Abstract
Three new species of Hypocrea/Trichoderma sect. Trichoderma (Hypocreaceae, Hypocreales, Ascomycota, Fungi) are described from recent collections in southern Europe and the Canary Islands. They have been characterized by morphological and molecular methods, including microscopic examination of the teleomorph in thin sections, the anamorph, growth rate experiments and phylogenetic analyses based on a part of the translation elongation factor 1-alpha encoding gene (tef1) containing the two last introns and a part of the rpb2 gene, encoding the second largest RNA polymerase subunit. Analyses involving tef1 did not unequivocally resolve the sister clade relationship of Hypocrea caerulescens relative to the Koningii and Viride clades, while analyses based on rpb2 clearly suggest a close relationship with the former, although the phenotype of H. caerulescens is similar to H. viridescens, particularly by its warted conidia and a coconut-like odor in CMD culture. Hypocrea hispanica and T. samuelsii however are clearly related to the Viride clade by both phylogenetic markers, despite their morphological similarity to H. koningii and its relatives. An apparently specific blue pigment is formed in CMD cultures by Hypocrea caerulescens but could not be obtained by extraction with organic solvents.
Introduction
Hypocrea (Hypocreaceae, Hypocreales), often addressed in the non-taxonomic literature under the anamorph name Trichoderma, is a well known genus due to its importance for humans (CitationHarman and Kubicek 1998). Many physiological or biochemical activities have been ascribed to the type species H. rufa and its anamorph T. viride ( CitationJaklitsch et al. 2006). The antagonistic action of many Trichoderma species mainly against asco-and basidiomycetous fungi and certain oomycetous Chromista has made them powerful biocontrol agents to fight plant diseases, thereby also acting as opportunistic plant symbionts enhancing systemic resistance of plants (CitationHarman et al. 2004, CitationHarman 2006), for example as endophytes (CitationSamuels et al. 2006b, CitationHanada et al. 2008, CitationChaverri et al. 2011).
Antimicrobial action is driven by mechanisms that comprise both enzymatic and antibiotic activities. Various industrial applications in food and textile processing involve Trichoderma enzymes such as β-glucanases, pectinases, xylanases, but industrially most lucrative or promising is mass production of cellulases and hemicellulases, on the one hand for the detergent industry, on the other for production of second generation biofuels from cellulosic waste (CitationSchuster and Schmoll 2010). More than 430 entries on different secondary metabolites are listed in the current version of the natural product database, Antibase (CitationLaatsch 2010), and 30 are known from Hypocrea; hence these fungi undoubtedly belong to the most prolific metabolite producers among the eukaryotes. Important secondary metabolites comprise antibiotics such as peptaibols and a broad array of volatile organic compounds including 6-pentyl-α-pyrone, which causes the coconut-like aroma of several species of sect. Trichoderma, but also trichothecene mycotoxins (CitationGhisalberti and Sivasithamparam 1991; CitationDegenkolb et al. 2006; Citation2008a, Citationb). Some species have gained particular attention due to their destructive action: Those that are able to grow at 37 C, mostly belonging to the Longibrachiatum clade (e.g. Hypocrea orientalis), are opportunistic human pathogens of immunocompromised patients (CitationKredics et al. 2003; CitationDruzhinina et al. 2008a, Citationb). Other species causing green mold disease are responsible for serious losses in commercial mushroom cultivation (CitationPark et al. 2006, CitationHatvani et al. 2007). Species of Trichoderma are susceptible to strain improvement and genetic engineering via transformation, opening a wide field of applications in addition to their wide array of intrinsic potential (CitationSchuster and Schmoll 2010).
Pigments of Hypocreales so far have not been reported to have potential importance for industrial applications. In contrast to many members of the Xylariaceae, brightly colored stromata of Hypocrea are devoid of pigments extractable with KOH. In culture Hypocrea species, particularly in sect. Hypocreanum and Longibrachiatum, often form yellow to orange, less commonly reddish pigments on PDA. On CMD however pigments are only rarely formed and mostly after prolonged storage, for example yellow in H. atroviridis (at 30 C), H. aureoviridis, H. petersenii, H. psychrophila or H. voglmayrii, brown in H. crystalligena and green in H. aeruginea (CitationJaklitsch 2009, Citation2011).
Blue pigments from fungi however are produced extremely rarely. Even though numerous lichenized as well as nonlichenized ascomycetes show blue, these colors cannot be extracted with organic solvents and might be due to the presence of metal ion complexes of certain secondary metabolites that do not constitute pigments themselves. For instance the characteristic blue of certain Penicillium species is probably due to the presence of dimeric zinc complexes of phenaleones (CitationRobinson et al. 1992). Likewise the blue and green pigments of certain hypoxyloid Xylariaceae coincide with the presence of “lichen compounds” of the lepraric acid type, which are major extractable stromatal constituents (CitationLæssøe et al. 2010), but are uncolored in their pure state.
CitationJaklitsch (2009, Citation2011) gave a comprehensive account of European taxa of Hypocrea. However southern Europe could be studied only after that work. Conidial colonies were collected from plant and fungal material in addition to Hypocrea teleomorphs in that region including the Canary Islands. As a preliminary result the most commonly encountered species belong to the section Trichoderma. This is the most complex and morphologically most conserved group of species within the genus. Three new species in the section Trichoderma are described below, of which Hypocrea caerulescens is the most common and most remarkable due to the formation of a blue pigment in CMD cultures.
Materials and methods
Isolates and specimens.
Ascospore and conidial isolates were prepared as described by CitationJaklitsch (2009). Specimen information is given for each species after its description; the letter S stands for southern Europe and is used for both specimens and cultures. Representative isolates have been deposited at the Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands (CBS). Specimens have been deposited in the Herbarium of the University of Vienna (WU).
Growth, morphology, DNA extraction, PCR and sequencing.
Growth rate experiments were carried out and teleomorph and anamorph morphology was studied as described by CitationJaklitsch (2009). Genomic DNA extraction, PCR and sequencing were performed as described in that work, except that before DNA extraction by the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) mycelium was grown in 2% liquid malt extract culture, harvested by filtration, freeze dried and ground according to CitationVoglmayr and Jaklitsch (2008). GenBank accession numbers of sequences generated in this study are provided (). Accession numbers of sequences retrieved from GenBank are given on the phylogenetic trees (, ).
Analysis of sequence data.
ITS, tef1 and rpb2 sequences of all available species of section Trichoderma were downloaded from GenBank and combined with those used by CitationJaklitsch (2009, Citation2011). For ITS only a sequence alignment was produced with MUSCLE 3.6 (CitationEdgar 2004) to enable sequence comparisons, but no further phylogenetic analyses were performed due to insufficient phylogenetic information. tef1 and rpb2 sequences were aligned for a first rough phylogenetic analysis to select representative sequences for the final analyses. A single representative sequence subsequently was selected for each species, preferably from the types. Because several type sequences were short these were replaced by identical or highly similar sequences from other authentic sources where available or by those determined for newly isolated strains. For the new species several representative sequences were included in the matrix. Sequence alignments were produced with MUSCLE3.6 (CitationEdgar 2004). The resulting alignments were checked and refined with BioEdit 7.0.9.0 (CitationHall 1999). After exclusion of excessive leading/trailing gap regions, the tef1/rpb2 matrices contained 1353/1067 characters from 85/44 sequences respectively.
Maximum parsimony (MP) analyses of the data matrices were performed with PAUP* 4.0 b10 (CitationSwofford 2002), using 1000 replicates of heuristic search with random addition of sequences and subsequent tbr branch swapping (multrees option in effect, collapse = maxbrlen, steepest descent option not in effect). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. BS analysis with 1000 replicates was performed in the same way, but using five rounds of random sequence addition and subsequent branch swapping during each BS replicate; the number of rearrangements per replicate was limited to 10 000 000 in the tef1 analyses.
Table I. GenBank accessions of sequences obtained in this study for the listed strains
For ML analyses 500 bootstrap replicates were computed with RAxML 7.0.4 (CitationStamatakis 2006) using the GTRCAT algorithm. For Bayesian analyses the computer program MrBayes 3.1.2 (CitationHuelsenbeck and Ronquist 2001) was used, implementing the well known general time reversible model and additionally assuming a proportion of invariant sites with gamma-distributed substitution rates of the remaining sites (GTR + I + G). Three parallel runs of four incrementally heated simultaneous Markov chains were performed over 5 000 000 generations from which every 500th tree was sampled in each run. The first 1000 trees were discarded. A 90% majority rule consensus of the remaining trees was computed to obtain estimates for the probabilities that groups are monophyletic given the sequence data (posterior probabilities).
Analytical studies on the pigments of H. caerulescens.
Studies were carried out with a well established methodology that had been used in the past to detect and identify secondary metabolites of Xylariaceae (CitationStadler and Fournier 2006, CitationStadler et al. 2008) and relying on one of the most comprehensive spectral libraries of natural products (CitationBitzer et al. 2007). Well grown mycelia from cultures of H. caerulescens were retrieved from the agar plates, transferred into glass vials and extracted with methanol or acetone for 1 h. The extracts were concentrated in vacuo, redissolved in methanol and subjected to HPLC-DAD/MS profiling. Because the results for the regular organic extracts appeared to be inconclusive the procedure was repeated by a modified extraction procedure. The methanol was adjusted to pH 2 or pH 11 by adding respectively concentrated trifluoroacetic acid or KOH solution, and the extraction was repeated in a similar manner as described above for the preparation of conventional methanol extracts.
Results
Molecular phylogenetic analyses.
The final alignments and the trees obtained were deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S11946). ITS sequences of H. hispanica and T. samuelsii were almost or completely identical with those of H. koningii, viridescens and H. petersenii, demonstrating the unsuitability of ITS as barcoding marker within this group, as already shown by other authors (CitationDruzhinina et al. 2005). On the contrary, the ITS of H. caerulescens was characteristically distinct from all other species of sect. Trichoderma, with sequence differences (including gaps) of at least 19 bp.
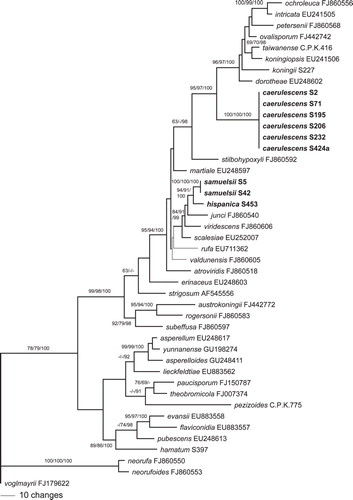
Of the 1353 characters of the tef1 alignment included in the analyses, 284 were parsimony informative. MP analyses revealed 224 MP trees 1191 bp long, one of which was selected and included herein (). Topologies of all MP trees were identical except for minor differences within the caerulescens, hispanica and samuelsii subclades, within the subclade containing H. rufa, T. scalesiae and T. martiale, and a slightly different position of the atroviridiserinaceus subclade (). The three Bayesian runs revealed almost identical posterior probabilities (PP) and were fully compatible with the MP strict consensus tree.
Of the 1067 characters of the rpb2 alignment included in the analyses, 227 were parsimony informative. MP analyses revealed two MP trees 847 bp long, one of which was selected and illustrated (). Topologies of the two MP trees differed only slightly in the phylogenetic position of H. rufa and H. valdunensis. The three Bayesian runs revealed almost identical posterior probabilities (PP) and were fully compatible with the MP strict consensus tree. Topologies of tef1 and rpb2 trees differed especially in the deeper nodes (, ); however most of these topological differences did not receive significant support in the tef1 tree (). The caerulescens, hispanica and samuelsii subclades were highly supported in both analyses, as was the sister group relationship of the latter two taxa. In the rpb2 analyses H. caerulescens is a closest relative to a core Koningii clade with high support, whereas its phylogenetic position remains unclear in the tef1 trees due to poor backbone support. In both phylogenetic trees H. hispanica and T. samuelsii are members of the Viride clade with medium to high support, but sister group relationship to H. junci lacks significant support (, ).
Characterization of the pigment formed in culture.
The native methanol and acetone extracts of mycelia of H. caerulescens grown in CMA as well as extracts prepared with acidified methanol remained largely uncolored, and the remaining mycelia remained grayish blue. Accordingly the HPLC analyses were inconclusive and no compounds featuring UV-visible maxima in the range of blue light were detected. A discoloration of the mycelia was observed only upon extraction with alkaline methanol. However in this case the extracts immediately turned yellowish, suggesting that the pigments of the mycelia had been dissolved. Treatments of these yellowish solutions by careful titration with trifluoroacetic acid or HCl to neutralize or acidify them in the hope of recovering blue pigment failed however, and the yellowish tint persisted. In the resulting HPLC profiles of such samples no distinct peaks with absorption maxima in the range of yellow light, but only a substantial tailing was observed, indicating that an original constituent of the mycelia might have decayed. The pigment of H. caerulescens is either an unstable compound that changed its chemical composition under the drastic extraction conditions or it could as well be an ion complex that is destroyed by high pH and cannot be reinstated by neutralization, according to these preliminary results.
Taxonomy
Hypocrea caerulescens Jaklitsch & Voglmayr sp. nov. ,
MycoBank MB563315
Etymology: Caerulescens for the blue CMD agar discoloration upon storage at 15 C.
Fig. 1. Phylogram of one of 224 MP trees of length 1191 revealed by PAUP from an analysis of the tef1 matrix of sect. Trichoderma, showing the phylogenetic position of H. caerulescens, H. hispanica and T. samuelsii (formatted in boldface). Thin branches represent nodes collapsing in the strict consensus tree of all MP trees. MP and ML BS above 60% and Bayesian PP above 90% are given respectively at first, second and third position, above or below the branches; GenBank accession or isolate numbers follow the taxon labels.
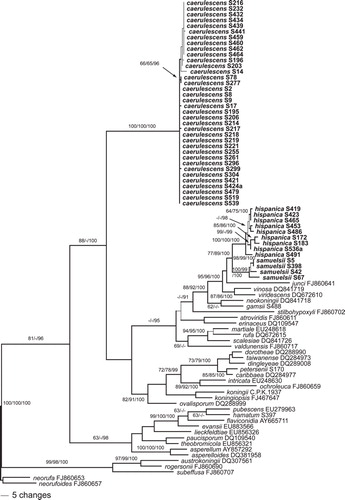
Fig. 2. Phylogram of one of two MP trees of length 847 revealed by PAUP from an analysis of the rpb2 matrix of sect. Trichoderma, showing the phylogenetic position of H. caerulescens, H. hispanica and T. samuelsii (formatted in boldface). Thin branches represent nodes collapsing in the strict consensus tree of the two MP trees. MP and ML BS above 60% and Bayesian PP above 90% are given respectively at first, second and third position, above or below the branches; GenBank accession or isolate numbers follow the taxon labels.
Stromata pulvinata, in vivo aurantiobrunnea, sicca fusce rubrobrunnea, tomentosa, 0.5–4 mm lata. Asci cylindrici, (72–)82–96(–102) × (5.0–)5.5–6.0(–6.5) μm. Ascosporae bicellulares, hyalinae, verruculosae, ad septum disarticulatae, pars distalis (sub)globosa, vel ellipsoidea, (4.0–)4.2–4.7(–5.0) × (3.0–)3.5–4.0(–4.5) μm, pars proxima oblonga vel ellipsoidea, (4.0–)4.8–5.7(–6.5) × (2.7–)3.0–3.7(–4.2) μm. Agarum CMD caerulescens in cultura ad 15 C. Conidiophora anamorphosis effuse disposita in agaris CMD at SNA, verticillio vel trichodermati similia. Phialides divergentes, anguste lageniformes, (4.5–)6.5–11(–16) × (2.0–)2.7–3.7 (–4.5) μm. Conidia ellipsoidea vel subglobosa, viridia, verruculosa, (3.0–)3.5–4.3(–5.5) × (2.7–)3.0–3.5(–3.8) μm.
Stromata when fresh 0.5–3(–4) mm, 0.5–1 mm thick, scattered or aggregated in small groups, first subeffuse, whitish to yellow, velutinous, later pulvinate with variable outline, pale orange, orange-brown or light brown. Surface becoming smooth and slightly tubercular; margin free, undulate. Ostiolar dots invisible, rarely appearing as diffuse minute dark spots. Spore deposits white. Stromata when dry (0.7–)1–2 mm diam, up to 4 mm long when young and subeffuse, 0.2–0.7 mm thick (n = 35), first appearing as white hairy tufts, turning yellow from the center, subeffuse or discoid when young, often wrinkled, with evanescent rust surface hairs and sometimes radiating white marginal mycelium; later flat pulvinate or discoid; outline variable, often angular or oblong; margin mostly free, sometimes lobed; surface smooth, uneven to tubercular; ostiolar dots invisible, only rarely visible under high magnification, 15–31(–55) μm diam, reddish to subhyaline. Stromata mostly dark reddish brown, or orange-brown, violet-brown to nearly black, 8–10F5–8, 8E5–8. Rehydrated stromata dark reddish or orange-brown, turning slowly dark red in 3% KOH.
Stroma anatomy: Cortical layer (17–)19–28(–35) μm thick (n = 30), orange-brown, paler and more yellow downward, comprising a dense t. angularis of indistinct, thin-to thick-walled cells (3.5–)4–8(–12) × (2.5–)3.0–6.0(–7.3) μm (n = 30) in section. Hairs, (7.5–)9–23(–37) × (2.5–)3.3–4.5(–5.0) μm (n = 30), scant on the surface, more frequent in lateral regions, cylindrical with narrowly rounded ends, 1–3-celled, light brown, thick-walled, warted. Subcortical tissue a t. intricata of hyaline, thin-walled hyphae (3–)4–6(–8) μm wide (n = 30), mixed with some angular cells. Subperithecial tissue a t. angularis-epidermoidea comprising hyaline, thick-walled cells (7.5–)9–21(–26) × (5–)6–13(–19) μm (n = 30). Stroma base mostly of parallel, often compressed, thick-walled yellow-brown hyphae (2–)3–5(–8) μm wide (n = 30). Perithecia (174–)195–235(–250) μm high, (127–)150–206(–230) μm wide (n = 30). Peridium (12–)15–21(–25) wide at the base, (10–)12–18(–20) μm at the sides (n = 30). Ostioles not projecting, (63–)70–84(–90) μm long, at the apex (19–)24–39(–47) μm wide inside, (40–) 45–60(–70) μm wide outside (n = 30), periphysate. Asci (72–)82–96(–102) × (5.0–)5.5–6.0(–6.5) μm; apex slightly thickened, with a minute ring; stipe (4–)5–11 (–15) μm long (n = 40). Ascospores hyaline, verruculose; cells dimorphic, distal cells (sub)globose or ellipsoid, (4.0–)4.2–4.7(–5.0) × (3.0–)3.5–4.0(–4.5) μm, l/w (1.0–)1.1–1.3(–1.5) (n = 95), proximal cells oblong or ellipsoid, (4.0–)4.8–5.7(–6.5) × (2.7–)3.0–3.7(–4.2) μm, l/w (1.2–)1.4–1.7(–2.1) (n = 95).
Anamorph on natural substrates: Colonies effuse or pulvinate, first white, turning light to dark green with or without white margin, often with bluish tone; from few millimeters to 9 cm long, granulose, farinose, hairy or fluffy, sometimes associated with yellow mycelium. Conidia and phialides, determined for the specimen of the holomorph, as given below under CMD cultures.
Cultures and anamorph: Optimal growth at 25 C on all media, slow and restricted growth at 30 C, no growth at 35 C. On CMD after 72 h 24–28 mm at 15 C, 30–41 mm at 25 C, 0.5–3.5 mm at 30 C; mycelium covering the plate after 5–6 d at 25 C. Colony hyaline, dense, circular or with ill-defined margin; mycelium not zonate, with conspicuous difference in width between primary and secondary hyphae, the latter short, thin and curly. Aerial hyphae only at the distal margin conspicuous and long, becoming fertile. Autolytic excretions absent, coilings inconspicuous. Chlamydospores detectable after 6–7 d, infrequent, (8–)9–13(–14) × (6–)8–12(–13) μm, l/w 0.9–1.3(–1.9) (n = 30). Within 2 wk no diffusing pigment detectable, but after 1–2 mo at 15 or 25 C a peculiar grayish blue pigment, 22–23B1–3, appearing in the agar in patches or on the entire plate. Often also mycelial spots or tufts or brown spots formed by pigmented hyphal segments. Coconut-like odor formed after more than 2 wk, rarely earlier (e.g. after 10 d). Conidiation appearing after 2 d, effuse, dry, short, in loose shrubs or on aerial hyphae, spreading over the entire plate, concentrated in one or several, broad, ill-defined, scarcely separated, loose, downy to floccose, later farinose zones including distal margin, with radial arrangement of shrubs within, after 4–6 d turning faintly greenish or dark bluish green 25–26EF4–6. Conidiation structure (four isolates, after 6–16 d on CMD and SNA at 25 C): Stipe or aerial hypha and primary branches of shrubs thick-walled, to 6–7 μm wide (with some thickenings and globose branching points to 8 μm), asymmetrically terminating in long and often broad, loosely branched, regularly tree-like conidiophores. Conidiophores verticillium-like with little rebranching, comprising loosely arranged side branches or phialides mostly in whorls of 2–4(–5), or singly, around a distinct main axis. Side branches 1.5–4 μm wide, consisting of 1–3 long cells, with some 1–2-celled terminal side branches up to ca. 130 μm long; side branches usually perpendicular to the main axis or aerial hypha, terminal branches also in steep angles upward. Thickenings inconspicuous, except for phialide origins that are slightly thickened terminally (e.g. 3.5 μm vs. 2.5 μm of the residual cell). Phialides solitary or in whorls of 2–4, (4.5–)6.2–10.8(–16) × (2.0–)2.7–3.7(–4.5) μm, l/w (1.2–)1.6–3.9(–7.1), (1.2–) 1.5–2.3(–3.2) μm wide at the base (n = 145), narrowly lageniform, less commonly ampulliform and then often with long neck, straight or curved or sometimes sigmoid, non-repetitive. Conidia (3.0–)3.7–4.5(–5.5) × (2.7–)3.0–3.5(–3.8) μm, l/w (1.0–)1.1–1.4(–1.9) (n = 120), ellipsoid, oval or subglobose, green, distinctly warted when young; warts less conspicuous when mature or old (e.g. after 12 d), scar inconspicuous.
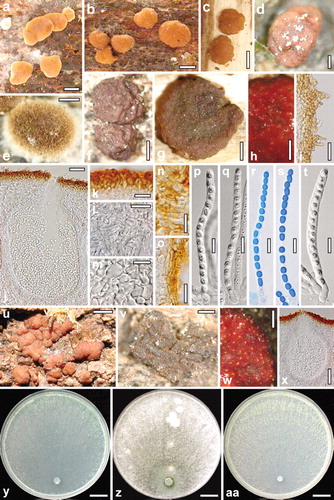
Fig. 3. a–s. Teleomorph of Hypocrea caerulescens. a–c. Fresh stromata. d–g. Dry stromata (d. with spore deposits on the surface; e. immature). h. Rehydrated stroma part in 3% KOH. i. Stroma surface with hairs. j. Perithecium in section. k. Cortex in section. l. Subcortical tissue in section. m. Subperithecial tissue in section. n, o. Stroma base in section (n. substrate-attached; o. free). p–s. Asci (r, s. in cotton blue/lactic acid). t–x. Teleomorph of Hypocrea hispanica. t. Ascus. u. Fresh stromata. v. Dry stroma. w. Rehydrated stroma part in 3% KOH. x. Perithecium in section. y–aa. Cultures of Hypocrea caerulescens after 10 d at 25 C (y. on CMD; z. on PDA; aa. on SNA). a–c, g–p, r. WU 31600. d. WU 31601. e, f, q, s. WU 31602. t–x. WU 31606. y, z. S8. aa. S252. Bars: a–c = 1 mm. d, e, w = 0.2 mm. f–h = 0.5 mm. i, l, m = 20 μm. j = 30 μm. k, n, o = 15 μm. p–t = 10 μm. u = 1.5 mm. v = 0.3 mm. x = 60 μm. y–aa = 15 mm.
On PDA after 72 h 19–24 mm at 15 C, 27–36 mm at 25 C, 0.5–2 mm at 30 C; mycelium covering the plate after 6–7 d at 25 C. Colony circular, dense. Aerial hyphae abundant, forming strands and a whitish hairy or floccose mat; plug and surroundings turning green. Autolytic excretions and coilings inconspicuous. Conidiation appearing after 1–2 d, remaining colorless or turning green after 3–5 d, effuse, inconspicuous, with low conidial yield, often only around the plug. Sometimes grayish bluish patches appearing from the center, also at 15 C. Odor indistinct.
On SNA after 72 h 17–22 mm at 15 C, 24–30 mm at 25 C, 0.7–3.5 mm at 30 C; mycelium covering the plate after 6–7 d at 25 C. Colony hyaline, dense, more or less circular, of hyphae as on CMD, colony not zoned. Aerial hyphae inconspicuous, common and long toward margin, becoming fertile. Autolytic excretions absent or rare; coilings frequent; Chlamydospores detected after 5–7 d, uncommon but more frequent than on CMD. No pigment produced; odor indistinct. Conidiation appearing after 2 d, abundant, effuse, spreading from the center over the entire colony, becoming concentrated in up to 5 indistinctly separated concentric zones, after 3–4 d successively turning green 26–27DE3–6 from the center outward, farinose and with radial arrangement within each zone; delicate, in loose but densely disposed shrubs and on aerial hyphae; conidia formed in mostly dry heads less than 20 μm diam; more abundant and faster than on CMD.
Distribution: Southern Europe (Croatia, Italy, Portugal (Madeira), Spain, including the Canaries).
Habitat: On wood and bark of various shrubs and trees and on fungi growing on them.
Holotype: SPAIN, CANARIAS, LA PALMA, Montaña Tagoja, 28°43′18″N, 17°47′07″W, 1040 m, on medium decayed branches 1–5 cm thick of Erica arborea, on wood, holomorph, soc. H. cf. minutispora, 14 Dec 2009, W. Jaklitsch (WU 31600; culture S195 = CBS 130011).
Other material examined (generally on branches or twigs, except where noted): Teleo-, holomorphs: ITALY, APULIA, Andria, Parco Nazionale dell’Alta Murgia, Castel del Monte, between SP234 and Masseria Savignano, 41°02′33″N, 16°18′24″E, 380 m, on medium to well decayed Olea europaea twigs, 0.5–1 cm diam, lying in moist grass and moss, on wood and a Hymenochaete sp. soc. effete pyrenomycete in wood, a Hysteriaceae, a coelomycete, Mollisia sp. and ample anamorph, 19 Nov 2009, W. Jaklitsch & H. Voglmayr (WU 31599; culture S71). Foggia, Gargano, NE from Mattinata, 41°44′48″N, 16°08′00″E, 80 m, on medium to well decayed twigs, 1–5 cm thick, of Olea europaea, on wood, teleomorph immature, 20 Nov 2009, H. Voglmayr & W. Jaklitsch (culture S76). PORTUGAL, MADEIRA, Portela, PR5, close to its start, 32°44′48″N, 16°49′16″W, 560 m, on twigs 0.7–2 cm thick of Clethra arborea, on medium decayed wood, soc. Biscogniauxia cf. capnodes, Stictis sp., white mold and algae, 21 Feb 2010, W. Jaklitsch & O. Sükösd (WU 31602; culture S206 = CBS 130012). Ribeiro Frio, Levada to Portela, 32°44′11″N, 16°53′11″W, 880 m, on well decayed, decorticated branches, 3–4 cm thick, of Persea indica, on bark, 16 Feb 2010, W. Jaklitsch & O. Sükösd (WU 31601; culture S196). SPAIN, CANARIAS, LA PALMA, Cumbre Nueva, 28°38′27″N, 17°49′50″W, 1040 m, on Erica arborea, 2 Dec 2010, W. Jaklitsch (WU 31603; culture S439); branching off LP-301 at Area Recreativa del Pilár, 28°37′24″N, 17°49′535″W, 1460 m, on branch, 4 cm thick, of Myrica faya, teleomorph scant and immature, 11 Dec 2009, W. Jaklitsch (culture S173); Mazo, LP2062, Pista Cabrita, 28°35′48″N, 17°48′04″W, 950 m, on Erica arborea, 5 Dec 2010, W. Jaklitsch (WU 31605; culture S460); El Paso, Castanea plantation at LP 301, close to crossing with LP 3, on Chamaecytisus proliferus, 2 Dec 2010, W. Jaklitsch (WU 31604; culture S441).
Anamorphs: CROATIA, ISTRIA, forest N of Barbariga, ca. 20 m, on Quercus ilex, 14 May 2010, W. Jaklitsch & H. Voglmayr (cultures S259, S261). Fažana, forest at Valbandon, on Phoenix canariensis, 26 Sep 2010, H. Voglmayr & W. Jaklitsch (culture S277); same place, on Cornus mas, soc. Stictis sp., 17 Oct 2010, W. Jaklitsch (culture S304); close to St Golaš, on Diatrype stigma/Quercus pubescens, 26 Sep 2010, W. Jaklitsch (culture S285). Vrsar, beach forest at Petalon Resort, 20 m, on Ulmus minor, Phillyrea angustifolia and Quercus ilex, 13 May 2010, H. Voglmayr & W. Jaklitsch (cultures S252–S255). Lošinj, hiking trail from Veli Lošinj to Javorna Uvala, on Laurus nobilis, 16 Oct 2010, W. Jaklitsch (culture S296); Jamna Uvala, on Arbutus unedo, 16 Oct 2010, W. Jaklitsch (culture S299). ITALY, APULIA, Foggia, Gargano, NE from Mattinata, 41°44′49″N, 16°07′51″E, 120 m, on 1 cm thick, medium decayed twigs of Phillyrea latifolia, on bark and wood, 20 Nov 2009, W. Jaklitsch & H. Voglmayr (culture S78). CALABRIA, Cosenza, Parco Nazionale del Pollino, above Morano Calabro, 39°50′48″N, 16°06′47″E, 650 m, on Ostrya carpinifolia, 3 cm diam, on wood, soc. Echinosphaeria aff. canescens, 18 Nov 2009, H. Voglmayr & W. Jaklitsch (culture S57). Lazio, close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before reaching the brook, to ½ m up, on corticated, standing branch, 2–4 cm thick, of Quercus virgiliana, soc. Peniophora sp., Vuilleminia, Steccherinum ochraceum, 25 Nov 2009, H. Voglmayr & W. Jaklitsch (culture S126). SARDINIA, roadside of SP 38 at junction to Isalle Orrule, 40°21′49″N, 09°31′00″E, 230 m, on Cistus monspeliensis, on bark, soc. old pyrenomycete, 2 Nov 2009, W. Jaklitsch (culture S2); at Su Gologone, 40°17′23″N, 09°29′38″E, 215 m, on Alnus glutinosa, 3 Nov 2009, W. Jaklitsch (culture S8) and 40°17′19″N, 09°29′36″E, 230 m, on Pistacia lentiscus, soc. Xylaria sp., 3 Nov 2009, W. Jaklitsch (culture S9); S of Oliena along SP22 to Orgosolo, 40°14′24″N, 09°23′59″E, 600 m, on Stereum hirsutum/Quercus ilex, 4 Nov 2009, W. Jaklitsch (culture S14); N of Sarule, 40°14′21″N, 09°09′57″E, 650 m, on Quercus virgiliana, on bark, 5 Nov 2009, W. Jaklitsch (culture S17). PORTUGAL, MADEIRA, Funchal, Botanical Garden, on ?Callistemon sp., 19 Feb 2010, W. Jaklitsch & O. Sükösd (culture S203). Portela, route PR5, close to its start, on ?Acacia melanoxylon, soc. Mollisia sp., 21 Feb 2010, W. Jaklitsch & O. Sükösd (culture S205). SPAIN, BALEARES, MALLORCA, Es Capdella, along Torrent de Galatzó, on a Diatrypaceae, 21 Nov 2010, W. Jaklitsch (culture S421); same place, same date, on Calicotome spinosa, soc. Hypocrea sp., W. Jaklitsch (culture S424a). CANARIAS, LA GOMERA, TF713, shortly after junction to Hermigua, on Myrica faya, 9 Dec 2010, W. Jaklitsch (culture S464). La Palma, El Paso, Castanea plantation at LP 301, close to crossing with LP 3, on Castanea sativa, 2 Dec 2010, W. Jaklitsch (culture S432); same place, same date, on Erica arborea, W. Jaklitsch (culture S434). Mazo, LP2062, beginning of the walking path Camino La Banda, on Erica arborea, 5 Dec 2010, W. Jaklitsch (culture S459). Montaña Tagoja, on Erica arborea, 7 Dec 2010, W. Jaklitsch (culture S462). TENERIFE, Macizo de Anaga, Pico del Ingles, on Erica platycodon, 11 Apr 2010, W. Jaklitsch (culture S214); El Pijaral, Chinobre, on moss on Erica platycodon, 15 Apr 2010, W. Jaklitsch (culture S232); Montaña Chamuscada, on Laurus novocanariensis, 16 Dec 2010, W. Jaklitsch (culture S479); at the road to Pedro Àlvarez shortly before the crossing Los Batanes, on stromata of a Nemania sp. on Persea indica, 15 Apr 2010, W. Jaklitsch (culture S237). Orotava, above Aguamansa/La Orotava, path at Mirador de Mataznos, on a Corticiaceae on Myrica faya, 12 Apr 2010, W. Jaklitsch (culture S221); Pista de Benijos, on a corticiaceous fungus on Myrica faya, 12 Apr 2010, W. Jaklitsch (culture S218); on Erica arborea, W. Jaklitsch (culture S219); slightly above Pista de Benijos/camping place La Caldera, on resupinate polypore on Eucalyptus globulus, 16 Apr 2010, W. Jaklitsch (culture S245). San Juan de la Rambla, Barranco Rambla de Ruíz, on Erica arborea, 12 Apr 2010, W. Jaklitsch (cultures S216, S217).
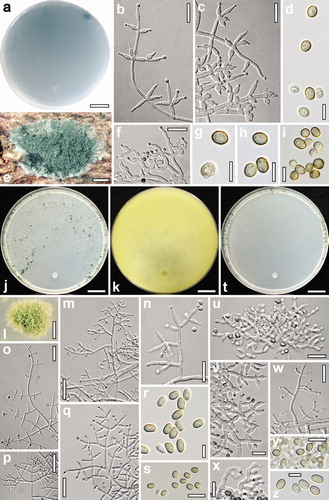
Fig. 4. a–i. Hypocrea caerulescens. a. Reverse of CMD culture after 64 d at 15 C. b, c, f. Conidiophores and phialides (b, c. from SNA after 7 d; b. at 15 C, c. at 25 C; f. from CMD after 16 d at 25 C). e. Anamorph on natural substrate. d, g–i. Conidia from SNA (d, g. after 6–7 d at 15 C; h, i. after 7–12 d at 25 C). j–s. Trichoderma samuelsii. Cultures and anamorph at 25 C on SNA except k. j, k. Cultures ( j. after 10 d; k. on PDA, yellow reverse after 7 d). l. Conidiation pustule (after 7 d). m–q. Conidiophores and phialides (m, o. after 4 d; n, p, q. after 5–7 d). r, s. Conidia after 4–6 d). t–z. Hypocrea hispanica. Cultures and anamorph on SNA at 25 C. t. Culture after 9 d. u–x. Conidiophores and phialides after 6 d (x. repetitive curved phialides). y, z. Conidia after 6 d. a, b, f. S8. c, g, h. S206. d, i. S252. e. S439. j–m, o, r. S5. n, p, q. S398. s. S67. t. S172. u, x, y. S450. v. S486. w, z. S423. Bars: a, j, k, t = 15 mm. b, c, f, n, u = 15 μm. d, g–i, r, z = 5 μm. e, l = 0.5 mm. m, o, p = 30 μm. q, w = 20 μm. s, v, x, y = 10 μm.
Notes: Hypocrea caerulescens is a common species; 50 specimens were collected within 17 mo. Most frequently this species is found as anamorph or in association with immature stromata (e.g. S71, S76) in the Mediterranean region. It is unknown whether the species also occurs farther north because no anamorphs have been collected earlier. CitationBlaszczyk et al. (2011) did not report this species from Poland.
The most conspicuous trait of Hypocrea caerulescens is the grayish blue discoloration of CMD agar upon storage 1–2 mo at 15 C. This has been observed in 46 of 50 isolates of this species and in none of any other species of the genus. Hypocrea caerulescens shares verruculose conidia with H. viridescens but differs from that species by more delicate and effuse conidiation, in non-repetitive phialides, a retarded formation of the coconut-like odor, poor growth at 30 C on all media, and particularly in the peculiar grayish blue pigment on CMD. Young conidia of H. caerulescens sometimes show wing-like projections as known from Trichoderma saturnisporum.
Hypocrea hispanica Jaklitsch & Voglmayr sp. nov. ,
MycoBank MB563316
Etymology: for its currently exclusive occurrence in Spain including the Canaries.
Stromata subeffusa vel pulvinata, fusce brunnea, tomentosa, 0.5–3 mm lata. Asci cylindrici, (72–)75–87(–96) × (5.5–)5.8–6.8(–7.2) μm. Ascosporae bicellulares, hyalinae, verruculosae, ad septum disarticulatae, pars distalis (sub)globosa, (3.8–)4.0–4.7(–5.0) × (3.5–)3.8–4.2(–4.5) μm, pars proxima subglobosa vel oblonga, (3.7–)4.2–5.3(–6.0) × (3.0–)3.3–3.8(–4.0) μm. Conidiophora in pustulis disposita in agaro CMD et SNA. Phialides divergentes, lageniformes, (4.5–)6–11(–17) × (2.0–) 2.5–3.2(–4.0) μm, interdum repetitae, saepe arcuatae. Conidia ellipsoidea vel oblonga, viridia, glabra, (3.5–)4.3–5.0(–6.0) × (2.8–) 3.0–3.5(–4.0) μm.
Stromata solitary, gregarious or densely aggregated into amorphous masses of ca. 6 mm diam, when fresh ca. 0.5–3 mm diam, subeffuse or flat pulvinate with irregular outline, velutinous, orange-brown. Stromata when dry (0.5–)1–2.4(–2.9) × (0.5–)0.8–1.7(–2.1) mm, (0.2–)0.3–0.6(–0.9) mm thick (n = 17), 20–25% larger after rehydration, pulvinate or forming thin crusts with stromata of irregular shapes; outline round, angular, oblong or irregular; margin free, often for a large part, sterile sides often covered by rust mycelium; surface tubercular, stroma tubercular, projecting ostioles involving overlying cortex; ostiolar openings hyaline, only visible after rehydration under strong magnification; dark brown or violaceous brown, turning dark red in 3% KOH, clothed with yellow-brown to rust hairs. Spore deposits white.
Stroma anatomy: Cortical layer (14–)17–30(–35) μm thick (n = 30), reddish brown, around entire stroma where unattached, comprising a t. angularis of indistinct small cells (2.5–)3.5–7.5(–9.0) × (2.0–)2.7–4.5(–5.5) μm in section (n = 30), lighter and more thin-walled downward. Hairs on stroma surface (8–)10–18(–22) × (2.5–)3.0–4.5(–5.7) μm (n = 30), numerous, cylindrical with rounded ends, smooth or warted, yellow-or light brown, narrow, thick-walled, sometimes basally widened. Subcortical tissue a hyaline, dense t. intricata of thin-walled hyphae (2.5–)3–5(–6) μm wide (n = 30). Subperithecial tissue a hyaline t. angularis-epidermoidea of thin-walled cells, (6–)8–20(–29) × (4–)6.5–12 (–15) μm (n = 30). Stroma base of thick-walled cells and (2.2–)2.5–5.5(–8.5) μm wide (n = 30), reddish brown hyphae; attached areas comprising a hyaline cellular tissue deeply emerging from bark. Perithecia ellipsoid to globose, (190–)210–250(–265) × (104–)120–192 (–246) μm (n = 20); peridium (14–)16–23(–29) μm wide at the base (n = 20), (9–)12–21(–24) μm at sides (n = 20), sometimes slightly yellowish at the base. Ostioles cylindrical, (59–)76–108(–120) μm long, projecting to ca. 40 μm including cortex, (13–)16–27(–35) μm wide at the apex inside. Asci (72–)75–87(–96) × (5.5–)5.8–6.8(–7.2) μm, stipe (6–)8–15(–17) μm long (n = 20). Ascospores hyaline, verruculose, cells dimorphic, distal cells globose or subglobose, (3.8–)4.0–4.7(–5.0) × (3.5–)3.8–4.2(–4.5) μm, l/w 1.0–1.2(–1.3) (n = 30), proximal cells oblong, subglobose or wedge-shaped, (3.7–)4.2–5.3(–6.0) × (3.0–)3.3–3.8(–4.0) μm, l/w (1.1–)1.2–1.5(–1.7) (n = 30), often subglobose and often smaller than the distal cell.
Cultures and anamorph: Optimal growth at 25 C on all media, no growth at 35 C. On CMD after 72 h 27–28 mm at 15 C, 50–51 mm at 25 C, 23–24 mm at 30 C; mycelium covering the plate after 4–5 d at 25 C. Colony hyaline, dense, not zonate, without distinct radial arrangement, margin ill defined. Hyphae with conspicuous difference in width, marginal primary surface hyphae wide, surface hyphae soon degenerating, hyper branching causing small white spots within the agar particularly in a broad marginal zone. Aerial hyphae inconspicuous. Autolytic excretions and coilings absent or infrequent. Agar within 3 wk turning at most slightly yellowish but after storage at 15 C for 6 mo or longer agar distinctly yellow (except S423). Odor strongly coconut-like. Chlamydospores infrequent, terminal. Conidiation after 6–7 d in loosely disposed, small shrubs and pustules on the entire surface, concentrated and larger, 1–3 mm diam, along the plate margin, turning first yellow then dark green after 7–9 d; phialides sometimes repetitive.
On PDA after 72 h 20–22 mm at 15 C, 38–42 mm at 25 C, 20–22 mm at 30 C; mycelium covering the plate after 5–6 d at 25 C. Colony circular, dense. Aerial hyphae abundant, forming a thick white floccose mat except for narrow plug surroundings, which turn yellowish. Autolytic excretions abundant, large; coilings inconspicuous. Odor unpleasant, with a coconut-like component. Conidiation starting after 2–3 d, scant, mostly in the center, also spreading on aerial hyphae, not turning green.
On SNA after 72 h 19–20 mm at 15 C, 34–37 mm at 25 C, 16–18 mm at 30 C; mycelium covering the plate after 6 d at 25 C. Colony hyaline, dense, margin ill defined, secondary hyphae fine, short, curly; surface hyphae scarcely degenerating. Aerial hyphae inconspicuous, more frequent and long at the distal margin. Autolytic excretions absent, coilings abundant. Chlamydospores infrequent. Conidiation starting after 3–5 d, short effuse and predominantly in small pustules 1–2 mm diam formed mostly at the lateral and distal plate margin, becoming densely aggregated or confluent, turning dark green 27–28F3–8 after 6–7 d. Pustules compact, velutinous, pulvinate to globose, with a smooth surface; consisting of a stipe ca. 6–8(–11) μm wide with irregularly or radially emergent primary branches up to 6.5 μm wide with often constricted septa, forming a reticulum. Main axes of conidiophores mostly embedded within the reticulum, difficult to separate, mostly short, resulting conidiophores therefore variable, irregular or tree-like, loosely or densely branched, often broad and with conspicuous curvatures particularly in terminal branches and phialides; branches mostly unpaired, often in right angles or inclined upward, 2–5 μm wide, thickening to 6.5 μm. Phialides arising singly or in whorls of 2–4, sometimes repetitive (i.e. supporting cell of similar shape). Phialides (4.5–)6–11(–17) × (2.0–)2.5–3.2(–4.0) μm, l/w (1.4–)2–4.1 (–7.2), (1.1–)1.5–2.2(–2.7) μm wide at the base (n = 160), lageniform or ampulliform, inequilateral when long and narrow, often distinctly curved. Conidia (3.5–) 4.3–5.0(–6.0) × (2.8–)3.0–3.5(–4.0) μm, l/w (1.1–)1.3–1.5(–2.0) (n = 200), green, smooth, oblong or mostly ellipsoid, thick-walled.
Distribution: Spain, including the Balearic and Canary Islands.
Habitat: On twigs and branches of shrubs and trees and stalks of herbaceous plants.
Holotype: SPAIN, CANARIAS, LA PALMA, Garafía, at LP1 close to the junction to El Tablado, 28°47′48″N, 17°53′40″W, 1110 m, on branch, 6 cm thick, of Chamaecytisus proliferus, stromata on bark and an effuse Diatrypaceae, holomorph, 4 Dec 2010, W. Jaklitsch (WU 31606; culture S453 = CBS 130540).
Other material examined: SPAIN, ANDALUCIA, Alcalá de los Gazules, Via de Servicio south of the exit at km 54 off the A7 (A381), 36°22′09″N, 5°38′41″E, 75 m, on Olea europaea subsp. sylvestris, 17 Mar 2011, W. Jaklitsch & H. Voglmayr (S491). El Colmenar, roadside of MA9300 at km 8, 36°31′39″N, 5°22′39″E, 330 m, on branches of Calicotome spinosa, soc. Hypocrea sp., 22 Mar 2011, H. Voglmayr & W. Jaklitsch (S536a). Jerez de la Frontera, pasture near the hotel La Cueva Park, 36°42′43″N, 6°01′23″E, 70 m, on Cynara sp., 16 Mar 2011, W. Jaklitsch & H. Voglmayr (S486). CANARIAS, LA PALMA, Cumbre Nueva, branching off LP-301 at Area Recreativa del Pilar, 28°37′24″N, 17°49′535″W, 1460 m, on Cistus symphytifolius, 11 Dec 2009, W. Jaklitsch & R.M. Dähncke (culture S172 = CBS 130538); Pista Cabrito, 28°35′47″N, 17°49′02″W, 1355 m, on Cistus symphytifolius, 13 Dec 2009, W. Jaklitsch & R.M. Dähncke (S183); Garafía, at LP1 close to the junction to El Tablado, 28°47′48″N, 17°53′39″W, 1110 m, on Chamaecytisus proliferus, 4 Dec 2010, W. Jaklitsch (S450). TENERIFE, Teno Alto, 28°20′28″N, 16°52′265″W, 775 m, on Teline canariensis, 10 Dec 2010, W. Jaklitsch (S465). ISLAS BALEARES, MALLORCA, W Santa Eugenia at Ma-3011, 39°36′00″N, 2°49′00″W, 115 m, on Pistacia lentiscus, 21 Nov 2010, W. Jaklitsch (S419); Es Capdella, along Torrent de Galatzó, 39°35′03″N, 2°29′01″W, 115 m, on Ceratonia siliqua, 21 Nov 2010, W. Jaklitsch (S423).
Notes: Hypocrea hispanica phylogenetically is closely related to H. junci and Trichoderma samuelsii. Hypocrea junci so far is known only from Juncus in Denmark and differs from H. hispanica also by its long, straight, radial conidiophores, larger conidiation pustules formed in a broad marginal zone on CMD and SNA, lack of a coconut-like odor on CMD, and by slower growth particularly on CMD and at 30 C on all media. Also in comparison with T. samuelsii, the anamorph of H. hispanica tends to have shorter conidiophores often with conspicuous curvatures in terminal branches and phialides and the conidia tend to be more ellipsoid. On SNA T. samuelsii grows faster and on CMD it sporulates much earlier (after 2 d vs. 6–7 d), on SNA in a conspicuous distal down and in pustules that are widely disposed on the agar surface, which is consistent in all examined strains. In H. hispanica pustules on SNA are generally formed only at the lateral and distal margin of the Petri dish. However the only ascospore isolate S453 deviates from all conidial isolates in the formation of some pustules within the colony, coinciding with a slightly different rpb2 sequence. Conidiation pustules are more compact than in T. samuelsii, with fewer projecting elements. Hypocrea hispanica produces a yellow pigment on PDA only after storage for several months at 15 C, while in T. samuelsii the reverse of PDA cultures already turns distinctly yellow within a week at 25 C.
Trichoderma samuelsii Jaklitsch & Voglmayr sp. nov.
MycoBank MB563317
Etymology: In honor of Gary J. Samuels for his excellent and comprehensive work on Hypocrea/Trichoderma, particularly on the section Trichoderma.
Conidiophora effusa et in pustulis disposita in agaris CMD et SNA, verticillio vel trichodermati similia. Phialides divergentes, lageniformes, (5–)7–11(–16) × (2.2–)2.5–3.0 (–3.5) μm. Conidia oblonga, interdum ellipsoidea, viridia, glabra, (3.7–)4.2–5.0(–5.7) × (2.4–)2.7–3.2(–3.7) μm.
Cultures and anamorph: Optimal growth at 25 C on all media, no growth at 35 C. On CMD after 72 h 25–27 mm at 15 C, 51–52 mm at 25 C, 44–48 mm at 30 C; mycelium covering the plate after 4 d at 25 C. Colony hyaline, dense, radial, not zonate; hyphae with conspicuous difference in width, marginal primary surface hyphae wide, surface hyphae soon degenerating from the center. Broad marginal zone becoming downy to floccose by numerous long aerial hyphae. Autolytic excretions and coilings nearly absent. Chlamydospores infrequent, terminal and intercalary. Diffusing pigment absent, odor coconut-like. After longer storage (e.g. 1 y) at 15 C on CMD agar evenly dull yellow. Conidiation starting after 2 d, first effuse in numerous delicate shrubs and on aerial hyphae in the broad distal zone, later also in some pustules to ca. 1.5 mm diam mostly laterally and at the distal margin of the same zone or pustules not formed, after 5–6 d turning bluish green, 25EF3–6, 26E3–5.
On PDA after 72 h 20–23 mm at 15 C, 50–51 mm at 25 C, 35–40 mm at 30 C; mycelium covering the plate after 4 d at 25 C. Colony circular, dense. Aerial hyphae abundant, forming a thick whitish mat except for the flat center, the latter turning light yellow to green. Autolytic excretions abundant and large, coilings absent. Reverse turning yellow, 3–4A3–5. Odor distinctly unpleasant, chemical, more or less pyridine-like, as in T. bavaricum, pronounced at 30 C. Conidiation starting after 2 d, effuse on flat delicate shrubs in the granulose center, turning greenish after 5–6 d; also slightly spreading on aerial hyphae.
On SNA after 72 h 21–22 mm at 15 C, 49–51 mm at 25 C, 34–39 mm at 30 C; mycelium covering the plate after 4–5 d at 25 C. Colony hyaline, circular, dense; marginal primary surface hyphae conspicuously wide, surface hyphae within the colony degenerating. Aerial hyphae abundant in a major distal part of the colony excluding the plug area or a broad marginal zone or several ill-separated zones becoming hairy or floccose after few days, turning greenish. Autolytic activity moderate, coilings frequent. Chlamydospores noticeable after 5–6 d, infrequent, terminal and intercalary. Diffusing pigment and distinct odor absent. Conidiation more abundant than on CMD, starting after 2 d, first effuse, mostly on aerial hyphae, later (after 5–6 d) in pustules ca. 0.5–2 mm diam first formed from proximal ends of a floccose central concentric zone, eventually in several ill separated zones, turning dark green 25–26EF3–6, 27F4–8, partly confluent to several mm. Pustule periphery long remaining white, surface loose, hairy, with projecting conidiophores. Conidiophores with the same structure on aerial hyphae, in shrubs and pustules, originating on 4–5 μm wide hyphae, loose, long and straight, regularly tree-like but often also broad due to long upper branches, typically comprising a distinct main axis and mostly unpaired side branches loosely arranged in right angles or inclined upward that give rise to phialides or short terminal branches; all branches narrow, 1.5–3.5 μm wide. Phialides non-repetitive, solitary or in whorls of 2–4, (5–)7–11(–16) × (2.2–)2.5–3.0(–3.5) μm, l/w (1.7–)2.5–4.6(–7.1), (1.2–)1.5–2.2(–2.7) μm wide at the base (n = 115), lageniform, straight, thickening median or above the middle, neck short, often inequilateral, infrequently curved. Conidia (3.7–) 4.2–5.0(–5.7) × (2.4–)2.7–3.2(–3.7) μm, l/w (1.2–)1.4–1.7(–2.1) (n = 130), green, typically oblong with distinctly parallel sides, but with a fraction of ellipsoid ones varying among strains, smooth, sometimes pinched at sides, with two or more small guttules; abscission scar indistinct.
Distribution: Southern Europe (Italy, Spain).
Habitat: On twigs and branches of shrubs and deciduous trees and on fungi growing on them.
Holotype: ITALY, SARDINIA, roadside of SP 38 at junction to Isalle Orrule, 40°21′49″N, 9°31′00″E, 190 m, on Hymenochaete sp./Cistus monspeliensis, 2 Nov 2009, W. Jaklitsch (dry culture WU 31607; living culture S5 = CBS 130537).
Other material examined: ITALY, APULIA, Andria, Parco Nazionale dell’Alta Murgia, Castel del Monte, between SP234 and Masseria Savignano, 41°02′34″ N, 16°18′02″ E, 510 m, on Crataegus laciniata, 19 Nov 2009, W. Jaklitsch & H. Voglmayr (S67). CAMPANIA, Roscigno, 40°24′26″N, 15°21′51″E, 745 m, on Quercus pubescens, 16 Nov 2009, H. Voglmayr & W. Jaklitsch (S42). SPAIN, MALLORCA, east of Calviá, Ma-1016 roadside, 39°34′54″N, 2°32′37″E, 190 m, on Stereum sp./Quercus ilex, 17 Nov 2010, W. Jaklitsch (S398).
Notes: Trichoderma samuelsii is a fungus so far known from four gatherings. This species is closely related phylogenetically to Hypocrea junci and H. hispanica. Morphological and other differences from the latter see under that species. Conidial morphology, conidiophores and cultures resemble that of H. junci, but that species so far has only been recorded from Juncus in Denmark, differs from T. samuelsii by lack of a coconut-like odor on CMD and by slower growth particularly on CMD and at 30 C. In the latter the distinctly oblong conidia are conspicuous, although this varies considerably among the studied strains. T. koningii has also oblong conidia of similar size (see CitationJaklitsch 2011, CitationSamuels et al. 2006a). Characteristic and so far unique in sect. Trichoderma is the distinctly unpleasant odor produced in PDA cultures, which is much reminiscent of PDA cultures of H. bavarica and H. leucopus.
Discussion
The taxonomy of the section Trichoderma was treated in detail by CitationSamuels et al. (2006a), CitationJaklitsch et al. (2006) and CitationJaklitsch (2011), and some species were added (CitationHanada et al. 2008; CitationSamuels and Ismaiel 2009; CitationSamuels et al. 2006b, Citation2010), but this phylogenetically complex and morphologically highly conserved section is still far from monographed.
Including the three newly described species, the section Trichoderma comprises 43 species, for which some DNA data are available. ITS sequences are identical or nearly identical for several species of the section (e.g. those of H. hispanica, H. koningii, H. viridescens and T. samuelsii), therefore this marker is not useful for phylogenetic reconstruction or for bar-coding (CitationDruzhinina et al. 2005, www.isth.info). CitationSamuels et al. (2006a), using tef1 introns, cal1 and act, included up to 20 named and some unnamed clades in their phylogenetic analyses. CitationJaklitsch et al. (2006) used tef1 introns to reconstruct the phylogeny of 29 named and five unnamed species, and CitationJaklitsch (2009, Citation2011), using rpb2 plus tef1 exon, included 27 species. tef1 introns have been shown in most analyses to permit a resolution that is superior to other phylogenetic markers. They are available for all species of the section, although they are short or of low quality in a few species. On the other hand rpb2 sequences are lacking for six species while cal, act and tef1 exon are unavailable for many species. In the current analysis 37 (rpb2 analysis) or 39 (tef1 analysis) species are included. The resolution of the tef1 tree has been considerably improved since earlier analyses, but backbone support is still not optimal for all nodes. The three new species are statistically highly supported in both phylogenetic analyses. However it has not been possible to determine the evolutionary (sister clade) relationships of H. caerulescens because the tef1 tree failed to show whether the species is closer related to the Koningii (containing H. koningii) or the Viride (containing H. rufa, anamorph T. viride) clade, which together with the comparatively long branches in the phylograms indicates an isolated position within sect. Trichoderma. This is also supported by distinct ITS sequences of H. caerulescens. On the other hand both analyses show that H. hispanica and T. samuelsii belong to the Viride clade.
Recognition based on phenotype is seriously impaired in Hypocrea hispanica and Trichoderma samuelsii, where tef1 sequences have proven to be basically the only reliable source of species recognition. Particularly notable is the distinctly unpleasant H. bavarica-like odor produced in PDA cultures of T. samuelsii, which is new to the sect. Trichoderma. Some other useful differences in anamorphs and cultures are given under the notes of the respective species. However conidiophore and conidial morphology, particularly in T. samuelsii, are much like those of T. koningii and some of its relatives (see CitationSamuels et al. 2006a), although these species are phylogenetically not closely related with that group.
The most remarkable of the three new species described above, H. caerulescens, is extremely common in southern Europe, but despite several studies (CitationMigheli et al. 2008, CitationZachow et al. 2008) it has not yet been reported. Morphological differentiation and recognition based on stromata has its limits because the light orange-brown fresh stromata drying dark reddish brown are shared with other species, particularly H. viridescens, which is even more common in the same habitats and regions, but also other species (e.g. H. hispanica or H. petersenii) may be similar. Most diagnostic of H. caerulescens is the blue pigment formed on CMD, but this occurs late, often only after storage for a month. This may be the reason why the species has not received its due attention. Another diagnostic set of traits is the combination of effuse conidiation, warted conidia, non-repetitive phialides on delicate, narrowly verticillate conidiophores and late emergence of a coconut-like odor.
The specific blue pigments of H. caerulescens were not identified in this study. It could be feasible to isolate the yellowish pigments that were extracted from the mycelia under alkaline conditions, elucidate their chemical structures and then study the compounds in the presence of metal ions.
Fungal pigment chemistry certainly should not be considered trivial. For instance the intense but highly polar and extremely unstable dark violet pigments of Cortinarius violaceus turned out to be conjugates of ferric ions with beta dopamine, following several decades of intensive work aimed at their identification (Citationvon Nussbaum et al. 1998), and many other pigments of these organisms still havel not been identified. The few true blue fungal pigments known to science include scleroderris blue from Gremmeniella abietina (CitationAyer et al. 1986, CitationAyer and Pedras 1987), asperopterin A from Aspergillus oryzae (CitationKaneko and Sanada 1969) and 1-(3,8-Dimethyl-azulen-5-yl)-ethanone from sporo-carps of Entoloma hochstetteri (CitationGill 1999). Several additional species of Entoloma also feature blue, and their pigments are likely azulene terpenoids as well. Basidiomata of the cobalt crust fungus Terana caerulea (syn. Pulcherricium caeruleum, Corticium caeruleum) are sky-blue owing to the presence of benzobisbenzofurans named corticins (CitationBriggs et al. 1976). Compounds that also are known from other biological sources such as indigo also were reported from fungi and can be produced using biotechnological systems (e.g. CitationFalanghe and Bobbio 1962); an indigo-like pigment is also known from cultures of Morchella (CitationEyal et al. 1991). Finally CitationGill and Steglich (1987) provided an overview on the manifold compounds from basidiomata of various Boletales, such as variegatic acid, which are converted enzymatically to blue anions upon injury of the fruiting bodies. A similar reaction also occurs in injured basidiomata of Lactarius indigo, yielding azulene pigments (CitationHarmon et al. 1980). Most of these compounds are unfortunately either not accessible in large quantities or they are highly unstable or insoluble, which has precluded their commercialization. In view of the increasing interest of both the food and cosmetics industries in natural pigments (see CitationMapari et al. 2005, Citation2009 and references therein), rapidly growing organisms such as species of Hypocrea/Trichoderma, which already have found wide biotechnological application, certainly would be better candidates for industrial production of natural pigments than the above mentioned mycorrhizal mushrooms. Therefore further studies on the pigments of H. caerulescens might be warranted.
Acknowledgments
We thank Begoña Aguirre-Hudson, Esperanza Beltran-Tejera, Liliana Bernardo, Marco Contu, Rose Marie Dähncke, Walter Gams, Quirico Migheli, Neven Matocec, Ivana Kušan, Barbara Scherm, Olga Sükösd, Luis Quijada for excursion support; Trix Merkx and Gerard Verkley (CBS) for patience with our cultures and Anton Hausknecht for insertion of specimens into WU. We also acknowledge the aid of Beata Schmieschek and Bärbel Köpcke (InterMed Discovery) for their help with the characterization of the pigment.
Financial support by the Austrian Science Fund (FWF; project P22081-B17) is gratefully acknowledged.
Literature cited
- AyerWAHoyanoYPedrasMSCvan AltenaI. 1986. Metabolites produced by the scleroderris canker fungus Gremmeniella abietina 1. Can J Chem 64:1585–1589, 10.1139/v86-262
- AyerWAPedrasMSC. 1987. Metabolites produced by the scleroderris canker fungus Gremmeniella abietina 3. Some further metabolites. Can J Chem 65:754–759, 10.1139/v87-128
- BitzerJKöpckeBStadlerMHellwigVJuYMSeipSHenkelT. 2007. Accelerated dereplication of natural products, supported by reference libraries. Chimia 51: 332–338, 10.2533/chimia.2007.332
- BlaszczykLPopielDChelkowskiJKoczykGSamuelsGJSobieralskiKSiwulskiM. 2011. Species diversity of Trichoderma in Poland. J Appl Genetics 52:233–243, 10.1007/s13353-011-0039-z
- BriggsLHCambieRCDeanICHodgesRIngramWBRutledgePS. 1976. Chemistry of fungi XI. Corticins A, B and C, benzobisbenzofurans from Corticium caeruleum. Austr J Chem 29:179, 10.1071/CH9760179
- ChaverriPGazisROSamuelsGJ. 2011. Trichoderma amazonicum, a new endophytic species on Hevea brasiliensis and H. guianensis from the Amazon basin. Mycologia 103:139–151, 10.3852/10-078
- DegenkolbTDieckmannRNielsenKFGräfenhanTTheisCZafariDChaverriPIsmaielABrücknerHvon DöhrenHThraneUPetriniOSamuelsGJ. 2008a. The Trichoderma brevicompactum clade: a separate lineage with new species, new peptaibiotics and mycotoxins. Mycol Prog 7:177–219, 10.1007/s11557-008-0563-3
- DegenkolbTGräfenhanTBergANirenbergHIGamsWBrücknerH. 2006. Peptaibiomics: screening for poly-peptide antibiotics (peptaibiotics) from plant-protective Trichoderma species. Chem Biodivers 3:593–610, 10.1002/cbdv.200690063
- DegenkolbTvon DöhrenHNielsenKFSamuelsGJBrücknerH. 2008b. Recent advances and future prospects in peptaibiotics, hydrophobin and mycotoxin research and their importance for chemotaxonomy of Trichoderma and Hypocrea. Chem Biodivers 5:671–680, 10.1002/cbdv.200890064
- DruzhininaISKomon-ZelazowskaMKredicsLHatvaniLAntalZBelaynehTKubicekCP. 2008a. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 154:3447–3459, 10.1099/mic.0.2008/021196-0
- DruzhininaISKopchinskiyAGKomonMBissettJSzakacsGKubicekCP. 2005. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet Biol 42:813–828, 10.1016/j.fgb.2005.06.007
- DruzhininaISLaFeKWillingerBKomon-ZelazowskaMAmmiratiJKubicekCPRogersJD. 2008b. An unknown species from Hypocreaceae isolated from human lung tissue of a patient with non-fatal pulmonary fibrosis. Clin Microbiol Newslett 29:180–184, 10.1016/j.clinmicnews.2007.11.002
- EdgarRC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797, 10.1093/nar/gkh340
- EyalJMabudMdAWalterJF. 1991. Production of indigotin in submerged culture using Morchella nov. ES-1. Appl Biochem Biotechnol 30:303–312, 10.1007/BF02922034
- FalangheHBobbioPA. 1962. Identification of indigo produced in submerged culture of Agaricus campestris mutant culture. Arch Biochem Biophys 96:430–433, 10.1016/0003-9861(62)90431-9
- GhisalbertiELSivasithamparamK. 1991. Antifungal antibiotics produced by Trichoderma spp. Soil Biol Biochem 23:1011–1020, 10.1016/0038-0717(91)90036-J
- GillM. 1999. Pigments of fungi (macromycetes). Nat Prod Rep 16:301–317, 10.1039/a705730j
- GillMSteglichW. 1987. Pigments of Fungi (macromycetes). Prog Ch Org Nat Prod 51:1–317.
- HallTA. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98.
- HanadaREde Jorge SouzaTPomellaAWVPrakash HebbarKPereiraJOIsmaielASamuelsGJ. 2008. Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. Mycol Res 112:1335–1343, 10.1016/j.mycres.2008.06.022
- HarmanGE. 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 96:190–194, 10.1094/PHYTO-96-0190
- HarmanGEHowellCRViterboAChetILoritoM. 2004. Trichoderma species—opportunistic, avirulent plant symbionts. Nat Rev Microbiol 2:43–56, 10.1038/nrmicro797
- HarmanGEKubicekCP. 1998. Trichoderma & Gliocladium. Vol. 2. Enzymes, biological control and commercial applications. London: Taylor & Francis Ltd. 393 p.
- HarmonADWeisgraberKHWeissU. 1980. Preformed azulene pigments of Lactarius indigo (Schw.) Fries (Russulaceae, Basidiomycetes). Experientia 36:54–56, 10.1007/BF02003967
- HatvaniLAntalZManczingerLSzekeresADruzhininaISKubicekCPNagyANagyEVagvolgyiCKredicsL. 2007. Green mold diseases of Agaricus and Pleurotus spp. are caused by related but phylogenetically different Trichoderma species. Phytopathology 97:532–537, 10.1094/PHYTO-97-4-0532
- HuelsenbeckJPRonquistF. 2001. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755, 10.1093/bioinformatics/17.8.754
- JaklitschWM. 2009. European species of Hypocrea I. The green-spored species. Stud Mycol 63:1–91, 10.3114/sim.2009.63.01
- JaklitschWM. 2011. European species of Hypocrea II: species with hyaline ascospores. Fungal Divers 48:1–250, 10.1007/s13225-011-0088-y
- JaklitschWMSamuelsGJDoddSLLuB-SDruzhininaIS. 2006. Hypocrea rufa/Trichoderma viride : a reassessment and description of five closely related species with and without warted conidia. Stud Mycol 56:135–177, 10.3114/sim.2006.56.04
- KanekoYSanadaM. 1969. Studies on the fluorescent substances produced by Aspergillus fungi VII. Purification and isolation of asperopterin B and chemical properties of asperopterin B and A. J Ferment Technol 47:8–19.
- KredicsLAntalZDócziIManczingerLKeveiFNagyE. 2003. Clinical importance of the genus Trichoderma. A review. Acta Microbiol Immunol Hung 50:105–117, 10.1556/AMicr.50.2003.2-3.1
- LaatschH. 2010. AntiBase—the natural compound identifier. Weinheim, Germany: Database, Wiley-VCH.
- LæssøeTSrikitikulchaiPFournierJKöpckeBStadlerM. 2010. Lepraric acid derivatives as chemotaxonomic markers in Hypoxylon aeruginosum, Chlorostroma sub-cubisporum and C. cyaninum sp. nov. Fungal Biol 114: 481–489, 10.1016/j.funbio.2010.03.010
- MapariSASMeyerASThraneUFrisvadJC. 2009. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb Cell Fact 8:24, 10.1186/1475-2859-8-24
- MapariSASNielsenKFLarsenTOFrisvadJCMeyerASThraneU. 2005. Exploring fungal biodiversity for the production of water-soluble pigments as potential natural food colorants. Curr Opin Biotechnol 16:231–238, 10.1016/j.copbio.2005.03.004
- MigheliQBalmasVKomon-ZelazowskaMSchermBFioriSKopchinskiyAGKubicekCPDruzhininaIS. 2008. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environ Microbiol 11:35–46, 10.1111/j.1462-2920.2008.01736.x
- ParkMSBaeKSYuSH. 2006. Two new species of Trichoderma associated with green mold of oyster mushroom cultivation in Korea. Mycobiology 34:111–113, 10.4489/MYCO.2006.34.3.111
- RobinsonNWoodKHylandsPJGibsonTM. 1992. Blue pigments of Penicillium herquei. J Nat Prod 55:814–817, 10.1021/np50084a019
- SamuelsGJDoddSLuB-SPetriniOSchroersH-JDruzhininaIS. 2006a. The Trichoderma koningii aggregate species. Stud Mycol 56:67–133, 10.3114/sim.2006.56.03
- SamuelsGJIsmaielA. 2009. Trichoderma evansii and T. lieckfeldtiae: two new T. hamatum-like species. Mycologia 101:142–156, 10.3852/08-161
- SamuelsGJIsmaielABonM-Cde RespinisSPetriniO. 2010. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia 102:944–966, 10.3852/09-243
- SamuelsGJSuarezCSolisKHolmesKAThomasSEIsmaielAEvansHC. 2006b. Trichoderma theobromicola and T. paucisporum: two new species isolated from cacao in South America. Mycol Res 110:381–392, 10.1016/j.mycres.2006.01.009
- SchusterASchmollM. 2010. Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol 87:787–799, 10.1007/s00253-010-2632-1
- StadlerMFournierJ. 2006. Pigment chemistry, taxonomy and phylogeny of the Hypoxyloideae (Xylariaceae). Rev Iberoamericana Micol 23:160–170, 10.1016/S1130-1406(06)70037-7
- StadlerMFournierJBeltrán-TejeraEGranmoA. 2008. The “red Hypoxylons” of the temperate and subtropical northern hemisphere. In: GlaweDAAmmiratiJF, eds., A Festschrift in honor of Professor Jack D. Rogers. North Am Fungi 3:73–125.
- StamatakisE. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690, 10.1093/bioinformatics/btl446
- SwoffordDL. 2002. PAUP*4.0b10: phylogenetic analysis using parsimony (*and other methods). Sunderland, Massachusetts: Sinauer Associates.
- VoglmayrHJaklitschWM. 2008. Prosthecium species with Stegonsporium anamorphs on Acer. Mycol Res 112:885–905, 10.1016/j.mycres.2008.01.020
- von NussbaumFSpitellerPRüthMSteglichWWannerGGamblinBStievanoLWagnerFE. 1998. Ein Eisen(III)-Catechol-Komplex als Pilzfarbstoff. Angew Chem 110: 3483–3485, 10.1002/(SICI)1521-3757(19981204)110:23 < 3483::AID-ANGE3483>3.0.CO;2-5
- ZachowCBergCMüllerHMeinckeRKomon-ZelazowskaMDruzhininaISKubicekCPBergG. 2008. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. ISME J 3:79–92, 10.1038/ismej.2008.87