HP6890)/isotope ratio mass spectrometer (IRMS) (Finnigan MAT Delta Plus) system and calculated for free organic acids using an isotopic mass balance equation based on the measured δ13C of the derivatives and the derivatising agent (1-butanol). Duplicate measurements for each sample were conducted and the difference in δ13C for major diacids for replicate analyses was generally below 1%. However, for minor species, the analytical accuracy was <2%.
The measurement of dicarboxylic acids, glyoxylic acid and glyoxal were done using a gas chromatography/flame ionisation detector (GC/FID) and GC/mass spectrometry (Kawamura and Yasui, Citation2005). Total concentrations of diacids were reported in a range of 289–362 ng m−3. Those of oxoacids and α-dicarbonyls were 37.8–53.7 ng m−3 and 5.7–7.8 ng m−3, respectively. Oxalic acid (C2) was found as the most abundant diacid species followed by succinic and/or malonic acids whereas glyoxylic acid and glyoxal were the dominant oxoacid and α-dicarbonyl, respectively in both seasons in PM2.5 and PM10 (Mkoma and Kawamura, Citation2013). The concentration of organic carbon (OC) was measured using a Sunset Laboratory carbon analyser following the method described in Wang et al. (Citation2005) whereas water-soluble organic carbon (WSOC) was measured using a Shimadzu carbon analyser (TOC-VCSH) (Miyazaki et al., Citation2011).
2.3. Meteorology and backward air mass trajectories
During the campaigns, meteorological parameters including ambient temperature, relative humidity and precipitation were recorded. The average ambient temperature was 25.0°C with minimum of 21.1°C and maximum of 29.1°C. The daily average relative humidity ranged from 65 to 96% in the morning hours and from 41 to 60% in the afternoon. The prominent wind pattern during sampling days was characterised by the south easterly (SE) monsoons with daytime average wind speed of 7.8 m s−1. The aerosol samplings were conducted mostly in days without rain or with a very weak rain.
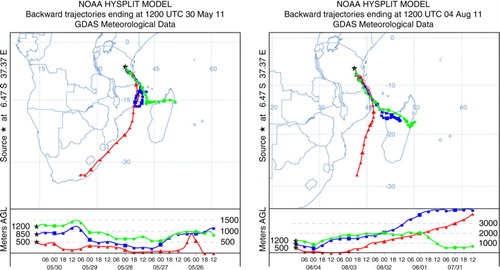
To characterise the air masses encountered at Morogoro during the campaign, 5-d back trajectory analyses were performed for each of the samples using the Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model of the NOAA/ARL (http://ready.arl.noaa.gov/HYSPLIT.php; Draxler and Rolph, Citation2012). The isentropic backward trajectories for 24 h samples indicated that the air masses in Morogoro were influenced by regional air masses from the Indian Ocean mixed with those from continental Madagascar, Mozambique and Tanzania ().
3. Results and discussion
3.1. Stable carbon isotopic compositions of dicarboxylic acids, glyoxylic acid and glyoxal
Table and 2 present the results of stable carbon isotope measurements for low-molecular-weight dicarboxylic acids (C2, C3, C4, C9), phthalic acid (Ph), glyoxylic acid (ωC2) and glyoxal (Gly) in PM2.5 and PM10 aerosols, respectively. δ13C of dominant diacids (C2, C3 and C4) ranged from −15.9 to −24.0‰ in PM2.5 with mean of −18.3‰, −19.6‰ and −21.8‰, respectively, whereas those in PM10 ranged from −15.7 to −32.2‰ with mean of −19.9‰, −20.2‰ and −23.3‰, respectively. The mean δ13C of glyoxylic acid (ωC2) (−22.5‰) and glyoxal (−20.2‰) in PM2.5 are larger than those (−26.7‰ and −23.7‰) of PM10 by about 4‰ and 2‰, respectively (Table and 2 ). Oxalic acid and Gly in PM2.5 showed similar trend of δ13C values in the samples during the campaign, suggesting that photochemical pathways for the formation of the acid and aldehyde are similar. Glyoxylic acid and Gly are reported as important precursors of oxalic acid via the oxidation of aromatic hydrocarbons and isoprene (Ervens et al., Citation2004; Lim et al., Citation2005).
Table 1. Stable carbon isotope ratios (δ13C, ‰) of dicarboxylic acids, glyoxylic acid and glyoxal in PM2.5 aerosols collected from Tanzania, in July and August 2011
Table 2. Stable carbon isotope ratios (δ13C, ‰) of dicarboxylic acids, glyoxylic acid and glyoxal in PM10 aerosols collected from Tanzania, in May through August 2011
On the other hand, C9 was abundantly observed in the aerosol samples collected in July to August. Its δ13C values (mean: −28.5‰) for PM10 are more depleted in 13C compared to C2–C4 diacids. The δ13C signature of C9 diacid suggests that the contributions of tropical organic emissions from C3 plant are more significant than those of C4 plant. Unfortunately, the δ13C measurements of C9 in PM2.5 samples were not available due to the lower concentrations. C9 diacid is mainly produced by atmospheric oxidation of unsaturated fatty acids that are emitted from either terrestrial or marine biological sources (Kawamura et al., Citation1996, Citation2000) and/or biomass burning (Kawamura et al., Citation1996). The smaller δ13C signature of C9 suggests that its major sources (unsaturated fatty acids) are terrestrial higher plants, not marine plankton, because terrestrial organic materials are more depleted in 13C compared to marine organic materials (Fang et al., Citation2002).
Phthalic acid (Ph) showed smaller δ13C values (mean: −27.5‰) than those of C2–C4 diacids, ωC2 and Gly. The observed 13C depletion of Ph in both PM2.5 and PM10 suggest an anthropogenic contribution from combustion sources (Kawamura and Kaplan, Citation1987) and/or atmospheric photochemical degradation of polycyclic aromatic hydrocarbons such as naphthalene (Kawamura and Ikushima, Citation1993). Open burning of municipal solid waste (large amounts of plastics) is very common in Tanzania (Kassim, Citation2006), together with local anthropogenic emissions. These sources should also be responsible for the aromatic diacid (Yassaa et al., Citation2001; Simoneit et al., Citation2005; Kawamura and Pavuluri, Citation2010).
The box plot in shows statistical distributions of δ13C of diacids, glyoxylic acid and glyoxal in PM2.5 and PM10. We found a decreasing trend in δ13C for C2 to C4 diacids in PM2.5 with C2 diacid being slightly more enriched in 13C than C3 and C4 diacids ( and ). The less negative δ13C of lower carbon number diacids can be explained by the isotopic fractionations that may occur during breakdown of diacids, e.g. iron-oxalate complex and, to a lesser extent, iron-malonate complex (Kawamura et al., Citation2012). Oxalic acid and Ph in both PM2.5 and PM10 show the largest and smallest median δ13C values, respectively. δ13C of glyoxylic acid (ωC2) and Gly showed fairly high values in PM2.5, but they are significantly lower than that of C2 diacid in PM10 (b). Although oxalic acid (most enriched with 13C) has been proposed as an end product of the atmospheric oxidation of longer-chain diacids and other precursors such as ωC2 and Gly (Kawamura et al., Citation1996; Sempéré and Kawamura, Citation2003), this study suggests that even C2 can be decomposed in the atmosphere.
Fig. 3 Box plot of stable carbon isotope ratios of diacids, glyoxylic acid (ωC2) and glyoxal (Gly) in PM2.5 and PM10 aerosols from Morogoro, Tanzania, collected during the campaign. Each box shows the median (black line), the interquartile range (box) and the minimum and maximum values. Open circles show the outliers.
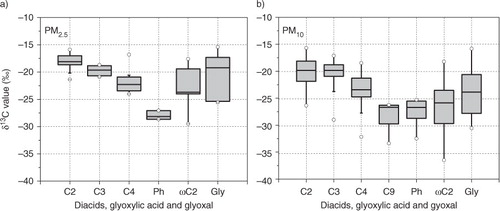
The range and average of δ13C for C2, C3 and C4 diacids in aerosols from Morogoro, Tanzania (Table and 2 ), are comparable to those reported for tropical aerosols from Chennai, India (range: −9.2‰ to −27.4‰, ave.: −17.1‰, −20.8‰ and −22.5‰, respectively) (Pavuluri et al., Citation2011) and for suburban aerosols from Sapporo, Japan (range: −14.0‰ to −25.3‰, ave.: −18.8‰, −21.7‰ and −22.7‰, respectively) (Aggarwal and Kawamura, Citation2008). It is likely that C2 and, to a lesser extent, C3 are photochemically aged during long-range atmospheric transport. The larger δ13C values of lower molecular weight diacids in particular C2 (a and b) indicate that the aerosols in Morogoro have significantly been subjected to photochemical processing in the tropical atmosphere during long-range transport from continental source regions in Tanzania, Mozambique and Madagascar ().
3.2. Relation of δ13C to relative abundance of oxalic acid
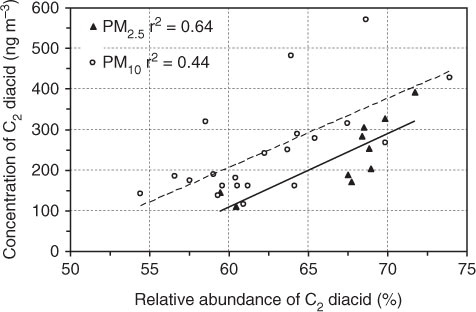
The relative abundance of C2 diacid (C2%) to total diacids has been proposed as a measure of photochemical processing (Kawamura and Sakaguchi, Citation1999). Concentrations of C2 positively correlated with C2% in the PM2.5 and PM10 aerosols from Morogoro (), indicating that preferential production of C2 is associated with photochemical processing of organic aerosols in the atmosphere, being similar to that reported in tropical aerosols from Chennai, India (Pavuluri et al., Citation2011). The air mass trajectories suggested that the air masses are mixed with fresh precursors along the transport pathway as they passed over the continent () where the biomass/biofuel burning emissions are significant.
Fig. 4 Relation between concentrations of oxalic (C2) acid and its relative abundance to total diacids in PM2.5 and PM10 during 2011 sampling period in Morogoro, Tanzania. Data from Mkoma and Kawamura (2013).
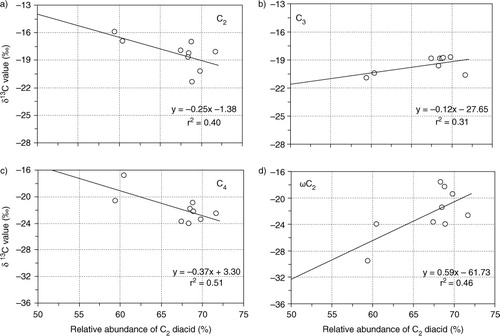
shows δ13C of C2, C3, C4 diacids and ωC2 as a function of relative abundance of oxalic acid (C2%) to total diacids in PM2.5. The δ13C of C2 negatively correlate with C2% (a) whereas those of C3 positively (although weak) correlate with C2% (b). Similar trend was obtained for C4 diacid (c), suggesting an enrichment of 13C in C4 as well. However, the δ13C of ωC2 did show a positive correlation with C2%, suggesting that photochemical decomposition of ωC2 is one of key factors responsible for the increase of δ13C (Kawamura et al., 2012). On the other hand, PM10 samples did not show any significant correlations, except for C4 diacid, which negatively correlated with C2% (r 2=0.38). These results may suggest that the isotopic enrichment of the remaining small organic acids occur in fine particles (PM2.5). The observed δ13C values for diacids in aerosols from Morogoro suggest that some organics (e.g. C3) were photochemically less aged (fresh), although average ambient temperatures (>25°C) and solar radiations were relatively high during the sampling periods.
3.3. δ13C of diacids, glyoxylic acid and glyoxal and photochemical processes
Atmospheric photochemical reactions, evaporation and isotope exchange with OC could contribute to the systematic differences in the isotopic composition of dicarboxylic acids (Anderson et al., Citation2004; Irei et al., Citation2006). However, under the ambient temperature and pressure, evaporation-related isotopic fractionations and isotope exchange between OC species and diacids are insignificant (Wang and Kawamura, Citation2006). Shorter-chain diacids can be formed by the photochemical breakdown of longer-chain diacids (Kawamura and Sakaguchi, Citation1999) and other precursors. The increasing trends of δ13C values for saturated diacids with a decrease in carbon numbers could be explained mainly by the kinetic isotope effect for the photochemical degradation of iron-oxalate/malonate complex (Wang and Kawamura, Citation2006; Kawamura et al., Citation2012). On the other hand, a laboratory study has provided an evidence for the isotopic enrichment of 13C in the remaining oxalic acid with photochemical aging; this approach is useful for better interpretation of atmospheric isotope measurements in terms of the extent of atmospheric processing of aerosols (Kawamura et al., Citation2012).
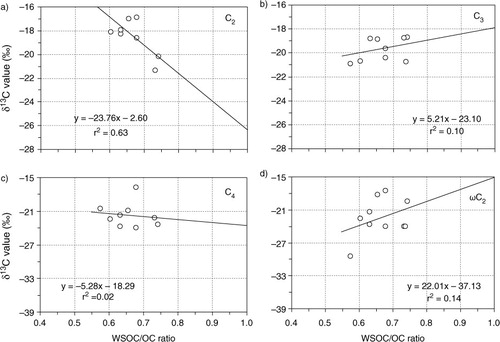
The ratio of WSOC/OC is a measure of photochemical processing or aging during atmospheric transport (Yang et al., Citation2004; Aggarwal and Kawamura, Citation2008), because prolonged photochemical oxidation of organics results in secondary organic aerosol (SOA) enriched with polar and thus water-soluble compounds. presents relations of the isotopic composition of diacids with WSOC/OC ratios in PM2.5. Interestingly, oxalic acid (C2) showed a negative correlation with WSOC/OC ratio whereas C3 and ωC2 showed a positive correlation (). Meanwhile, C4 showed no correlation. However, such a trend was not observed for PM10, except for C4 diacid that showed a negative correlation with WSOC/OC ratio (r 2=0.14). The negative correlation between the isotopic composition of C2 and WSOC/OC ratios supports that photochemical breakdown of C2 would increase δ13C of the remaining C2. C2 is one of the important contributors to WSOC.
4. Conclusions
In this study, we determined for the first time stable carbon isotope ratios of diacids, glyoxylic acid and glyoxal in PM2.5 and PM10 tropical aerosol samples that were collected between May and August 2011 in Tanzania (East Africa). Oxalic acid (C2) showed larger δ13C values than other species, suggesting photochemical processing of water-soluble organic aerosols. A slight decrease of δ13C was observed with an increase in carbon numbers from C2 to C4 diacids in PM2.5. Higher δ13C values of C2 and C3 diacids and Gly in PM2.5 and C2–C3 diacids in PM10 were found whereas C9 and Ph were depleted with 13C in both fractions. These observations suggest that the higher δ13C of C2–C3 diacids compared to C4 diacid may be associated with kinetic isotope effects for photochemical degradation of diacids and related compounds. Although the initial δ13C value of diacids can depend on their original (precursor) sources, enrichment of 13C caused by aerosol aging is more important in fine particles to control the stable carbon isotopic composition of lower molecular weight diacids in the ambient atmosphere.
6.Acknowledgements
We acknowledge the financial support by the Japan Society for the Promotion of Science (JSPS) to S.L.M. and the Environment Research and Technology Development Fund (B-0903) from the Ministry of the Environment, Japan. We thank Mr. Filbert T. Sogomba of the Department of Physical Sciences (SUA) for aerosol sample collection. The authors also thank the NOAA Air Resources Laboratory (ARL) for the provision of the HYSPLIT transport and dispersion model and/or READY website (http://www.arl.noaa.gov/ready.php) used in this publication.
References
- Aggarwal S. G. , Kawamura K . Molecular distributions and stable carbon isotopic compositions of dicarboxylic acids and related compounds in aerosols from Sapporo, Japan: implications for photochemical aging during long-range atmospheric transport. J. Geophys. Res. 2008; 113 D14301.
- Anderson R. S. , Iannone R. , Thompson A. E. , Rudolph J. , Huang L . Carbon kinetic isotope effects in the gas-phase reactions of aromatic hydrocarbons with the OH radical at 296±4 K. Geophys. Res. Lett. 2004; 31 L15108.
- Draxler R. R. , Rolph G. D . HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model Access via NOAA ARL READY Website . 2012. NOAA Air Resources Laboratory, Silver Spring, MD http://ready.arl.noaa.gov/HYSPLIT.php .
- Ervens B. , Feingold G. , Frost G. J. , Kreidenweis S. M . A modelling study of aqueous production of dicarboxylic acids: 1. Chemical pathways and speciated organic mass production. J. Geophys. Res. 2004; 109 D15205.
- Fang J. , Kawamura K. , Ishimura Y. , Matsumoto K . Carbon isotopic composition of fatty acids in the marine aerosols from the western North Pacific: implication for the source and atmospheric transport. Environ. Sci. Technol. 2002; 36: 2598–2604.
- Fisseha R. , Dommen J. , Sax M. , Paulsen D. , Kalberer M. , co-authors . Identification of organic acids in secondary organic aerosol and the corresponding gas phase from chamber experiments. Anal. Chem. 2004; 76: 6535–6540.
- Irei S. , Huang L. , Collin F. , Zhang W. , Hastie D. , co-authors . Flow reactor studies of the stable carbon isotope composition of secondary particulate organic matter generated by OH-radical-induced reactions of toluene. Atmos. Environ. 2006; 40: 5858–5867.
- Kassim S. M . Sustainability of Private Sector in Solid Waste Collection-A Case of Dar es Salaam, Tanzania.
- Kassim S. M . Sustainability of Private Sector in Solid Waste Collection-A Case of Dar es Salaam, Tanzania.
- Kawamura K. , Kaplan I. R . Motor exhaust emission as a primary source of dicarboxylic acids in Los Angeles ambient air. Environ. Sci. Technol. 1987; 21: 105–110.
- Kawamura K. , Kasukabe H. , Barrie L. A . Source and reaction pathways of dicarboxylic acids, ketoacids and dicarbonyls in arctic aerosols: one year of observations. Atmos. Environ. 1996; 30: 1709–1722.
- Kawamura K. , Ono K. , Tachibana E. , Charriére B. , Sempéré R . Distributions of low molecular weight dicarboxylic acids, ketoacids and α-dicarbonyls in the marine aerosols collected over the Arctic Ocean during late summer. Biogeoscience. 2012; 9 4725–4737.
- Kawamura K. , Pavuluri C. M . New directions: need for better understanding of plastic waste burning as inferred from high abundance of terephthalic acid in South Asian aerosols. Atmos. Environ. 2010; 44: 5320–5321.
- Kawamura K. , Sakaguchi F . Molecular distributions of water soluble dicarboxylic acids in marine aerosols over the Pacific Ocean including tropics. J. Geophys. Res. 1999; 104(D3): 3501–3509.
- Kawamura K. , Steinberg S. , Kaplan I. R . Homologous series of C1-C10 monocarboxylic acids and C1-C6 carbonyls in Los Angeles air and motor vehicle exhausts. Atmos. Environ. 2000; 34: 4175–4191.
- Kawamura K. , Watanabe T . Determination of stable carbon isotopic compositions of low molecular weight dicarboxylic acids and ketocarboxylic acids in atmospheric aerosol and snow samples. Anal. Chem. 2004; 76: 5762–5768.
- Kawamura K. , Yasui O . Diurnal changes in the distribution of dicarboxylic acids, ketocarboxylic acids and dicarbonyls in the urban Tokyo atmosphere. Atmos. Environ. 2005; 39: 1945–1960.
- Kundu S. , Kawamura K. , Andreae T. W. , Hoffer A. , Andreae M. O . Molecular distributions of dicarboxylic acids, ketocarboxylic acids and α-dicarbonyls in biomass burning aerosols: implications for photochemical production and degradation in smoke layers. Atmos. Chem. Phys. 2010; 10: 2209–2225.
- Lichtfouse E . Compound-specific isotope analysis: application to archaeology, biomedical sciences, biosynthesis, environment, extraterrestrial chemistry, food science, forensic science, humic substances, microbiology, organic geochemistry, soil science and sport. Rapid Commun. Mass Spectrom. 2000; 14: 1337–1344.
- Lim H.-J. , Carlton A. G. , Turpin B. J . Isoprene forms secondary organic aerosol through cloud processing: model simulations. Environ. Sci. Technol. 2005; 39: 4441–4446.
- Maenhaut W. , François F. , Cafmeyer J . The Gent stacked filter unit sampler for the collection of atmospheric aerosols in two size fractions: description and instructions for installation and use. Applied Research on Air Pollution Using Nuclear-Related Analytical Techniques. 1994; 249–263. IAEA Report NAHRES-19, Vienna.
- Miyazaki Y. , Kawamura K. , Jung J. , Furutani H. , Uematsu M . Latitudinal distributions of organic nitrogen and organic carbon in marine aerosols over the western North Pacific. Atmos. Chem. Phys. 2011; 11: 3037–3049.
- Mkoma S. L. , Kawamura K . Molecular composition of dicarboxylic acids, ketocarboxylic acids, α-dicarbonyls and fatty acids in atmospheric aerosols from Tanzania, East Africa during wet and dry seasons. Atmos. Chem. Phys. 2013; 13: 2235–2251.
- Mochida M. , Kawamura K. , Umemoto N. , Kobayashi M. , Matsunaga S. , co-authors . Spatial distribution of oxygenated organic compounds (dicarboxylic acids, fatty acids and levoglucosan) in marine aerosols over the western Pacific and off coasts of East Asia: continental outflow of organic aerosols during the ACE-Asia campaign. J. Geophys. Res. 2003; 108(D23): 8638.
- Narukawa M. , Kawamura K. , Takeuchi N. , Nakajima T . Distribution of dicarboxylic acids and carbon isotopic compositions in aerosols from 1997 Indonesian forest fires. Geophys. Res. Lett. 1999; 26(20): 3101–3104.
- Pavuluri C. M. , Kawamura K. , Swaminathan T. , Tachibana E . Stable carbon isotopic compositions of total carbon, dicarboxylic acids and glyoxylic acid in the tropical Indian aerosols: implications for sources and photochemical processing of organic aerosols. J. Geophys. Res. 2011; 116 D18307.
- Saxena P. , Hildemann L. M . Water-soluble organics in atmospheric particles: a critical review of the literature and application of thermodynamics to identify candidate compounds. J. Atmos. Chem. 1996; 24: 57–109.
- Schmidt T. C. , Zwank L. , Elsner M. , Berg M. , Meckenstock R. U. , co-authors . Compound-specific stable isotope analysis of organic contaminants in natural environments: a critical review of the state of the art, prospects, and future challenges. Anal. Bioanal. Chem. 2004; 378: 283–300.
- Sempéré R. , Kawamura K . Trans-hemispheric contribution of C2-C10 α, ω-dicarboxylic acids and related polar compounds to water-soluble organic carbon in the western Pacific aerosols in relation to photochemical oxidation reactions. Global Biogeochem. Cycles. 2003; 17: 1069.
- Shilling J. E. , King S. M. , Mochida M. , Worsnop D. R. , Martin S. T . Mass spectral evidence that small changes in composition caused by oxidative aging processes alter aerosol CCN properties. J. Phys. Chem. A. 2007; 111: 3358–3368.
- Simoneit B. R. T. , Medeiros P. M. , Didyk B. M . Combustion products of plastics for refuse burning in the atmosphere. Environ. Sci. Technol. 2005; 39: 6961–6970.
- Sun J. , Ariya P. A . Atmospheric organic and bio-aerosols as cloud condensation nuclei (CCN): a review. Atmos. Environ. 2006; 40: 795–820.
- Wang H. , Kawamura K . Stable carbon isotopic composition of low-molecular-weight dicarboxylic acids and ketoacids in remote marine aerosols. J. Geophys. Res. 2006; 111 D07304.
- Wang H. , Kawamura K. , Shooter D . Carbonaceous and ionic components in wintertime atmospheric aerosols from two New Zealand cities: implications for solid fuel combustion. Atmos. Environ. 2005; 39: 5865–5875.
- Wang H. , Kawamura K. , Yamazaki K . Water-soluble dicarboxylic acids, ketoacids and dicarbonyls in the atmospheric aerosols over the Southern Ocean and Western Pacific Ocean. J. Atmos. Chem. 2006; 53: 43–61.
- Yang H. , Xu J. , Wu W.-S. , Wan C. H. , Yu J. Z . Chemical characterization of water-soluble organic aerosols at Jeju Island collected during ACE-Asia. Environ. Chem. 2004; 1: 13–17.
- Yassaa N. , Meklati B. Y. , Cecinato A. , Marino F . Organic aerosols in urban and waste landfill of Algiers metropolitan area: occurrence and sources. Environ. Sci. Technol. 2001; 35: 306–311.