Abstract
Recent molecular phylogenetic studies have revealed the existence of at least 50 species of Morchella worldwide and demonstrated a high degree of continental endemism within the genus. Here we describe 19 phylogenetic species of Morchella from North America, 14 of which are new (M. diminutiva, M. virginiana, M. esculentoides, M. prava, M. cryptica, M. frustrata, M. populiphila, M. sextelata, M. septimelata, M. capitata, M. importuna, M. snyderi, M. brunnea and M. septentrionalis). Existing species names (M. rufobrunnea, M. tomentosa, M. punctipes and M. angusticeps) are applied to four phylogenetic species, and formal description of one species (M. sp. “Mel-8”) is deferred pending study of additional material. Methods for assessing morphological features in Morchella are delineated, and a key to the known phylogenetic species of Morchella in North America is provided. Type studies of M. crassistipa, M. hotsonii, M. angusticeps and M. punctipes are provided. Morchella crassistipa is designated nomen dubium.
Introduction
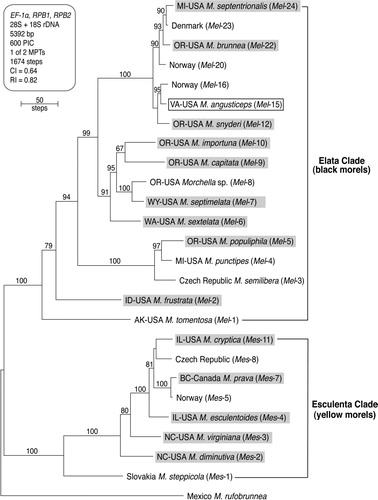
Morels (species of Morchella Dill. ex Pers.) are highly valued edible mushrooms in the northern hemisphere, especially in Europe and North America (CitationWeber 1995, CitationKuo 2005), but we still lack an understanding of many aspects of their biology, taxonomy and distribution. Molecular phylogenetic analyses of 590 Morchella collections (CitationO'Donnell et al. 2011) revealed at least 41 phylogenetic species worldwide, 19 of which appeared to be endemic to North America. Results additionally indicated a high degree of continental endemism for the genus, with only two of the 41 species occurring naturally in both Europe and Asia and none occurring naturally in both North America and Eurasia (although a few North American species appear to have been introduced into Turkey; see CitationTaşkın et al. 2010, Citation2012). Because extensive sampling has indicated strict North American endemism, existing European and Asian species names (including among others the well known names M. esculenta [L] Pers., M. elata Fr., M. semilibera DC and M. conica Pers.) could not be applied to North American morels. We studied the type collections of the six species originally described from North America (M. tomentosa M. Kuo, M. rufobrunnea Guzmán & F. Tapia, M. angusticeps Peck, M. punctipes Peck, M. hotsonii Snyder, M. crassistipa Snyder) to investigate the contemporary applicability of these species names (see Supplementary materials). We also studied morphological, ecological and distributional data from 244 North American collections identified phylogenetically to assess whether these species can be defined on the basis of morphology, ecology and distribution. Herein, five species within the Esculenta Clade (yellow morels) and nine species within the Elata Clade (black morels) from North America are formally described and M. angusticeps is epitypified ().
Fig. 1 One of two equally parsimonious phylograms 1674 steps long depicting phylogenetic relationships among 19 North American and seven European species of Morchella inferred from a five-gene dataset containing 600 parsimony informative characters (PIC). Sequences of M. rufobrunnea were used to root the phylogram based on more inclusive analyses (CitationO'Donnell et al. 2011). The 17 species within the Elata Clade (black morels) and eight species within the Esculenta Clade (yellow morels) are identified respectively by Mel and Mes followed by a unique Arabic number. Highlight is used to identify nine species within the Elata Clade and five species within the Esculenta Clade that are formally described herein. In addition M. angusticeps (Mel-15) and M. punctipes (Mel-4) are epitypified. Numbers above internodes represent bootstrap values ≥ 65%. CI = consistency index, RI 5= retention index.
Materials and methods
Collections were accessioned into the Morel Data Collection Project (MDCP) database (www.mushroomexpert.com/mdcp/mdcprecords.pl), together with collectors' notes and photographs. Phylogenetic identification of 244 collections was accomplished following the methods for DNA isolation, PCR amplification and sequencing, and phylogenetic analysis described in CitationO'Donnell et al. (2011). Macromorphology of specimens was determined through observation of fresh specimens, dried specimens, collectors' notes and photographs of material in the fresh state. (See Supplementary materials for details on specific features.) For micromorphological study horizontal scalp sections taken from the hymenia and sterile ridges were squash-mounted in 2% KOH. Measurements of asci, paraphyses and elements on sterile ridges were made at 400× magnification; ascospore measurements were made at 1000×. At least 10 mature ascospores were measured for each specimen. Collections were deposited in the Mycology Collection of the Field Museum of Natural History in Chicago (F) using MDCP accession numbers or were studied and deposited in the Fungarium at the Royal Ontario Museum in Toronto (TRTC). Others were returned to lending herbaria. DNA sequence data from type specimens described in this study were deposited in GenBank under accession numbers JQ670119–JQ670132.
Results
Study of 244 North American collections identified phylogenetically (Supplementary table I) revealed five species within the Morchella esculenta Clade as defined by CitationO'Donnell et al. (2011) and nine species within the Morchella elata Clade (). Among these, a few phylogenetic species (Morchella rufobrunnea, M. tomentosa, M. importuna, M. prava, M. frustrata) could be differentiated on the basis of morphological characters, and several others (M. punctipes, M. populiphila, M. brunnea, M. capitata, M. snyderi) could be distinguished when geographic range and/or ecological factors are combined with morphological characters. However, several phylogenetic species (including the pair M. sextelata and M. septimelata and the pair M. esculentoides and M. cryptica) remain morphologically and ecologically cryptic based on present data. Type studies (see Supplementary materials) supported applying four of the six previous North American species names (M. angusticeps, M. punctipes, M. rufobrunnea, M. tomentosa) to phylogenetic species. However, two previous North American names were problematic: (i) the type collection of M. crassistipa was determined to be mixed, obliging us to designate this name as nomen dubium; and (ii) preliminary DNA sequence data on M. hotsonii suggested that it might represent a novel phylogenetic species, apparently undocumented since the 1935 type collection. As a result of our analyses 14 new species are described below. Description of another new species was deferred pending additional material for study. In addition, we identified a suite of morphological characters for study of Morchella specimens (see Supplementary materials).
Taxonomy
Morchella rufobrunnea Clade
The Morchella rufobrunnea Clade (CitationO'Donnell et al. 2011) is basal to the remaining Morchella lineage and consists of a single species. Morphologically M. rufobrunnea resembles the “yellow morels” as they have been defined traditionally (CitationKuo 2005) and most members of the Morchella esculenta Clade.
Morchella rufobrunnea Guzmán & F. Tapia, Mycologia 90:706. 1998.
Figs. 2–10 Morchella species. 2. M. rufobrunnea F 03080501. 3. M. diminutiva HOLOTYPE F 05030404, Mes-2. 4. M. virginiana HOLOTYPE BPI 880503, Mes-3. 5. M. esculentoides F 04250405, Mes-4. 6. M. prava HOLOTYPE F 05100602, Mes-7. 7. M cryptica HOLOTYPE F 04220401, Mes-11. 8. M. tomentosa HOLOTYPE F 06150405. 9. M. frustrata UC 1860809, Mel-2. 10. M. punctipes F 04240304, Mel-4. Bars = 5 cm. Mel and Mes numbers refer to the phylogenetic species reported in CitationO'Donnell et al. (2011).

The original description of Morchella rufobrunnea appeared in CitationGuzmán and Tapia (1998). (For an augmented description based on material studied by the authors see Supplementary materials.)
Comments. Morchella rufobrunnea is easily distinguished on the basis of “its abruptly conical young cap with pale ridges and nearly black pits, and its rufescence” (CitationKuo 2008). It appears in woodchips and landscaping settings on the West Coast from California to Seattle. (For a discussion of species names frequently misapplied to M. rufobrunnea see CitationKuo 2008.) Molecular phylogenetic analysis of F 03110601 and several other commercially produced morels (O'Donnell unpubl data) confirms M. rufobrunnea as the morel cultivated commercially (US Patent numbers 4594809, 4757640).
Morchella esculenta Clade
The Morchella esculenta Clade (CitationO'Donnell et al. 2011) is sister to the M. elata Clade and corresponds fairly well with the traditional morphological concept of “yellow morels” (CitationKuo 2005), amended to exclude M. rufobrunnea, which has pale ridges like yellow morels but is phylogenetically distinct, and to exclude M. frustrata and M. snyderi, which have the colors usually associated with yellow morels but are nested within the M. elata Clade. The latter two species, however, possess pits that primarily are elongated vertically and feature a notable sinus where the hymenophore attaches to the stipe; most members of the M. esculenta Clade have less vertically arranged pits and feature hymenophores that are typically adnate.
Morchella diminutiva M. Kuo, Dewsbury, Moncalvo & S.L. Stephenson, sp. nov.
MycoBank MB 563948
Ascomata 35–94 mm alta; capitulum conicum; costae pallidae, colocatae recte; hymenium sufflavum colore subcaesio; biotopium in silva frondosa in orienti America septentrionalis, praesertim in alveis ubi Fraxinus americana L aut Liriodendron tulipifera L praesens est; sporae 20–24 × 11–16 μm. Holotypus: Biotopium in silva frondosa; USA, in Illinoisense, ad Coles County; M. Kuo col.; specimen typicum in herbarium F (05030404) conservatum.
Etymology. The epithet refers to the size of the ascomata.
Ascomata 35–94 mm high. Hymenophore 20–41 mm high; 10–27 mm wide at the widest point; usually conical to subconical, occasionally ovoid or subcylindrical with a subacute apex; pitted and ridged, with 8–16 primary vertical ridges and occasional secondary vertical ridges, with scattered to frequent, sunken, transecting horizontal ridges; adnate at the point of attachment, or when young attached to stipe with a slight sinus. Ridges glabrous; yellowish to nearly whitish when young, becoming pale brownish yellow or pale yellowish brown with maturity; widely rounded to nearly flat when young, but with age often becoming sharpened or eroded. Pits primarily vertically elongated; glabrous; medium to dark gray or grayish brown when young, becoming pale brownish yellow or pale yellowish tan. Stipe 10–68 mm high; 3–18 mm wide; more or less equal, or sometimes basally subclavate; finely mealy with whitish granules or nearly glabrous; whitish. Context whitish; about 1 mm thick in the hollow hymenophore; in the stipe sometimes slightly chambered near the base. Sterile inner surface whitish and pubescent. Ascospores (18–)20–24(−26) × (10–)11–16(−18) μm; elliptical; smooth; contents homogeneous; orangish yellow in deposit. Asci 175–325 × 12.5–25 μm; eight-spored; cylindrical; hyaline. Pa-raphyses 125–250 × 7.5–20 μm; cylindrical; apices generally merely rounded but occasionally subfusiform, subcapitate, or irregular; septate; brown to brownish in KOH (2%) on immature ascomata, becoming hyaline with maturity. Elements on sterile ridges 75–175 × (7.5–) 10–30 μm; hyaline to brownish or brown in KOH (2%); septate; terminal cell subfusiform by maturity but sometimes merely cylindrical, with a rounded or subcapitate apex.
Ecology. Appearing in eastern North American hardwood forests, especially in association with Fraxinus americana L and Liriodendron tulipifera L but also found under Carya spp. and other hardwoods; widely distributed east of the Rocky Mountains, although the northern limits of its range are undetermined; April and May. Specimens examined (Supplementary table I) were collected in Illinois, Massachusetts, Mississippi, Missouri, Pennsylvania, Tennessee, Virginia and West Virginia.
Comments. Morchella diminutiva corresponds to phylogenetic species Mes-2 in CitationO'Donnell et al. (2011). The species is characterized by its small average size; its conical to subconical hymenophore, which features vertically elongated pits and ridges, and the elements on its sterile ridges, which usually are subfusiform. It is widely distributed and common east of the Great Plains under ash, tulip trees and other hardwoods. Larger specimens are similar to smaller specimens of M. virginiana, which has a limited southeastern range and scattered elements on sterile ridges that are variably shaped. Morchella esculentoides, along with M. cryptica and M. prava, can be separated from M. diminutiva on the basis of their larger size, less conical hymenophores, and their pits and ridges, which are not primarily vertically elongated. Morchella diminutiva corresponds to “type 1” of the “North American Deliciosas” in CitationKuo (2005) and, in part, to M. deliciosa in CitationWeber (1995).
Morchella virginiana O'Donnell & S.A. Rehner, sp. nov.
MycoBank MB 563949
Ascomata 50–125 mm alta; capitulum late conicum; costae pallidae; hymenium sufflavum colore subcaesio; biotopium in silva frondosa in America vulturna septentrionalis, praesertim in alveis ubi Liriodendron tulipifera L. praesens est; sporae 18–25 × 10–16 μm Holotypus: Biotopium in alveo se conjungens cum Liriodendron tulipifera L; USA, in Virginiaense, ad Fairfax County; S. Rehner col.; specimen typicum in Herb. BPI (880503) conservatum.
Etymology. The epithet refers to the location of the holotype collection.
Ascomata 50–125 mm high. Hymenophore 30–70 mm high; 22–35 mm wide at the widest point; usually ovoid with a bluntly conic to subconic apex, but occasionally subcylindrical with a rounded apex; pitted and ridged, with 12–16 primary vertical ridges and scattered secondary vertical ridges, with scattered, sunken, transecting horizontal ridges; adnate at the point of attachment. Ridges finely tomentose to glabrous; pale yellowish when young, becoming pale brownish yellow or yellowish brown with maturity; flattened when young but often becoming sharpened or eroded in age. Pits primarily elongated vertically; glabrous or finely tomentose; grayish brown when young, becoming pale brownish yellow or pale yellowish tan but often retaining grayish hues. Stipe 25–55 mm high; 10–20 mm wide; more or less equal, or basally subclavate; finely mealy with whitish granules or nearly glabrous; whitish to pale yellowish. Context whitish; about 1 mm thick in the hollow hymenophore; in the stipe sometimes slightly chambered near the base. Sterile inner surface whitish and pubescent. Ascospores 18–25(−28) × 10–16 μm; elliptical; smooth; contents homogeneous. Asci 200–325 × 15–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 125–200 × 5–15 μm; cylindrical; apices generally merely rounded but occasionally subclavate, subcapitate or subfusiform; septate; hyaline to brownish in KOH (2%). Elements on sterile ridges scattered and infrequent (often difficult to locate or distinguish from paraphyses), 100–175 × 10–30 μm; hyaline to brownish or brown in KOH (2%); septate; terminal cell widely cylindrical, with a rounded, subcapitate, subclavate or subfusiform apex.
Ecology. Appearing in southeastern hardwood forests, especially in association with L. tulipifera in river bottoms, coastal plains and drainage areas, often in sandy soil; North Carolina, South Carolina, Mississippi and Virginia; April and May. Specimens examined (Supplementary table I) were collected in Mississippi, North Carolina, South Carolina and Virginia.
Comments. Morchella virginiana corresponds to phylogenetic species Mes-3 in CitationO'Donnell et al. (2011). Based on current data it can be characterized as intermediate in stature between M. diminutiva and M. esculentoides; its dimensions and proportions approximate large specimens of M. diminutiva and small specimens of M. esculentoides. It is usually less sharply conical than M. diminutiva, however, and its stipe is proportionally longer than that of M. esculentoides, from which it also differs in the primarily vertical (rather than more or less random) orientation of the pits. While M. diminutiva is found in a variety of eastern hardwood habitats in association with Fraxinus spp., L. tulipifera and other hardwoods, M. virginiana apparently is limited to association with L. tulipifera in riparian and upland ecosystems from Virginia to northern Mississippi (CitationO'Donnell et al. 2011). Elements on sterile ridges in M. virginiana specimens examined were scattered and often difficult to isolate; when present they were variably shaped, cylindrical to subcapitate, subcylindrical or subfusiform. Elements on sterile ridges of M. diminutiva, by contrast, were easily demonstrated and primarily widely fusiform. Morchella virginiana corresponds with “type 2” of the “North American Deliciosas” in CitationKuo (2005).
Morchella esculentoides M. Kuo, Dewsbury, Moncalvo & S.L. Stephenson, sp. nov. , Supplementary fig. 1
MycoBank MB 563950
Ascomata 36–220(−415) mm alta; capitulum ovoideum cum apice late conico aut convexo; costae pallidae; hymenium sufflavum aetate; biotopiumin silva; sporae 18–22 × 11–13 μm. Holotypus: Biotopium in silva frondosa; USA, in Oregonense, ad Linn County; N. S. Weber 7114 col.; specimen typicum in Herb. OSC (138364—138367) conservatum.
Etymology. The epithet refers to similarity with the iconic European species M. esculenta L.
Ascomata 36–220(−415) mm high. Hymenophore 23–110(−220) mm high; 15–42(−126) mm wide at the widest point; usually ovoid with a bluntly conical or convex apex but occasionally subcylindrical with a convex apex or subglobose to pyriform; pitted and ridged, with approximately 12–30 vertical ridges and numerous horizontal and oblique ridges, along with scattered, sunken, transecting ridges; adnate at the point of attachment. Ridges glabrous or nearly so; white to pale yellowish when young, becoming pale brownish yellow or remaining whitish with maturity; bluntly rounded or nearly flattened when young, usually becoming sharpened or eroded in age. Pits usually more or less vertically elongated but not strictly so and not infrequently subglobose to irregular in outline; glabrous or finely tomentose; grayish brown to dark brown or nearly black when young, becoming pale brownish yellow. Stipe 20–120(−240) mm high; 16–92(−140) mm wide; usually basally clavate to subclavate; glabrous or finely mealy with scattered whitish to yellowish granules; whitish to pale yellowish or brownish. Context whitish; 1–3 mm thick in the hollow hymenophore; in the stipe often becoming thickened and chambered near the base with maturity. Sterile inner surface whitish and pubescent. Ascospores (17–)18–22(−24) × 11–13(−15) μm; elliptical; smooth; contents homogeneous. Asci 225–325 × 15–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 75–180 × 5–15 μm; cylindrical; apices generally merely rounded but occasionally subclavate to clavate or subfusiform; septate; hyaline to ochraceous or faintly brownish in KOH (2%). Elements on sterile ridges 75–160 × 10–27.5(−37.5) μm; hyaline to ochraceous in KOH (2%); septate; terminal cell subclavate to clavate, or subfusiform to widely cylindrical with a rounded or subcapitate apex.
Ecology. Widely distributed and common; often found under living and dead hardwoods (especially living F. americana and dead or dying Ulmus americana L) but also found in apparent association with Populus deltoides Bartr., P. balsamifera L, P. grandidentata Michx., L. tulipifera, Platanus occidentalis L and Quercus spp. in a variety of habitats (from riparian cottonwood-sycamore lowlands to oak-hickory forests, mixed woods and northern hardwood forests); also appearing in association with Malus spp. in old orchards and occasionally in association with conifers (especially Pinus strobus L but also with P. resinosa Ait., Abies balsamea [L] Mill. and Picea abies [L] Karst.); rarely found in areas with no trees present; widely distributed and common east of the Rocky Mountains from Kansas to Ontario and the northeastern United States, south to Texas, Arkansas and South Carolina; in western North America occurring under hardwoods in river bottoms or in urban settings in association with apple trees or ornamental ash plantings; appearing in spring (March–June, depending on latitude and altitude). Specimens examined (Supplementary table I) were collected in Arkansas, California, Colorado, Illinois, Iowa, Kansas, Massachusetts, Minnesota, Missouri, Nebraska, New York, Ohio, Oklahoma, Ontario, Oregon, Pennsylvania, South Carolina, South Dakota, Texas, Vermont, Virginia, West Virginia and Wisconsin.
Comments. Morchella esculentoides is the most widely distributed member of the genus in North America and corresponds to phylogenetic species Mes-4 in CitationO'Donnell et al. (2011). Mature ascomata that have developed normally can be distinguished morphologically from all other North American species of Morchella, except M. cryptica, by its medium to large ascomata, together with the non-rufescent, pale ridges, the generally rounded apex of the hymenophore, and the pits, which are not strictly vertical in arrangement but are not as asymmetrical and irregular as those of M. prava (however, occasional aberrant specimens of M. esculentoides, such as F 04150501, can appear similar to M. prava and must be identified with molecular analysis). In the Great Lakes region, where the ranges of M. cryptica and M. esculentoides overlap, the two species cannot be separated reliably based on current data without molecular analysis. (See the comments under M. cryptica for details.) In western regions M. esculentoides has no close look-alikes, although M. rufobrunnea, which has rufescent ridges, and M. frustrata, which has a conical hymenophore featuring vertically arranged pits, have similarly colored hymenophores. Morchella esculentoides corresponds (in part) to M. esculenta in CitationWeber (1995) and to the “Classic North American Yellow Morel” in CitationKuo (2005).
Morchella prava Dewsbury, Moncalvo, J.D. Moore & M. Kuo, sp. nov.
MycoBank MB 563951
Ascomata 50–100 mm alta; capitulum ovoideum cum apice subconico; costae pallidae; hymenium pravium, canae vel atrae, pallescens aetate; biotopium in silva; sporae 17–21 × 10–12 μm. Holotypus: Biotopium in humo harenoso sub Pinus strobus L et Acer saccharum Marsh.; USA, in Michiganense, ad Emmet County; M. Kuo & J.D. Moore col.; specimen typicum in Herb. F (05100602) conservatum.
Etymology. The epithet means crooked, irregular or deformed and reflects the contorted aspect of the hymenophore.
Ascomata 50–100 mm high. Hymenophore 30–60 mm high; 20–50 mm wide at the widest point; irregularly shaped but often more or less ovoid with a slightly narrowed or subconic apex; pitted and ridged; with 12–18 vertical ridges intersecting a line across the widest portion but with numerous horizontal and oblique ridges as well, along with scattered, sunken, transecting ridges; adnate at the point of attachment or occasionally attached with an inconsistent, poorly defined sinus. Ridges glabrous or very finely tomentose; thick; whitish to pale yellowish or pale tan when young, becoming pale brownish yellow with maturity and eventually darkening to yellowish brown or reddish brown in places; flattened or widely rounded when young but with age sometimes becoming sharpened or eroded. Pits asymmetrical and irregular in outline and size; glabrous or finely tomentose; gray to dark gray, gray-brown or nearly black when young and often remaining dark for a long time before becoming pale brownish yellow or pale yellowish tan with maturity. Stipe 25–40 mm high; 10–30 mm wide; more or less equal above a subclavate to clavate base; glabrous or nearly so; whitish to pale yellowish, often discoloring reddish brown; usually developing indistinct ridges and folds near the base. Context whitish; about 1–2 mm thick in the hollow hymenophore; usually chambered or layered near the base. Sterile inner surface whitish and pubescent. Ascospores (16–)17–21(−24) × (8–)10–12(−13) μm; ellipsoid to slightly subfusiform; smooth; contents homogeneous. Asci 200–300 × 15–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 100–175 × 5–12.5 μm; cylindrical; apices generally merely rounded or subclavate but occasionally clavate or subfusiform; septate; hyaline to brownish or brown in KOH (2%). Elements on sterile ridges scattered and infrequent (often difficult to locate or distinguish from paraphyses); 75–125 × 7.5–25(−37.5) μm; hyaline to ochraceous, brownish, or brown in KOH (2%); septate; terminal cell widely cylindrical with a rounded apex, subclavate, clavate, subcapitate, capitate or widely subfusiform.
Ecology. The ecology of Morchella prava collections studied was not consistent. Among the seven collections studied, few if any common ecological denominators were apparent. Collections were made approximately 43–50°N across North America, in April, May and early June. (See Supplementary materials for further ecological data from the collections studied.) Specimens examined (Supplementary table I) were collected in Michigan, Montana, Ontario, Saskatchewan and South Dakota.
Comments. Morchella prava corresponds to phylogenetic species Mes-7 in CitationO'Donnell et al. (2011). The species usually can be identified on the basis of its esculenta-like stature and its contorted, asymmetrical and irregular pits and ridges. The pits are highly irregular in outline and size, and the thick, bluntly rounded ridges are less likely to become eroded and sharpened with maturity than the ridges in M. esculentoides and M. cryptica. The hymenophore of M. prava often appears like a contorted or somewhat deformed version of the M. esculentoides hymenophore. Significant differences in microscopic features between M. prava, M. esculentoides and M. cryptica were not observed, although elements on sterile ridges in the M. prava specimens studied generally were sparsely scattered or nearly absent, in contrast to the easily located elements of the other two species. Morchella prava undoubtedly has been misidentified as “M. esculenta” and “M. deliciosa” in many North American treatments, and it probably corresponds to what has been labeled “M. vulgaris” in Québec (CitationLincoff 1981). It appeared in CitationKuo (2005) as the “Classic North American Morel III”.
Morchella cryptica M. Kuo & J.D. Moore, sp. nov.
MycoBank MB 563952
Ascomata 60–200 mm alta; capitulum ovoideum cum apice late conico aut convexo; costae pallidae; hymenium sufflavum aetate; biotopium in silva frondosa in meditulio occidente Americae septentrionalis, alveis ubi Fraxinus americana L praesens est; sporae 18–23 × 10–13 μm. Holotypus: Biotopium in silva frondosa cum Fraxinus americana L et Acer spp. praesentes; USA, in Illinoisense, ad Coles County; M. Kuo col.; specimen typicum in Herb. F (04220401) conservatum.
Etymology. The epithet refers to the cryptic morphology of the species.
Ascomata 60–200 mm high. Hymenophore 40–75 mm high; 32–62 mm wide at the widest point; usually ovoid with a convex, bluntly conical, or conical apex; pitted and ridged; with 10–18 vertical ridges intersecting a line across the widest portion but with numerous horizontal and oblique ridges as well, along with scattered, sunken, transecting ridges; adnate at the point of attachment. Ridges glabrous or nearly so; pale yellowish when young, usually remaining pale yellowish with maturity but occasionally darkening somewhat to brownish yellow; flattened when young, becoming sharpened or eroded in age. Pits often vertically elongated but not strictly so and occasionally subglobose to irregular in outline; glabrous; grayish yellow to gray or pale grayish brown when young, becoming pale yellowish or pale brownish yellow (usually concolorous with the ridges at maturity). Stipe 50–130 mm high; 14–60 mm wide; usually basally subclavate to clavate when mature but occasionally more or less equal throughout development; sometimes developing wide, shallow ridges; finely mealy with whitish granules or nearly glabrous; whitish. Context whitish; about 1–2 mm thick in the hollow hymenophore; often becoming thickened and chambered near the base. Sterile inner surface whitish and pubescent. Ascospores 18–23 × 10–13(−15) μm. ellipsoid; smooth; contents homogeneous. Asci 175–300 × 15–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 100–160 × 5–12.5(−15) μm; cylindrical; apices usually rounded or subclavate but occasionally clavate or widely subfusiform; septate; hyaline to brownish in KOH (2%). Elements on sterile ridges scattered (occasionally difficult to locate or distinguish from paraphyses); 75–125(−175) × 10–30 μm; hyaline in KOH (2%); septate; terminal cell widely cylindrical with a rounded apex, subcapitate, capitate, subclavate, clavate or widely subfusiform.
Ecology. Appearing in Midwestern hardwood forests, especially in apparent association with F. americana but also reported under L. tulipifera and Acer spp.; fairly common in the Great Lakes region from Ontario to central Illinois and western Pennsylvania; April, May and June. Specimens examined (Supplementary table I) were collected in Illinois, Michigan, Ontario and Pennsylvania.
Comments. Morchella cryptica corresponds to phylogenetic species Mes-11 in CitationO'Donnell et al. (2011). Based on current data the species cannot be reliably separated from M. esculentoides on the basis of morphological characters, although the hymenophore of M. cryptica is frequently somewhat paler and its ridges are usually more flattened. Microscopic features studied for the two species are virtually identical. M. cryptica is not found as frequently as M. esculentoides; however, both species were collected under hardwoods, often in apparent association with Fraxinus spp. Further collections of M. cryptica might lead to a reliable means of separating these phylogenetic species. It undoubtedly has been labeled “M. esculenta” in North American treatments that cover the Great Lakes region (e.g. CitationWeber 1995). It corresponds with the “Classic North American Yellow Morel II” in CitationKuo (2005).
Morchella Elata Clade
The Morchella elata Clade (CitationO'Donnell et al. 2011) is sister to the M. esculenta Clade and corresponds with the traditional morphological concept of “black morels” (CitationKuo 2005) amended to include M. semilibera-like species and three species (M. tomentosa, M. frustrata, M. snyderi) that can manifest pale, esculenta-like coloration of the hymenophore. The North American species in the M. elata Clade have pits that primarily are elongated vertically at maturity and generally feature a notable sinus where the hymenophore attaches to the stipe.
Morchella tomentosa M. Kuo, Mycotaxon 105:441. 2008. , Supplementary fig. 2
The original description of Morchella tomentosa appeared in CitationKuo 2008. (For an augmented description based on material studied by the authors see Supplementary materials.)
Comments. Morchella tomentosa is a postfire morel featuring densely tomentose surfaces. Because of the dark, young ascomata it frequently has been called the “gray morel” by western commercial collectors. With prolonged exposure to sunlight its colors often fade dramatically, approximating those typical of M. esculentoides-like morels; these specimens, however, still feature hairs that are brown in KOH (2%) and thus can be identified microscopically. CitationStefani et al. (2010) described an underground, root-like structure beneath three Alaskan ascomata of M. tomentosa. It is unclear whether this structure, which they termed a “radiscisclerotium”, is consistently attached to M. tomentosa ascocarps; it was not documented by any of the collectors of the specimens we examined, and it has not been reported by mycologists investigating the species (CitationMcFarlane et al. 2005, CitationPilz et al. 2007) or by commercial collectors for whom M. tomentosa is a popular species. The three radiscisclerotia documented by Stephani et al. (2010) extended to about 80 mm into the substrate, branching several times; individual branches of the structures were 5–15 mm thick. Morchella tomentosa has been treated as the “gray morel” (CitationPilz et al. 2004, Citation2007; CitationMcFarlane et al. 2005) and as the “Black Foot Morel” (CitationKuo 2005).
Morchella frustrata M. Kuo, sp. nov.
MycoBank MB 563953
Ascomata 60–90 mm alta; capitulum conicum; costae minime spadice, non nigrescens; hymenium levis, minime spadix; biotopium in silvis multigeneri; sporae 20–29 × 14–19 μm. Holotypus: Biotopium in silvis coniferibus; USA, in Californiaense, ad Placer County; T. Bruns 3643 col.; specimen typicum in Herb. UC (1860811) conservatum.
Etymology. The epithet reflects the frustrating combination of black and yellow morel features that characterize the species.
Ascomata 60–90 mm high. Hymenophore 40–60 mm high; 25–40 mm wide at the widest point; conical; pitted and ridged; with 16–22 primary vertical ridges and few shorter, secondary vertical ridges, with frequent, sunken, transecting horizontal ridges; attached to stipe with a sinus about 2–4 mm deep and 2–4 mm wide. Ridges glabrous; pale yellowish to nearly whitish when young, becoming pale tan with maturity; slightly flattened when young but often becoming sharpened or eroded with age. Pits primarily elongated vertically; glabrous; dull grayish to pale yellowish or nearly whitish when young, becoming pale tan to pale pinkish tan. Stipe 20–40 mm high; 10–25 mm wide; more or less equal or sometimes basally subclavate; glabrous or finely mealy with whitish granules; whitish. Context whitish; 1–2 mm thick in the hollow hymenophore; in the stipe sometimes slightly chambered near the base. Sterile inner surface whitish and pubescent. Ascospores 20–29 × 14–19 μm; elliptical; smooth; contents homogeneous. Asci 225–300 × 15–25 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 100–225 × 10–25 μm; cylindrical; apices rounded to subclavate or infrequently subfusoid; septate; hyaline to brownish in KOH (2%). Elements on sterile ridges 100–175 × 12.5–20 μm; septate; terminal cell clavate or subclavate; hyaline or with brownish contents in KOH (2%).
Ecology. Appearing at various altitudes in mixed forests dominated by various trees, including Arbutus menziesii Pursh, Quercus spp., Pseudotsuga menziesii (Mirb.) Franco, Pinus ponderosa Laws., Pinus lambertiana Dougl. and Abies concolor (Gord. & Glend.) Lindl.; California and Oregon; April. Specimens examined (Supplementary Table I) were collected in California and Oregon.
Comments. Morchella frustrata corresponds to phylogenetic species Mel-2 in CitationO'Donnell et al. (2011). The species is one of a few North American members of the M. elata Clade with pale colors that can approximate the ones traditionally associated with members of the M. esculenta Clade. However, despite its colors M. frustrata manifests the stature typical of black morels; its hymenophore is conical, its pits are vertically oriented and the hymenophore is attached to the stipe with a notable sinus. Unlike M. snyderi, which also can feature pale, M. esculenta-like colors, M. frustrata lacks a conspicuously lacunose stipe, its ridges eventually darken in old age or upon drying and its pits are glabrous rather than finely tomentose. Ascospores of M. frustrata examined for the present work were substantially smaller than those examined for M. snyderi; analysis of further collections might determine whether the difference in ascospore sizes is consistent between the species. Morchella frustrata is likely the same as the morel informally designated the “mountain blond morel” (CitationPilz et al. 2004, Citation2007).
Morchella punctipes Peck, Bull. Torrey Bot. Cl. 30:99. 1903.
Holotypus. USA, Michigan, Agricultural College, Iunius, B. O. Longyear col.; in herbarium NYS (NYSF2511) conservatum.
Epitypus (hic designatus). Biotopium sub Liriodendron tulipifera L et Fraxinus americana L; USA, in Virginiaense, ad Rappahannock County; T. Geho col.; specimen epitypicum in herbarium F (05020502) conservatum.
Etymology. Peck's epithet refers to the surface of the stipe, which often is punctated with mealy granules that can darken with maturity; he used this feature to distinguish M. punctipes from what we now know is the strictly European M. semilibera, which according to his concept, had a glabrous stipe.
Ascomata 25–180 mm high. Hymenophore 20–45 mm high; 20–45 mm wide at the widest point; broadly to narrowly conical or occasionally nearly convex; pitted and ridged; with 14–26 primary vertical ridges and infrequent shorter, secondary vertical ridges and transecting horizontal ridges; attached in a skirt-like manner to the stipe, roughly halfway from the apex, with a sinus 8–20 mm deep. Ridges glabrous; pale yellow to dull yellowish brown when young; darkening to brown, dark brown, or black with maturity; when young up to 1 mm wide, and flat with sharp edges, but with age often rounded, sharp or eroded. Pits vertically elongated; glabrous; whitish to pale yellowish when immature, becoming brownish to yellowish brown at maturity. Stipe 15–150 mm high; 8–45 mm wide; more or less equal, or tapered to apex; often hidden by the hymenophore when young, but lengthening dramatically by maturity; often developing shallow longitudinal furrows; fragile; in warm, wet conditions sometimes becoming inflated, especially near the base; white to whitish or watery brownish; occasionally nearly glabrous but more commonly mealy with whitish granules that sometimes darken to brownish or brown. Context whitish to watery tan; 1–2 mm thick in the hollow hymenophore; in the stipe sometimes chambered or layered near the base. Sterile inner surface whitish to brownish; mealy; sometimes grooved. Ascospores elliptical; smooth; contents homogeneous; 20–27 × (10–)14–18 μm; whitish to bright yellowish orange in deposit. Asci eight-spored; 175–350 × 15–25 μm; cylindrical; hyaline. Paraphyses cylindrical with rounded, subcapitate, clavate, mucronate or irregularly inflated apices; septate; hyaline in KOH (2%); 120–275 × 10–22 μm. Elements on sterile ridges 50–100 × 10–25 μm; septate; tightly packed in an even layer; brownish in KOH (2%); terminal cell broadly clavate to sub-rectangular with a flattened to broadly rounded or submucronate apex.
Ecology. Appearing in eastern North American hardwood forests, especially those containing F. americana, L. tulipifera and U. americana; widely distributed east of the Rocky Mountains (although CitationWeber and Smith 1985 reported that it is “to be expected primarily in the northern and montane parts of the South”); from late March in southern areas to late May in northern areas. Specimens examined (Supplementary table I) were collected in Illinois, Michigan, Missouri, Pennsylvania and Virginia.
Comments. Morchella punctipes corresponds to phylogenetic species Mel-4 in CitationO'Donnell et al. (2011). Most North American treatments (e.g. CitationOverholts 1934, CitationWeber 1995) regard the present species as “Morchella semilibera DC,” distinct from other morels on the basis of its “half-free” cap attachment. However, results from CitationO'Donnell et al. (2011) support two semilibera-like morels in North America (species Mel-4 and species Mel-5), morphologically similar but clearly separated on the basis of their range and ecology. Morchella populiphila (phylogenetic species Mel-5 in CitationO'Donnell et al. 2011) is a western species associated with Populus trichocarpa Torr. & Gray; the eastern species (phylogenetic species Mel-4 in CitationO'Donnell et al. 2011) is widely distributed in hardwood forests east of the Rocky Mountains. Neither is the same as the European species (phylogenetic species Mel-3 in CitationO'Donnell et al. 2011), M. semilibera. Although we were unable to obtain informative phylogenetic results from the holotype of M. punctipes Peck, Peck's description (1903) and the morphology of the holotype specimens (see M. punctipes, type studies) match the morphology of the specimens examined for the present work, which are conspecific with phylogenetic species Mel-4 in CitationO'Donnell et al. (2011).
Morchella populiphila M. Kuo, M.C. Carter & J.D. Moore, sp. nov.
Figs. 11–19 Morchella species. 11. M. populiphila HOLOTYPE F 03240401, Mel-5. 12. M. sextelata HOLOTYPE F 07130403, Mel-6. 13. M. septimelata HOLOTYPE F 06150404, Mel-7. 14. M. capitata HOLOTYPE F 08070406, Mel-9. 15. M. importuna HOLOTYPE F 04130401, Mel-10. 16. M. snyderi F 05140401, Mel-12. 17. M. angusticeps EPITYPE F 04090601, Mel-15. 18. M. brunnea F 04100401, Mel-22. 19. M. septentrionalis F 05110306, Mel-24. Bars = 5 cm. Mel numbers refer to the phylogenetic species reported in CitationO'Donnell et al. (2011).

MycoBank MB 563954
Ascomata 40–150 mm alta; capitulum conicum, remisse adherum stipiti; costae perpendicules, atrae; hymenium fulvum; biotopium in alveis occidentis Americae septentrionalis sub Populus trichocarpa Torr. & Gray; sporae 20–25 × 12–16 μm. Holotypus: Biotopium in alveo sub Populus trichocarpa Torr. & Gray; USA, in Oregonense, ad Jackson County; N. Selbicky col.; specimen typicum in Herb. F (03240401) conservatum.
Etymology. The epithet refers to the association with Populus trichocarpa.
Ascomata 40–150 mm high. Hymenophore 20–50 mm high; 20–50 mm wide at the widest point; broadly to narrowly conical; pitted and ridged, with 12–20 primary vertical ridges and infrequent shorter, secondary vertical ridges and transecting horizontal ridges, attached in a skirt-like manner to the stipe, roughly halfway from the apex, with a sinus 10–25 mm deep. Ridges glabrous; yellowish brown to honey brown when young, darkening to brown, dark brown or black with maturity; when young up to 1 mm wide and flat with sharp edges but often rounded, sharp or eroded in age. Pits vertically elongated; glabrous; whitish to pale brown when immature, becoming brownish to yellowish or grayish brown at maturity. Stipe 25–110 mm high; 10–50 mm wide; more or less equal, or tapered to apex; often hidden by the hymenophore when young but lengthening dramatically with maturity; often developing shallow longitudinal furrows; fragile; in warm, wet conditions sometimes becoming inflated, especially near the base; white to whitish or watery brownish; occasionally nearly glabrous but more commonly mealy with whitish granules that sometimes darken to brownish or brown. Context whitish to watery tan; 1–2 mm thick in the hollow hymenophore; sometimes chambered or layered near the base; fragile. Sterile inner surface whitish to brownish; mealy. Ascospores 20–25(−29) × 12–16(−18) μm; elliptical; smooth; contents homogeneous; bright yellowish orange in deposit. Asci 225–325 × 15–22.5 μm; eight-spored; cylindrical; hyaline. Paraphyses 150–275 × 7–15 μm; cylindrical with subclavate, clavate or subcapitate apices; septate; hyaline in KOH (2%). Elements on sterile ridges 100–175 × 10–25 μm; septate; tightly packed in an even layer; brownish to brown in KOH (2%); terminal cell broadly clavate to sub-rectangular with a flattened to broadly rounded apex.
Ecology. Occurring under P. trichocarpa in western North American river bottoms; distributed from Oregon to Nevada and northern California; March and April. Specimens examined (Supplementary table I) were collected in California, Nevada and Oregon.
Comments. Morchella populiphila corresponds to phylogenetic species Mel-5 in CitationO'Donnell et al. (2011). Western North American field guides (e.g. CitationSmith 1975, CitationArora 1986) regard this species as “Morchella semilibera DC”, distinct from other morels on the basis of its “half-free” hymenophore attachment, and identical to the half-free morel of eastern North America, which is phylogenetically distinct and is reported as M. punctipes Peck in the present work. On the basis of the specimens examined, comments in western field guides and the Seattle area collecting experience of one of us (Moore), the association with P. trichocarpa is consistent; the range of M. populiphila may correspond to the range of P. trichocarpa. Morchella populiphila was featured as one of two “North American Half-Free Morels” in CitationKuo (2005).
Morchella sextelata M. Kuo, sp. nov.
MycoBank MB 563955
Ascomata 40–105 mm alta; capitulum conicum; costae brunneae, interdum nigrescens; hymenium minime brunum usque minime puniceum; biotopium in silvis coniferibus incensis in occidentali America septentrionalis; sporae 18–25 × 10–16 μm. Holotypus: Biotopium in silvis coniferibus incensis; USA, in Montanaense, ad Missoula County; S. Engstrom col.; specimen typicum in Herb. F (07130403) conservatum.
Etymology. The epithet reflects the fact that this is the sixth phylogenetic species (Mel-6) in the M. elata Clade enumerated in CitationO'Donnell et al. (2011).
Ascomata 40–105 mm high. Hymenophore 25–75 mm high; 20–50 mm wide at the widest point; conical to widely conical; pitted and ridged, with 12–20 primary vertical ridges and numerous shorter, secondary vertical ridges and sunken transecting horizontal ridges; attached to stipe with a sinus about 2–4 mm deep and 2–4 mm wide. Ridges glabrous or finely tomentose; pallid when young; becoming dark grayish brown to nearly black with maturity; bluntly flattened when young, sometimes becoming sharpened or eroded with age. Pits primarily vertically elongated; glabrous; dull brownish to yellowish tan, pinkish, or nearly buff. Stipe 20–50 mm high; 10–22 mm wide; more or less equal or sometimes basally subclavate; glabrous or finely mealy with whitish granules; whitish. Context whitish; 1–2 mm thick in the hollow hymenophore; sometimes slightly chambered near the base. Sterile inner surface whitish and pubescent. Ascospores 18–25 × 10–16(−22) μm; elliptical; smooth; contents homogeneous. Asci 200–325 × 5–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 175–300 × 2–15 μm; cylindrical with rounded, subacute, subclavate or subfusoid apices; septate; hyaline in KOH (2%). Elements on sterile ridges 50–180 × 5–25 μm; septate; terminal cell cylindrical with a rounded apex, subfusoid, subcapitate or block-like; with brown to brownish contents in KOH (2%).
Ecology. Appearing at 1000–1500 m in lightly to moderately burned conifer forests, including forests dominated by Ps. menziesii and P. ponderosa. Found primarily in years immediately following forest fires but often appearing in dwindling numbers for several seasons thereafter; Washington, Idaho, Montana, Wyoming and Yukon Territory; April–July. Specimens examined (Supplementary table I) were collected in Idaho, Montana, Washington, Wyoming and Yukon Territory.
Comments. Morchella sextelata corresponds to phylogenetic species Mel-6 in CitationO'Donnell et al. (2011). From a strictly morphological perspective the species is virtually identical to several members of the M. elata Clade (M. septimelata, M. brunnea, M. angusticeps, M. septentrionalis), but because it apparently is limited to conifer burn sites in western North America it can be easily separated from all but M. septimelata, from which it is morphologically and ecologically indistinguishable on the basis of currently available data. Elements on sterile ridges in the latter species were primarily subclavate to clavate in the specimens examined, while elements in M. sextelata were cylindrical with a rounded apex, subfusoid, subcapitate or block-like, but this distinction is too tentative and based on too few specimens examined for us to express confidence that the difference is consistent. Because several of the collections studied for the present work (F 07130403, F 07070405) had pinkish pits, M. sextelata probably was included in the concept of the “pink morel” set forth in CitationPilz et al. (2004, Citation2007); however, M. septimelata specimens also demonstrated pinkish pits. Morchella sextelata was treated in CitationKuo (2005) as one of several “Other North American Black Morels” appearing in burn sites.
Morchella septimelata M. Kuo, sp. nov.
MycoBank MB 563956
Ascomata 75–200 mm alta; capitulum conicum; costae brunneae, interdum nigrescens; hymenium minime brunum usque leviter veridis aut minime puniceum; biotopium in silvis coniferibus incensis in occidenti America septentrionalis; sporae 18–25 × 10–15 μm. Holotypus: Biotopium in silvis coniferibus incensis; USA, in Montanaense, ad Mineral County; S. Engstrom col.; specimen typicum in Herb. F (06150404) conservatum.
Etymology. The epithet reflects the fact that the species is the seventh phylogenetic species (Mel-7) in the M. elata Clade enumerated in CitationO'Donnell et al. (2011).
Ascomata 75–200 mm high. Hymenophore 40–100 mm high; 30–70 mm wide at the widest point; conical to subconical; pitted and ridged, with 14–22 primary vertical ridges and numerous shorter, secondary vertical ridges and transecting horizontal ridges; attached to stipe with a sinus about 1–3 mm deep and 1–3 mm wide. Ridges glabrous or finely tomentose; brownish to brown when young, becoming dark brown to black at maturity; bluntly flattened when young but sometimes becoming sharpened or eroded with age. Pits primarily vertically elongated; glabrous; progressing from olive to olive-brown, pinkish or brownish when immature to brownish or tan at maturity. Stipe 35–100 mm high; 20–50 mm wide; occasionally basally subclavate; flared slightly to apex; mealy with whitish granules; white, darkening to brownish in older specimens. Context whitish; 1–2 mm thick in the hollow hymenophore; sometimes slightly chambered near the base. Sterile inner surface whitish and pubescent. Ascospores (17–)18–25(−30) × 10–15(−20) μm; elliptical; smooth; contents homogeneous. Asci 175–275 × 12–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 100–200 × 5–12.5 μm; cylindrical; apices subacute or subfusiform (occasionally merely rounded, or subclavate); septate; hyaline in KOH (2%). Elements on sterile ridges 60–200 × 7–18 μm; septate; with brown contents in KOH (2%); cylindrical; terminal cell clavate (rarely subcapitate or irregular).
Ecology. Appearing at 1000–2000 m in lightly to moderately burned conifer forests, often near creek beds, springs and seeps. Found primarily in years immediately following forest fires but often appearing in dwindling numbers for several seasons thereafter; April–July. Specimens examined (Supplementary table I) were collected in British Columbia, Montana and Oregon.
Comments. Morchella septimelata corresponds to phylogenetic species Mel-7 in CitationO'Donnell et al. (2011). On the basis of present data the species is morphologically and ecologically indistinguishable from M. sextelata. (See the comments for the latter species for further information and comparison with other elata-like species.) Because several of the M. septimelata collections studied had greenish pits (F 06150404, F 07140404, F 07070401, F 07070408) or pinkish pits (F 07070408), M. septimelata probably has been included in commercial collectors' concept of the “pickle”, and in the concepts of the “pink morel” and the “green morel” set forth in CitationPilz et al. (2004, Citation2007); however, several M. capitata specimens also had greenish pits and specimens of M. sextelata also had pinkish pits. Morchella septimelata was treated in CitationKuo (2005) as one of several “Other North American Black Morels” appearing in burn sites.
Morchella sp. Mel-8
Commentary and a description of the sole ascoma representing this phylogenetic species for the present work are included (Supplementary materials). A formal description is deferred pending identification of further specimens.
Morchella capitata M. Kuo & M.C. Carter, sp. nov. , Supplementary fig. 4
MycoBank MB 563957
Ascomata 60–110 mm alta; capitulum conicum vel subconicum; costae brunneae, interdum nigrescens; hymenium aerugineum vel fulvum; biotipium in silvis coniferibus incensis; sporae 18–25 × 12–17 μm; elementa in costis sterilibus capitata. Holotypus: Biotopium in silvis coniferibus incensis; 1200 m. altitudinis; USA, in Oregonense, ad Jefferson County; M.C. Carter col.; specimen typicum in Herb. F (08070406) conservatum.
Etymology. The epithet refers to the capitate elements on the sterile ridges.
Ascomata 60–110 mm high. Hymenophore 40–80 mm high; 25–80 mm wide at the widest point; conical to subconical (occasionally subglobose); pitted and ridged, with 18–28 primary vertical ridges and numerous shorter, secondary vertical ridges, often with prominent and regular transecting horizontal ridges; attached to stipe with a sinus about 1–7 mm deep and 1–7 mm wide. Ridges glabrous or finely tomentose; dull olive, brown, brownish black or brownish when young, becoming dark brown to black at maturity; somewhat flattened when young but with age sometimes becoming sharpened or eroded. Pits primarily vertically elongated; glabrous; progressing from olive to olive brown or brownish when immafoture to brownish or tan at maturity. Stipe 25–50 mm high; 20–50 mm wide; occasionally basally subclavate; often mealy with whitish granules; whitish, darkening to brownish in older specimens. Context whitish; 1–2 mm thick in the hollow hymenophore; in the stipe sometimes layered or chambered, especially near the base. Sterile inner surface whitish and pubescent. Ascospores 18–25 × 12–17(−19) μm; elliptical; smooth; contents homogeneous. Asci 175–300 × 15–27 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 125–225 × 7.5–13 μm; cylindrical; septate; apices subclavate, subfusiform or merely rounded (rarely subcapitate); hyaline to faintly brownish in KOH (2%). Elements on sterile ridges 75–200 × 10–35 + μm; septate; brown in KOH (2%); terminal cell usually subcapitate, capitate or strongly swollen and subglobose but occasionally clavate or merely cylindrical with a rounded apex.
Ecology. Appearing at approximately 1200 m in lightly burned spruce and fir forests. Found in the year following summer forest fires; August. Specimens examined (Supplementary table I) were collected in Oregon.
Comments. Morchella capitata corresponds to phylogenetic species Mel-9 in CitationO'Donnell et al. (2011). Among the confusingly similar burn-site morels in western North America, Morchella capitata is distinct microscopically, based on the specimens examined; elements on its sterile ridges are overwhelmingly capitate, often dramatically so (Supplementary fig. 4). Specimens we studied of M. sextelata and M. septimelata, which are nearly identical to M. capitata in macroscopic features and which also occur in conifer burn sites, had sterile ridge elements from cylindrical with a rounded apex to subclavate, subfusiform or subcapitate. Because several of the collections studied (08070401, 08070408) had greenish pits, M. capitata probably has been included in commercial collectors' concept of the “pickle” and in the concept of the “green morel” set forth in CitationPilz et al. (2004, Citation2007); however, several M. sextelata and M. septimelata specimens also had greenish pits. Morchella capitata was treated in CitationKuo (2005) as one of several “Other North American Black Morels” appearing in burn sites.
Morchella importuna M. Kuo, O'Donnell & T.J. Volk, sp. nov.
MycoBank MB 563958
Ascomata 60–200 mm alta; capitulum conicum vel ovatum; costae aequabiles, perpendicules, atrae, similes scalae; hymenium fulvum; biotopium in hortis et terra perturbata; sporae 18–24 × 10–13 μm. Holotypus: Biotopium in horto; USA, in Washingtonense, ad King County; A. Thrailkill col.; specimen typicum in Herb. F (04130401) conservatum.
Etymology. The epithet means “assertive” or “inconsiderate” in character; the species often is the cause of consternation and distress among gardeners and homeowners whose territory has been invaded.
Ascomata 60–200 mm high. Hymenophore 30–150 mm high; 20–90 mm wide at the widest point; conical to widely conical or occasionally ovoid; pitted and ridged, with 12–20 primary vertical ridges and numerous transecting horizontal ridges, creating a laddered appearance; attached to stipe with a sinus about 2–5 mm deep and 2–5 mm wide. Ridges glabrous or finely tomentose; pale to dark gray when young, becoming dark grayish brown to nearly black with maturity; bluntly rounded when young, but with age becoming sharpened or eroded. Pits vertically elongated in all stages of development; glabrous or finely tomentose; opening and deepening with development; progressing from gray to dark gray when immature to grayish brown, grayish olive or brownish yellow at maturity. Stipe 30–100 mm high; 20–60 mm wide; often basally clavate to subclavate; glabrous or finely mealy with whitish granules; developing longitudinal ridges and channels with maturity, especially basally; whitish to pale brownish. Context whitish to watery tan; 1–3 mm thick in the hollow hymenophore; in the stipe sometimes chambered or layered. Sterile inner surface whitish and pubescent. Ascospores 18–24 × 10–13 μm; elliptical; smooth; contents homogeneous. Asci 220–300 × 12–25 μm; eight-spored; cylindrical; hyaline. Paraphyses 150–250 × 7–15 μm; septate; cylindrical with rounded to subclavate, subcapitate, subacute or subfusoid apices; hyaline or brownish in KOH (2%). Elements on sterile ridges 125–300 × 10–30 μm; septate; terminal cell cylindrical with a rounded apex, subclavate, clavate, subcapitate or subfusiform; hyaline or brownish to brown in KOH (2%).
Ecology. Appearing in gardens, planters, woodchip beds and urban landscaping settings in the Pacific Northwest and northern California; March–May. Specimens examined (Supplementary table I) were collected in British Columbia, California, Nevada, Oregon and Washington.
Comments. Morchella importuna corresponds to phylogenetic species Mel-10 in O'Donnell et al. (2010). The species is distinguished from other morels in the M. elata Clade on the basis of its regularly laddered, vertically oriented pits and ridges, combined with its urban habitat in landscaping areas, planters, woodchip beds and gardens, primarily in western North America. One of us (Volk) collected morels matching the morphology of M. importuna in a woodchip bed on the campus of the University of Wisconsin at La Crosse in 1999. Morchella hotsonii Snyder (so far known only from the 1935 type collection) is similar and also apparently appeared in woodchip beds in the Pacific Northwest, although it differs morphologically by having finely tomentose surfaces (Supplementary materials, Type studies, for further information). Morchella importuna was treated and illustrated erroneously in CitationKuo (2005) as conspecific with the “Classic North American Black Morel”; the top-middle, top-right and bottom-middle photos on p 179 actually represent M. importuna.
Morchella snyderi M. Kuo & Methven, sp. nov.
MycoBank MB 563959
Ascomata 60–140 mm alta; capitulum conicum; costae pallidae, brunnescens aetate; hymenium sufflavum vel fulvum; stipes lacunosus aetate; biotopium in silvis coniferibus in occidenti America septentrionalis; sporae 25–37 × 15–23 μm. Holotypus: Biotopium in silvis coniferibus; USA, in Idahoense, ad Kootenai County; N. S. Weber 6554 col.; specimen typicum in Herb. OSC (139277) conservatum.
Etymology. The epithet honors Leon Carlton Snyder (1908–1987), who named M. hotsonii and M. crassistipa from Washington state in the 1930s.
Ascomata 60–140 mm high. Hymenophore 35–80 mm high; 30–50 mm wide at the widest point; conical; pitted and ridged, with 16–22 primary vertical ridges and frequent shorter, secondary vertical ridges, with occasional sunken, transecting horizontal ridges; attached to stipe with a sinus about 2–4 mm deep and 2–4 mm wide. Ridges glabrous or very finely tomentose; pale yellowish, becoming pale tan, then grayish brown with maturity and darkening to nearly black when dried; flattened when young but with age sometimes becoming sharpened or eroded. Pits more or less vertically elongated, at least at maturity; finely tomentose; yellowish when young, becoming pale tan to pale grayish brown. Stipe 35–70 mm high; 25–40 mm wide; more or less equal, or sometimes basally subclavate; at first finely mealy with whitish granules, becoming prominently granulated; whitish to pale brownish; usually becoming prominently ridged and/or lacunose with maturity. Context whitish; 1–2 mm thick in the hollow hymenophore, becoming layered and chambered, especially near the base of the stipe. Sterile inner surface whitish and pubescent. Ascospores 25–37 × 15–23 μm; elliptical; smooth; contents homogeneous. Asci 225–300 × 17.5–32.5 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 100–200 × 7.5–20 μm; cylindrical; apices rounded to subclavate, clavate, or occasionally subcapitate or widely fusiform; septate; hyaline to faintly brownish in KOH (2%). Elements on sterile ridges 75–175 × 10–20 μm; septate; terminal cell subclavate, clavate, subcapitate or widely fusiform; with hyaline to brownish or brown contents in KOH (2%).
Ecology. Appearing under non-burned, montane conifers, including Ps. menziesii, P. ponderosa and A. concolor; April, May and early June. Specimens examined (Supplementary table I) were collected in California, Idaho, Montana, Oregon and Washington.
Comments. Morchella snyderi corresponds to phylogenetic species Mel-12 in CitationO'Donnell et al. (2011). A combination of ecological and morphological features separates the species: it appears in non-burned conifer forests in western North America; its stipe is often ridged and lacunose (although the stipes of many morels can become ridged and sublacunose, especially near the base, when mature, the stipe of M. snyderi is often prominently lacunose and ridged throughout most of its development) and prominently granulated; the colors of the hymenophore approximate those of esculenta-like morels when young, but the ridges become smoky brown to black with development or upon drying; its hymenophore is conical, with longitudinally arranged pits and a sinus at the point of attachment to the stipe; and its ascospores are comparatively large. Young specimens of M. snyderi can appear similar to M. frustrata, but the latter species has ridges that do not darken and has smaller ascospores. Morchella brunnea also is similar, but its hymenophore is more brown when young and its stipe is not regularly lacunose. We found one ascoma fragment in the putative holotype of M. crassistipa Snyder to be phylogenetically conspecific with M. snyderi, but we were unable to recognize Snyder's name because the type collection was mixed as to species (see Supplementary materials, Type studies, for full discussion). Morchella snyderi was not treated in CitationKuo (2005).
Morchella angusticeps Peck, Ann. Rep. New York St. Mus. 32:44. 1879. , Supplementary fig. 5
Holotypus. USA, New York, West Albany et Center, Aprilis et Maius, C. H. Peck col.; in herbarium NYS (NYSF268) conservatum.
Epitypus (hic designatus). Biotopium sub Fraxinus americana; USA, in Illinoisense, ad Coles County; M. Kuo col.; specimen epitypicum in herbarium F (04090601) conservatum.
Etymology. Peck's epithet refers to the narrow hymenophore.
Ascomata 50–140 mm high. Hymenophore 30–80 mm high; 25–50 mm wide at the widest point; conical or bluntly conical; pitted and ridged, with 16–24 primary vertical ridges and occasional shorter, secondary vertical ridges, with frequent sunken, transecting horizontal ridges; attached to stipe with a sinus about 2–5 mm deep and 2–5 mm wide (however, at maturity the stipe sometimes becomes swollen, obscuring the sinus). Ridges finely tomentose; tan to dark brown, blackening with maturity; flattened when young but with age sometimes becoming sharpened or eroded. Pits primarily vertically elongated; glabrous; pale tan to dull brownish yellow (occasionally with olive shades). Stipe 20–80 mm high; 10–30 mm wide; more or less equal or sometimes basally subclavate to clavate; finely mealy with whitish granules; whitish to pale brownish; developing folds and channels, especially near the base, at maturity; in warm, wet conditions sometimes becoming swollen to reach, or nearly reach, the width of the hymenophore, obscuring the sinus at the point of attachment. Context whitish; 1–2 mm thick in the hollow hymenophore; in the stipe becoming layered and chambered near the base. Sterile inner surface whitish and pubescent. Ascospores 22–27 × 11–15 μm; elliptical; smooth; contents homogeneous. Asci 225–400 × 17.5–30 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 125–250 × 5–12.5 μm; septate; cylindrical with a rounded, subclavate, clavate, subcapitate, or occasionally widely fusiform apex; hyaline in KOH (2%). Elements on sterile ridges 100–200 × 7.5–35 μm; septate; terminal cell widely cylindrical with a rounded apex, clavate, subcapitate or occasionally irregular; with hyaline to brownish or brown contents in KOH (2%).
Ecology. Appearing under diverse hardwoods, especially F. americana and L. tulipifera; widely distributed east of the Rocky Mountains; March, April and May. Specimens examined (Supplementary table I) were collected in Arkansas, Illinois, Massachusetts, Mississippi, Pennsylvania and West Virginia.
Comments. Morchella angusticeps corresponds to phylogenetic species Mel-15 in CitationO'Donnell et al. (2011). Results from CitationO'Donnell et al. (2011) support two elata-like morels in North America east of the Rocky Mountains. One of these (Mel-24 in CitationO'Donnell et al. 2011) is Morchella septentrionalis, characterized by its small size (ascomata 40–75 mm high), small ascospores ([19–]20–22[−25] × 11–15 μm), northern distribution (from about 44°N northward) and frequent association with the deadwood of hardwoods (especially that of P. grandidentata); the second (Mel-15 in CitationO'Donnell et al. 2011) has larger ascomata and ascospores and is widely distributed east of the Rocky Mountains. Although we were unable to obtain informative phylogenetic results from the holotype of M. angusticeps, Peck's description and illustration (1879) and the macromorphology of the holotype roughly match the specimens examined and cited above, which are phylogenetically consistent with Mel-15 in CitationO'Donnell et al. (2011). However, there are several potentially unresolved complications presented by the holotype and by Peck's description (see Supplementary materials, type studies). From a strictly morphological perspective M. angusticeps is virtually indistinguishable from several western North American species, including M. sextelata, M. septimelata and Morchella sp. Mel-8 (all of which occur in conifer burn sites), as well as M. brunnea, which appears in unburned forests. Morchella angusticeps has been treated as M. angusticeps by many authors (e.g. CitationSeaver 1928, CitationOverholts 1934, CitationWeber 1995) who probably combined it with M. septentrionalis. It appeared as the “Classic North American Black Morel” in CitationKuo (2005), where it was combined erroneously with M. importuna. We have selected a robust, contemporary collection (F 04090601) as an epitype representative of our concept of the phylogenetic species we are labeling M. angusticeps.
Morchella brunnea M. Kuo, sp. nov.
MycoBank MB 563960
Ascomata 45–90 mm alta; capitulum conicum; costae fulvum, nigrescentes aetate; hymenium fulvum, subtiliter tomenteum; biotopium in silva in occidenti America septentrionalis; sporae 22–36 × 14–20 μm. Holotpus: Biotopium in silvis; USA, in Oregonense, ad Jefferson County; N.S. Weber 6199 col.; specimen typicum in Herb. OSC (138686) conservatum.
Etymology. The epithet refers to the brown hymenophore.
Ascomata 45–90 mm high. Hymenophore 30–50 mm high; 25–35 mm wide at the widest point; conical; pitted and ridged, with 16–22 primary vertical ridges and occasional shorter, secondary vertical ridges, with frequent sunken, transecting horizontal ridges; attached to stipe with a sinus about 2–3 mm deep and 2–3 mm wide. Ridges glabrous or finely tomentose; dark brown to nearly black, blackening with maturity and when dried; flattened when young but sometimes becoming sharpened or eroded with age. Pits primarily vertically elongated; finely tomentose; pale tan to brownish yellow. Stipe 20–35 mm high; 8–15 mm wide; more or less equal, or sometimes basally subclavate; finely mealy with whitish granules; whitish; developing fine ridges, or a few folds near the base, with maturity. Context whitish; 1–2 mm thick in the hollow hymenophore, becoming layered and chambered near the base of the stipe. Sterile inner surface whitish and pubescent. Ascospores 22–36(–40) × 14–20(−25) μm; elliptical; smooth; contents homogeneous. Asci 225–300 × 17.5–22.5 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 150–250 × 7.5–17.5 μm; cylindrical; apices rounded to subclavate, clavate, or widely fusiform; septate; hyaline in KOH (2%). Elements on sterile ridges 75–160 × 12.5–27.5 μm; septate; terminal cell clavate (sometimes strikingly so), subcapitate or widely subfusiform; with hyaline to brownish contents in KOH (2%).
Ecology. Appearing under hardwoods, including A. menziesii and Quercus spp.; probably also to be expected in non-burned conifer forests; April. Specimens examined (Supplementary table I) were collected in Oregon.
Comments. Morchella brunnea corresponds to phylogenetic species Mel-22 in CitationO'Donnell et al. (2011). The species is morphologically similar to several morels in the M. elata Clade (including M. sextelata, M. septimelata, M. capitata, Morchella sp. Mel-8, M. angusticeps, M. septentrionalis) but can be differentiated when its ecology (in non-burned forests) and distribution (in western North America) are considered. Among the species that are similar in appearance to M. brunnea, only the poorly known Morchella sp. Mel-8 apparently inhabits similar western habitats. The sole specimen we examined for Morchella sp. Mel-8 had glabrous pits, as compared to the finely tomentose pits of M. brunnea specimens examined; further collections of both phylogenetic species may determine whether this difference is consistent. Morchella brunnea is mentioned as M. angusticeps in CitationWeber (1995) and is treated as the “natural black morel” in CitationPilz et al. (2004, Citation2007). It was treated as one of several “Classic North American Black Morels” in CitationKuo (2005).
Morchella septentrionalis M. Kuo, J.D. Moore & Zordani, sp. nov.
MycoBank MB 563961
Ascomata 40–75 mm alta; capitulum conicum vel subclavatum; costae fuscae, nigrescentes aetate; hymenium fulvum usque fuscum; biotopium in silva frondosa in alta America septentrionalis; sporae 20–22 × 11–15 μm. Holotypus: Biotopium sub Populus grandidentata Michx.; USA, in Michiganense, ad Cheboygan County; R. Zordani col.; specimen typicum in Herb. F (05110405) conservatum.
Etymology. The epithet reflects the northern distribution of the species.
Ascomata 40–75 mm high. Hymenophore 30–45 mm high; 15–25 mm wide at the widest point; conical, bluntly conical, or subclavate; pitted and ridged, with 14–20 primary vertical ridges and occasional shorter, secondary vertical ridges, with occasional or frequent sunken, transecting horizontal ridges; attached to stipe with a sinus about 2–3 mm deep and 2–3 mm wide. Ridges finely tomentose or nearly glabrous; tan to brown or dark brown, blackening with maturity; flattened when young but with age sometimes becoming sharpened or eroded. Pits primarily vertically elongated; glabrous; pale tan to dull brownish yellow or grayish brown. Stipe 20–30 mm high; 8–15 mm wide; more or less equal or sometimes basally subclavate; finely mealy with whitish granules; whitish to pale brownish; sometimes developing a few folds near the base with maturity. Context whitish; up to about 1 mm thick. Sterile inner surface whitish and pubescent. Ascospores (19–)20–22(−25) × 11–15 μm; elliptical; smooth; contents homogeneous. Asci 225–350 × 15–30 μm; eight-spored; cylindrical; hyaline in KOH (2%). Paraphyses 75–225 × 7.5–17.5 μm; septate; cylindrical with a rounded, subacute, subclavate, clavate, subfusiform, sublecythiform, subcapitate or occasionally irregular apex; hyaline in KOH (2%). Elements on sterile ridges 75–175 × 7.5–25 μm; septate; terminal cell widely cylindrical with a rounded apex, subclavate, clavate, widely fusiform, utriform, or occasionally irregular; with hyaline to brownish or brown contents in KOH (2%).
Ecology. Appearing under hardwoods, especially P. grandidentata and F. americana; usually growing near woody debris or growing directly from rotted hardwoods; known from north of 44°N; May. Specimens examined (Supplementary table I) were collected in Michigan and New York.
Comments. Morchella septentrionalis corresponds to phylogenetic species Mel-24 in CitationO'Donnell et al. (2011). Based on available data the species can be characterized by the comparatively small size of the ascomata, its northern distribution in eastern North America (from approximately 44°N northward), its frequent association with the deadwood of hardwoods and its relatively small ascospores. It is easily confused with M. angusticeps, which is morphologically similar and overlaps with M. septentrionalis in range, size of ascomata and ascospores. (See the comments for M. angusticeps for further discussion.) Morchella septentrionalis probably has been misidentified as M. angusticeps (e.g. CitationSeaver 1928, CitationOverholts 1934, CitationWeber 1995). It appears as the “Classic North American Black Morel II” in CitationKuo (2005).
Key to 20 phylogenetic species of Morchella in the United States and Canada
umyc_a_11831932_sm0001.pdf
Download PDF (147.1 KB)umyc_a_11831932_sm0002.pdf
Download PDF (144.2 KB)umyc_a_11831932_sm0003.pdf
Download PDF (322.8 KB)Acknowledgments
We are grateful for the help of Cathie Aime, Cathy Cripps, Darvin DeShazer, Ron Kerner and Carol Schmudde for presubmission reviews. We thank Stacy Sink who provided excellent technical assistance and John Haines who examined Peck's type collections and provided morphological data.
We wish to thank and acknowledge Nancy Weber for her many years of invaluable contributions to the study of Morchella in North America and to mycological study in general.
We are grateful for the following collectors who contributed specimens and data cited in this work to the MDCP: Terry Allen, William Andrew, Keith Arquilla, Rex Bartlett, Dent Benjamin, Lesley Bergemann, Glenn Brown, Tommy Chiu, Norris Coit, Darvin DeShazer, Tanya Duchild, Tom Emig, Chris England, Scott Engstrom, Gretchen Fitzgerald, Tim Geho, Jim George, Ken Greger, Gaston Guzmán, Bill Hartwig, Donna Herbold, Matthew Hill, John Holmes, Julia Hoskins, John Jennemann, Sherry Kay, Dan Kimberling, Toff Kobylarz, Dave Kowalishen, Judy Latchaw, Curt Leitz, Jim Lessard, Rowlin Lichter, Douglas Ling, Jeff Linkinhoker, Patrick Lyon, Ray Mason, James Mattan, Cornelius McHugh, Ron Meyers, Keith Miller, Nathan Mueller, Mary Munch, Kristin Musgnug, Bill Neill, Carl Nielsen, Carolina Nurik, Miles Oleskiw, Ron Pastorino, Greg Pecchia, Jamie Petersen, John Plischke, Judy Provo-Klimek, Rebecca Rader, Morgan Sailors, John Schaefer, Wendy Schaefer, Floyd Schmidt, Steve Schwartzman, Bob Sears, Neil Selbicky, Tony Sepulveda, Robert Sharman, Mike Sheller, Noah Siegel, Scott Smith, Dave Steortz, Greg Stevenson, Don Stokes, Jeanne Storm, Alan Thrailkill, Dave Trummer, Nancy Walker, Mike Wood, Joe Ziolkowski and Bob Zordani.
We thank the following individuals and herbaria for kindly lending or processing specimens: the Stover-Ebinger Herbarium at Eastern Illinois University (EIU, Gordon Tucker, curator), the Harry D. Thiers Herbarium of San Francisco State University (SFSU, Dennis Desjardin, curator), the Mycology Collection of the Field Museum of Natural History (F, Robert Lücking, curator), the Oregon State University Mycological Collection (OSC, Joey Spatafora, curator), the University Herbarium of the University of California (UC, Tom Bruns, curator), the University of Washington Herbarium (WTU, Richard Olmstead, curator) and the U.S. National Fungus Collections (BPI, Shannon Dominick, curator).
We also thank Joe Ammirati, Alija Bajro Mujic, Dick Bishop, Roy Halling, Patrick Leacock, Lorinda Leonardi, Drew Minnis, Tom Nauman, Vicky Nauman, Lorelei Norvell, Dennis Oliver, Ron Petersen, Alice Piller, Jean Toothman and Sue Yocum for invaluable assistance in various aspects of this study.
Studies of morels in National Capital Region Parks were supported by Cooperative Agreement H3992050001 (Protecting resources: sustaining wild mushrooms in four NCR Parks) from the National Park Service, which provided financial support to the University of Arkansas and (through sub-award agreement SA0604193) to the University of Toronto. The logistical support provided by park personnel on many different aspects of the overall project is greatly appreciated.
The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned. The United States Department of Agriculture is an equal opportunity provider and employer.
Literature cited
- AroraD. 1986. Mushrooms demystified: a comprehensive guide to the fleshy fungi. Berkeley, California: Ten Speed Press. 959 p.
- FriesEM. 1822. Systema mycologicum. Vol. 2. Reprint, Italy: Confederatio Europaea Mycologiae Mediterraneensis, 1994. 621 p.
- GuzmánGTapiaF. 1998. The known morels in Mexico, a description of a new blushing species, Morchella rufobrunnea, and new data on M. guatemalensis. Mycologia 90:705–714, doi:10.2307/3761230
- KuoM. 2005. Morels. Ann Arbor: Univ. Michigan Press. 205 p.
- KuoM. 2008. Morchella tomentosa, a new species from western North America, and notes on M. rufobrunnea. Mycotaxon 105:441–446.
- LincoffG, ed. 1981. Simon & Schuster's guide to mushrooms. New York: Simon & Schuster Inc. 512 p.
- McFarlaneEMPilzDWeberNS. 2005. High-elevation gray morels and other Morchella species harvested as non-timber forest products in Idaho and Montana. Mycologist 19:62–68, doi:10.1017/S0269915X0500203X
- McKnightKHMcKnightVB. 1987. A field guide to mushrooms of North America. Peterson Field Guides. Boston, Massachusetts: Houghton Mifflin. 429 p.
- O'DonnellKRooneyAPMillsGLKuoMWeberNSRehnerSA. 2011. Phylogeny and historical biogeography of true morels (Morchella) reveals an early Cretaceous origin and high continental endemism and provincialism in the Holarctic. Fungal Genet Biol 48:252–265, doi:10.1016/j.fgb.2010.09.006
- OverholtsLO. 1934. The morels of Pennsylvania. Proc Acad Sci (PA) 8:108–114.
- PeckCH. 1879. Report of the botanist. Annu Rep NY St Mus 32:17–72.
- PeckCH. 1903. New species of fungi. Bull Torrey Bot Cl 30:95–101, doi:10.2307/2478879
- PersoonCH. 1801. Synopsis methodica fungorum. Göttingen.
- PersoonCH. 1818. Traité sur les champignons comestibles. Paris.
- PilzDMcLainRAlexanderSVillareal-RuizLBerchSWurtzTLParksCGMcFarlaneEBakerBMolinaRSmithJE. 2007. Ecology and management of morels harvested from the forests of western North America. USDA Gen. Tech. Rep. 161 p.
- PilzDWeberNSCarterMCParksCGMolinaR. 2004. Productivity and diversity of morel mushrooms in healthy, burned and insect-damaged forests of northeastern Oregon. For Ecol Manage 198:367–386, doi:10.1016/j.foreco.2004.05.028
- SeaverFJ. 1928. The North American cup-fungi (operculates). New York: Hafner Publishing Co. 377 p.
- SmithAH. 1975. A field guide to western mushrooms. Ann Arbor: Univ. Michigan Press. 279 p.
- SnyderLC. 1938. The operculate Discomycetes of western Washington. Univ Wash Pub Biol 8:1–64.
- StefaniFOPSokolskiSWurtzTLPichéYHamelinRCFortinJABerube′JA. 2010. Morchella tomentosa: a unique belowground structure and a new clade of morels. Mycologia 102:1082–1088, doi:10.3852/09-294
- TaşkınHBüyükalacaSDoğanHHRehnerSAO'DonnellK. 2010. A multigene molecular phylogenetic assessment of true morels (Morchella) in Turkey. Fungal Genet Biol 47:672–682, doi:10.1016/j.fgb.2010.05.004
- TaşkınHBüyükalacaSHansenKO'DonnellK. 2012. Multilocus phylogenetic analysis of true morels (Morchella) reveals high levels of endemics in Turkey relative to other regions of Europe. Mycologia. (In press).
- WeberNS. 1995. A morel hunter's companion: a guide to the true and false morels. Lansing, Michigan: Thunder Bay Press. 209 p.
- WeberNSSmithAH. 1985. A field guide to southern mushrooms. Ann Arbor: Univ. Michigan Press. 280 p.