Abstract
A survey of wild boar shot during two consecutive years (hunting seasons 2002–2004) was carried out in order to evaluate which somatic measurements are most significant in identifying and discriminating among different morphotypes in central Italy. Biometric data from 688 wild boars was collected in three different areas of central Italy, two in Viterbo and one in the Province of Rieti. The following somatic measurements were individually recorded for each specimen: head-body length, height at withers, hind-foot length, ear length, ear-snout distance and ear-shoulder distance. Body weight was registered, and age was estimated from tooth eruption and wear. The animals were divided into three age classes; young (aged less then 12 months), sub-adults (aged between 12 and 36 months), and adults (36 months and older). After a preliminary ANOVA procedure, which did not give satisfactory results, a statistical analysis was performed using a canonical discriminant procedure, given an a priori classification (geographical area) and several quantitative variables (somatic measurements and weight). The separation between areas was estimated calculating the squared distance of Mahalanobis. The data referring to all 688 specimens was subjected to factor analysis. The results of the canonical discriminant analysis highlight the existence of two distinct groups within all three age classes. There is a statistically significant difference between the southern-Maremma (SM) vs the Apennine (A) and sub-Apennine (SA) areas, for young (P<0.0001), sub-adults (P<0.001) and adults (P<0.001). The difference between the A and SA areas was significant only for sub-adults (P<0.05). The first canonical variable account for 92.5, 92.7 and 89.9% of the total variance for the three age classes respectively, but this is unequally correlated with the original variables suggesting that the separation between the two areas is due to differences in conformation rather than in body size. On the basis of the discriminant analysis large part of the animals were correctly categorised in the sampling areas. As regards the factor analysis results for the adult group, the first three common factors are able to explain 78, 92, and 64% of the covariance for the data of the SM, A and SA groups respectively. These results suggest that, for the SM group, a differentiation among morphotypes may be possible on the basis of a few somatic measurements. These results confirm the need for biochemical and genetic studies to identify if the different morphotypes refer to the autochthonous wild boar strain.
Introduction
Wild boar numbers in Europe underwent a severe decline from the Middle Ages to the Second World War. In contrast, in the post-war period a massive expansion took place both in Europe and in Italy (CitationDe Beaux and Festa, 1927; CitationBoitani et al., 1995a; CitationDanilkin, 2001) due to many cofactors difficult to isolate, including land cover changes, feed availability and land management (CitationSchley and Roper, 2003; CitationBieber and Ruf, 2005; CitationGeisser and Reyer, 2005). The current increase in wild boar numbers and their expansion, into unusual habitats (CitationCocca et al., 2007) as well as throughout Italy (CitationAmici et al., 2008), have important management implications concerning crop damage (CitationGeisser and Reyer, 2004) and vehicle collision (CitationPrimi et al., 2009). The spread of wild boar was due to the massive restocking which was done using animals from central and eastern Europe (CitationSaez-Royuela and Telleriia, 1986; CitationApollonio et al., 1988) and spontaneous colonisation from France. The reproductive potential of animals from central Europe (CitationNahlik and Sandor, 2003) is of major importance. They show a higher prolificacy in comparison to autochthonous Italian wild boar (CitationPerco, 1987) and this plays an important role in population increase (CitationMassei and Toso, 1993).
According to CitationRandi (1995), Italian and European wild boar can be distinguished into different genotypes on the basis of mitochondrial DNA analyses. Nevertheless, the systematics of wild boar is controversial and recent studies have shown a limited impact of the massive restocking on mitochondrial DNA translocations (CitationVernesi et al., 2003).
Firstly, it should be underlined that the wild boar populations of central Italy have undergone a massive increase, greater than in other European countries. Secondly, it is interesting to note that this expansion has (probably) helped to maintain pre-glacial diversity (CitationScandura et al., 2008). In fact, as reported by CitationScandura et al. (2008), restocking with non-autochthonous animals has not produced a strong impact on genetic variation, thus only 7% of the individuals studied was characterised by having a significant proportion of their genome related to central European wild boar. These considerations support the adoption of species management policies aimed at avoiding both accidental escapes from wild boar breeding farms and hybridisation with free-range domestic pigs (CitationScandura et al., 2008). This also confirms a great interest for local group conservation since genetic differences are evident among wild boar populations in Italy (CitationVernesi et al., 2003).
In recent decades, several studies on wild boar morphology have been performed with the aim of distinguishing between autochthonous and introduced genotypes (CitationGenov et al., 1995; CitationTinelli et al., 1999), studying growth patterns ( CitationGallo Orsi et al., 1995; CitationPedone et al., 1995) and obtaining a morphological characterisation (CitationMassei and Genov, 1993). A large part of these studies was based on craniometrical measures only (CitationGenov et al., 1991; CitationGallo Orsi et al., 1995; CitationGenov, 2004); even if this type of measurement is one of the most adequate systematic techniques, it has two main limitations. First, it requires very accurate measurements, implying the use of appropriate equipment. Secondly, such measurements are difficult to perform in field conditions, and the specimens should be transferred to a laboratory. Moreover, in the case of wild boar, hunters tend to hand over females heads only, since males heads are in demand for trophies (CitationRandi et al., 1987). On the basis of the results reported in a large part of the studies performed in Italy in order to discriminate the Sus scrofa majori, it is possible to hypothesise that a limited number of somatic measurements match the differences among the phenotypes (CitationAmici et al., 2003, Citation2005; CitationAdriani et al., 2005). The aim of the present study was i) to determine which somatic measurements allow different morphotypes to be distinguished; ii) to select the somatic measurements to be registered after drive hunting sessions for the purpose of monitoring wild boar population morphotypes; and iii) to point out the different morphotypes for further genetic analysis.
Materials and methods
Biometric data from 688 wild boars (319 males, 379 females) were collected in three different areas of central Italy (), during two consecutive years (2002–2003 and 2003–2004, the hunting period lasting from November throughout January). The first of the three areas is a flat zone of the southern-Maremma (SM) in the Province of Viterbo, the second is a hilly sub-Apennine (SA) area (Province of Viterbo) near the borders with Tuscany and Umbria. The third, in the province of Rieti, is a mountainous zone of the Apennines (A) near the Abruzzo border. The first two are divided by a wide urban area and intensively cultivated lands. All three zones match wild boar habitat preferences (CitationAbaigar et al., 1994; CitationFonseca, 2008).
The data were collected after shooting and before slaughtering. All the animals legally shot in the area during the hunting seasons were included in the data set. Nine animals were excluded from the statistical analysis on the basis of signs of cross-breeding with domestic pigs (CitationAndersson-Eklund et al., 1998; CitationRandi, 2005). As no selective hunting is practised in the three areas, the animals were shot with the technique of dog drive hunting (CitationMassei and Toso, 1993).
On the basis of the literature available (CitationMayer and Brisbin, 1991; CitationMoretti, 1995; CitationTinelli et al., 1999) selected morphological traits were measured, using a flexible meter with a mm scale (), and recorded in an appropriately designed format. The following somatic measurements were individually recorded for all the specimens: head-body length, measured from the snout to the first caudal vertebra; height at withers, from the distal extreme of the fore leg to the upper part of the wither; hind-foot length, from the cal-caneum process to the distal part of the nail; ear length, from the base to the tip; ear-snout distance, from the base of the ear to the snout; ear-shoulder distance, from the basis of the ear to scapula-humerus joint. Body weight was measured by a dynamometer (CAMI S.r.l., DIN 1, Accuracy: ± 0.5%), and age was estimated from tooth eruption and wear (CitationIff, 1978; CitationDzieciolowski et al., 1989; CitationBoitani and Mattei, 1992).
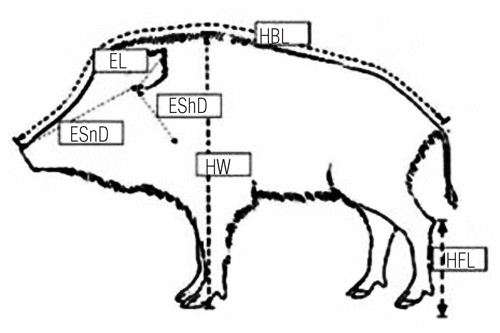
The animals were divided into three age classes; young (12 months or less), sub-adults (between 12 and 36 months), and adults (36 months and older). This was performed with reference to the results obtained in previous studies (CitationAmici et al., 2003; CitationAmici et al., 2005).
All the data, separated on the basis of the age class, have been preliminarily analysed with an ANOVA (GLM procedure) including year, sex, area and the interactions area × year, sex × year and sex × area as fixed factors. Since the analysis showed no significant differences between sex and years and contrasting results concerning the areas, the data were merged and a different statistical approach was adopted. The statistical analysis was then performed using a canonical discriminant procedure (CitationSPSS, 2007) that, given an a priori classification (geographical area) and several quantitative variables (somatic measures and weight), derives linear uncorrelated combinations of the original variables. In the space defined by the first two canonical variables, the separation among areas was estimated by the squared distance of Mahalanobis that is non affected by linear transformations and accounts for the correlations among the original variables. Discriminant analysis also allowed to categorise the animals in the areas. It should be underlined that the result could be biased, since the same data that has been classified is also used to derive the discriminant function. As a second step the data underwent factor analysis (CitationSPSS, 2007). This procedure is based on the assumption that so called common factors (unobservable latent variables) are able to explain the variance – covariance structure of the original variables. On the basis of the comparison between simple (each pair of variables) and partial (each pair of variables controlling for all other variables) Pearson correlation coefficients, it is possible to calculate the Kaiser measure of sampling adequacy (MSA) indicating the suitability of the data structure for factor analysis.
Results and discussion
The results refer to 688 animals, 253 from the SA area (119 males, 134 females), 301 from the SM area (139 males, 162 females), and 135 from the A area (62 males, 73 females). The sex ratio is similar to that observed in field surveys by CitationPerco (1987) and CitationBoitani et al. (1995b). Raw means of the somatic measurements are reported in , according to specimen age class. The analysis of variance showed no significant effect of the year and sex (unreported results). With reference to the sampling areas () the results of the statistical analysis put in evidence that some variables were significantly different (ear-shoulder distance, height at withers, hind-foot length), but these differences were not homogenous for all the age classes and were unable to highlight differences among the areas.
Table 1 Mean ± SD of somatic measurements of wild boar, and F probability of the difference among the areas.
These mean values are comparable to those observed in other Italian populations (CitationRandi et al., 1987; CitationMassei and Genov, 1993; CitationTinelli et al., 1999). However, it should be considered that a large part of the studies reported by various authors, both in Italy (CitationRandi et al., 1987; CitationApollonio et al., 1988; CitationGenov et al., 1995; CitationMartinoli et al., 1997; CitationTinelli et al., 1999) and in other countries (CitationBrisbin et al., 1977; CitationMayer and Brisbin, 1991), refer to variables not directly comparable with those of the present study.
Concerning total length of young animals, CitationMoretti (1995) registered higher values in the Ticino area (Switzerland), compared with the present study (males 107.9 vs 100.1; females: 112.5–99.0). A similar trend for adults was registered by CitationPerco (1987), compared with the present study (males 155.0–137.4; females 135.0–132.8). Live weight showed wide differences comparing different studies, and a wide individual variability (CitationGallo Orsi et al., 1995; CitationMoretti, 1995; CitationPedone et al., 1995). In addition differences in live weight between sexes can be put in evidence only after two years of age, and do not show statistic significance (CitationBoitani et al., 1995a).
The canonical discriminant analysis reveals the existence of two different area groups within the three age classes, as shown by the quadratic Mahalanobis distance (). Specifically, there are statistically significant differences among the SM and the A and SA areas, for young, sub-adults and adults. A difference between A and SA is clearly evident only for the sub-adults (P<0.05).
Table 2 Mahalanobis quadratic distance between the three areas.
The first canonical variable accounts for 92.5, 92.7 and 89.9 percent of the total variance for young, sub-adults and adults respectively, but is unevenly correlated with the original variables (). This suggests that the separation between the areas (SM vs SA and A) is due to differences in conformation rather than in body size. In more detail, the first canonical variable, for the young and sub-adults, shows the highest correlations with height at withers and ear-shoulder distance. For adults, height at withers and ear-shoulder distance but also hind-foot length and body weight (negative correlations) are relevant. In the simple and partial correlation coefficients for all the variables are reported. The differences indicate that unobservable factors, able to control the dependence structure of the observed variables, exist. This is also confirmed by Kaiser’s Measure of Sampling Adequacy (MSA), which is considered acceptable with values over 0.8 (CitationCerny and Kaiser, 1977). In fact, the MSA was 0.810, 0.829 and 0.816 for young, sub-adults and adults respectively. shows the results of the discriminant analysis in the correct classification of the data in the groups. It is useful to underline that a large part of SM animals are correctly classified and a higher percentage of misclassification can be observed for A and SA groups. This result is not easily explainable but the effect of artificial restocking for hunting purposes can be supposed.
Table 3 Correlations between canonical and original variables.
Table 4 Simple (above the diagonal) and partial (below the diagonal) correlation coefficients between the somatic variables.
Table 5 Percent of animals correctly categorised with the discriminant analysis, within age class.
Concerning the factor analysis results, only the data concerning the adults () are reported. For this age class, the first three common factors are able to explain 78, 92, and 64% of the covariance for the data of SM, A and SA respectively. The first common factor ( A) is clearly associated with body weight, head-body length, height at withers and hind-foot length, which expresses the largest portion of communality. The percentage of covariance explained by the first common factor was 75, 72 and 68% for A and SA and SM respectively, of the total explained variance. The second common factor ( B) accounts for a limited part of communality (14%), mainly related to ear length and earsnout distance. The third factor accounts only for 9% of communality ( C), and an explanation of the role of somatic variables was impossible due to the confusion of the results obtained. For the age classes of young and sub-adults, a factorial analysis showed that the first common factor is able to explain large part of the covariance (82%, 72% and 76% for SM, A and SA areas respectively). Also for these age classes the first common factor is associated with head-body length, height at withers and hind-foot length (mainly for sub-adults), but is not associated with body weight.
From an analysis of the results relative to the SM, it can be inferred that a differentiation among morphotypes is possible on the basis of a few selected somatic measures. The animals from SM tend to be shorter and have more developed fore portions (i.e. they are trapezoidal in shape) and thus correspond to a typical wild boar (CitationMauget, 1979). On the other hand, the animals from the SA area are more rectangular in shape, suggesting hybridisation with central European animals or with domestic swine (CitationMassei and Toso, 1993). The somatic measurements allowed to differentiate morphotypes of two areas and suggest the existence of two populations. The effect of the environment should be evident with difference in size rather than in conformation, although this component is impossible to separate. To this regard interesting results have been reported by CitationBrisbin et al. (1977) indicating that in two feral pig populations height at shoulder contributed in a meaningful proportion to discriminate between two populations. The same Authors also reported that no statistical differences were put in evidence for weight and total length between sexes within populations.
Conclusions
These results confirm that different morphotypes of wild boar are detectable in some different areas of Central Italy. These morphotypes are differentiated on the basis of height and length measurements (head-body length, height at withers and hind-foot length) and body weight can be relevant only for animals over three years of age.
The above mentioned somatic measurements allow different morphotypes to be distinguished and are easily registered after drive hunting sessions, implying that a wide number of field data can be collected. This study highlights the need to perform biochemical and genetic studies to identify the autochthonous wild boar strain presently populating Central Italy. These studies should be done taking into consideration the results of a field survey on population morphology.
References
- Abaigar T. del Barrio G. Vericad J.R. 1994 Habitat preferences of wild boar (Sus scrofa L 1758) in a Mediterranean environment. Indirect evaluation by signs Mammalia 58 201–210
- Adriani S. Grifoni G. Ricci V. Calderini P. 2005 Biometric study on wild boars (Sus scrofa) in Rieti province, Lazio, Italy Page 63 (abstr.) Proc. 4th Int. Symp. Wild Fauna Tatranska Lomnica, Slovakia
- Amici A. Adriani S. Serrani F. 2008 Monitoring wild boar (Sus scrofa) reproductive traits in Rieti Province, Central Italy Page 65 (abstr.) Proc. 7th Int. Symp. Wild Boar (Sus scrofa) and on Suborder Suiformes Sopron, Hungary
- Amici A. Adriani S. Serrani F. Sperduti A. Sabatini A. 2005 An approach to the interpretation of morphological differences among wild boar (Sus scrofa) in different areas of Central Italy Page 63 (abstr.) Proc. 4th Int. Symp. Wild Fauna Tatranska Lomnica, Slovakia
- Amici A. Serrani F. Faggiani M. Ronchi B. 2003 Biometric study on wild boars (Sus scrofa L.) in two areas of Viterbo Province, Italy 275–279 Proc. 3rd Int. Symp. Wild Fauna Ischia, Italy
- Andersson-Eklund L. Marklund L. Lundstrom K. Haley C.S. Andersson K. Hansson I. Moller M. Andersson L. 1998 Mapping quantitative trait loci for carcass and meat quality traits in a wild boar x Large White intercross J. Anim. Sci 76 694–700
- Apollonio M. Randi E. Toso S. 1988 The systematics of the wild boar (Sus scrofa L.) in Italy B. Zool 3 213–221
- Bieber C. Ruf T. 2005 Population dynamics in wild boar Sus scrofa: ecology, elasticity of growth rate and implications for the management of pulsed resource consumers J. Appl. Ecol 42 1203–1213
- Boitani L. Mattei L. 1992 Aging wild boar by toot eruption 419–421 Proc. Int. Conf. Ongulés/Ungulates 91 Paris -Toulouse, France
- Boitani L. Trapanese P. Mattei L. 1995a Demographic patterns of a wild boar (Sus scrofa L.) population in Tuscany, Italy Ibex Journal of Mountain Ecology 3 197–201
- Boitani L. Trapanese P. Mattei L. Nonis D. 1995b Demography of a wild boar (Sus scrofa L.) population in Tuscany, Italy Game and Wildilife Science 12 109–132
- Brisbin I.L. Geiger R.A. Graves H.B. Pinder J.E.III Sweeney J.M. Sweeney J.R. 1977 Morphological characterizations of two population of feral swine Acta Theriol 22 75–85
- Cerny A. Kaiser H.F. 1977 A study of a measure of sampling adequacy for factor-analytic correlation matrices Multivar. Behav. Res 12 43–47
- Cocca G. Sturaro E. Dal Compare L. Ramanzin M. 2007 Wild boar (Sus scrofa) damages to mountain grassland. A case study in the Belluno province, eastern Italian Alps Ital. J. Anim. Sci 6 (Suppl. 1) 845–847
- Danilkin A.A. 2001 The wild boar: an unprecedented spread or restoration of the species range? Dokl. Biol. Sci 380 457–460
- De Beaux O. Festa E. 1927 La ricomparsa del cinghiale nell'Italia settentrionale-occidentale Memorie della Società Italiana di Scienze Naturali e Museo Civico di Storia Naturale di Milano 3 263–322
- Dzieciolowski R.M. Clarke C.M.H. 1989 Age structure and sex ratio in a population of harvested feral pigs in New Zealand Acta Theriol 37 259–270
- Fonseca C. 2008 Winter habitat selection by wild boar Sus scrofa in south-eastern Poland European. J. Wildlife Res 54 361–366
- Gallo Orsi U. Macchi E. Perrone A. Durio P. 1995 Biometric data and growth rates of a wild boar population living in the Italian Alps Ibex Journal of Mountain Ecology 3 60–63
- Geisser H. Reyer H.U. 2004 Efficacy of hunting, feeding, and fencing to reduce crop damage by wild boar J. Wildlife Manage 68 939–946
- Geisser H. Reyer H.U. 2005 The influence of food and temperature on population density of wild boar Sus scrofa in the Thurgau (Switzerland) J. Zool 267 89–96
- Genov P. 2004 Craniometric characteristics of the subgenus Sus Linnaeus, 1758 and a systematic conclusion Galemys 16 9–23
- Genov P. Massei G. Nikolov H. 1995 Morphometrical analysis of two Mediterranean wild boar populations Ibex Journal of Mountain Ecology 3 69–70
- Genov P. Nikolov H. Massei G. Gerasimov S. 1991 Craniometrical analysis of Bulgarian wild boar (Sus scrofa) population J. Zool 225 309–325
- Iff U. 1978 Détermination de l'âge chez le sanglier Diana, Suisse 10 377–381
- Martinoli A. Zilio A. Cantini M. Ferrario G. Schillaci M. 1997 Distribution and biometry of the wild boar (Sus scrofa) in the Como and Varese provinces Hystrix 9 78–83
- Massei G. Genov P. 1993 Variabilità morfologica nel cinghiale maremmano Ric. Biol. Selvaggina 21 (Suppl.) 615–617
- Massei G. Toso S. 1993 Biologia e gestione del Cinghiale. Documenti Tecnici 5 INFS Bologna, Italy
- Mauget R. 1979 Quelques problèmes de biologie et d'éco-éthologie chez le sanglier Office National de Chasse Bulletin Mensuel 3 14–23
- Mayer J.J. Brisbin I.L.Jr 1991 Wild pigs in the United States: their history, comparative morphology, and current status University of Georgia Press Athens, USA
- Moretti M. 1995 Biometric data growth rates of mountain population of wild boar (Sus scrofa L.), Ticino, Switzerland Ibex Journal of Mountain Ecology 3 56–59
- Nahlik A. Sandor G. 2003 Birth rate and offspring survival in a free-ranging wild boar Sus scrofa population Wildlife Biol 9 37–42
- Pedone P. Mattioli S. Mattioli L. 1995 Body size and growth patterns in wild boars of Tuscany, Central Italy Ibex Journal of Mountain Ecology 3 66–68
- Perco F. 1987 Ungulati Lorenzini Carlo Udine, Italy
- Primi R. Pelorosso R. Ripa M.N. Amici A. 2009 A statistical GIS-based analysis of Wild boar (Sus scrofa) traffic collisions in a Mediterranean area Ital. J. Anim. Sci 8 (Suppl. 2) 649–651
- Randi E. 1995 Conservation genetics of the genus Sus Ibex Journal of Mountain Ecology 3 6–12
- Randi E. 2005 Management on wild ungulate population in Italy; captive-breeding, hybridisation and genetics consequences of translocation Vet. Res. Commun 29 71–75
- Randi E. Apollonio M. Toso S. 1987 The systematics of some Italian populations of Wild boar (Sus scrofa L.): a craniometric and electrophoretic analysis Z. Säeuge -terkd 54 40–56
- Saez-Royuela C. Telleriia JL. 1986 The increased population of the Wild Boar (Sus scrofa L.) in Europe Mammal Rev 16 97–101
- Scandura M. Iacolina L. Crestanello B. Pecchioli E. Di Benedetto M.F. Russo V. Davoli R. Apollonio M. Bertorelle G. 2008 Ancient vs. recent processes as factors shaping the genetic variation of the European wild boar: are the effects of the last glaciation still detectable? Molec. Ecol 17 1745–1762
- Schley L. Roper T.J. 2003 Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops Mammal Rev 33 43–56
- SPSS 2007 Base System User's Guide. Statistics 13.0 SPSS Inc Chicago, IL, USA
- Tinelli A. Pietrelli L. Focardi S. 1999 Dati biometrici della popolazione di cinghiale (Sus scrofa L.) di Castelporziano Proc. Società Italiana Scienze Naturali Museo Civico Storia Naturale 2 171–177
- Vernesi C. Crestanello B. Pecchioli E. Tartari D. Caramelli D. Hauffe H. Bertorelle G. 2003 The genetic impact of demographic decline and reintroduction in the wild boar (Sus scrofa): A microsatellite analysis Molec. Ecol 12 585–595