Abstract
Eurasian perch (P. fluviatilis) is a very important fish species in Varese lake (N-W Italy). Since the second half of 20th century, perch catches in the lake have steadily decreased and by the end of the ‘80s the species resulted clearly endangered. The purpose of this study was to investigate growth, mortality and feeding conditions of perch post-larvae, reared in illuminated floating cage in Varese lake, to obtain fingerlings for a restocking program. In June 2006 and 2007, groups of 280 and 300 pre-weaned post-larvae (average body weight 0.64±0.09 g and 0.25±0.08 g respectively P<0.01) were held in an illuminated net cage for 90 days. The cage was illuminated inside from 20:30 p.m. to 6:30 a.m. During the trial, the nightly zooplankton accumulation inside the cage was assessed weekly. At night time the zooplankton biomass, which resulted dominated by Cladocera family, was higher inside the cage than in the lake. In 2006, 322±36 zooplankters L−1 were observed, compared to 945±600 observed in 2007 (P<0.05). In the lake, the number of zooplankters per litre was similar in both years, resulting in 63.3±50.30 and 61.10±45 zooplankters L−1, respectively on 2006 and 2007. In order to assess perch growth performances, 25 fishes were sampled from the cage every 15–20 days and length (cm) and weight (g) were assessed for each sample. At the end of September, specific growth rate (SGR) and survival rate were assessed. In 2006 the final mean body weight of the perch fry was 4.65±1.47 g and that results significantly lower (P<0.05) than of 2007 (6.3±1.69 g). The SGR was 2.04% and 3.42%, respectively. The higher growth rate observed in 2007 was influenced by a higher zooplankton accumulation in the cage due to an improved cage management. In order to assess the cage efficiency, in September 2006 and 2007, the weight of young-of-year perch (n=50) captured in the lake were compared to those of reared fish. Wild fry showed a mean body weight significantly higher (P<0.05) than reared ones (15.90±4.25 g and 17.86±4.47 g in 2006 and 2007, respectively). The survival percentages resulted 50.7% in 2006 and 60.7% in 2007. The results demonstrate that the rearing of perch in illuminated floating cages enables the possibility to produce fry for restocking programs in Varese lake.
Introduction
Eurasian perch (Perca fluviatilis), because of its white flesh, delicate texture and mild flavour, is among the most appreciated and popular freshwater fish in Northern Italy, as well as in the Central Europe regions (CitationGandolfi and Zerunian, 1987). Despite this fact, in comparison with other farmed freshwater fish, perch production in Italy is currently low and often limited to the wild capture (CitationWatson, 2008).
During the ‘60s, Eurasian perch population in Varese lake was quite abundant representing an important economic resource for the professional fishing. Perch catches increased consistently during the ‘60s, reaching a peak at the beginning of the ‘70s with a capture of 25,000 kg y−1 (nearly 30% of the total commercial fishery in the lake). From 1973 to 1985, although the fishing effort remained essentially constant, a considerable decline of perch biomass (from 25,000 kg y−1 to about 200 kg y−1) was observed in the lake (CitationCeccuzzi, 2007). Although in 2007 the capture improved, with the total perch catch estimated at 2800 kg, a further recovery strategy for this population was recommended. In Italy, the conservation and restocking of autochthonous fresh-water species is a relevant topic. In order to improve the effectiveness of the lakes restocking, fish are reared from hatching until they grow to a size that reduces their vulnerability to predation by ichthyophagous fish and birds (CitationŽiliukiené, 2005).
Preliminary studies have demonstrated that Eurasian perch behaviour in confined environment (gregarious behaviour, low aggressiveness, congregating under feeders, opportunistic feeding behaviour) is quite compatible with rearing in floating cages (CitationFontaine et al.,1996). Moreover, rearing fish in floating cages allows the exploitation of water resources situated in unavailable fishing grounds and facilitates suitable performance monitoring of the fish stock, with regard to health and feeding habits (CitationSchmittou, 1970). Several studies on Eurasian perch breeding and rearing have demonstrated that thereis a great potential to rear this species under controlled conditions and the rearing technologies such as water recirculating systems or floating cages (CitationKestemont et al., 1996; CitationBaras et al., 2003) may be applied. In fact, Eurasian perch is already intensively reared in several European countries, such as Switzerland, France, Ireland and Belgium (CitationWatson, 2008).
A key aspect of the fish farming cycle is represented by the production of high quality stocking material, including the feeding of fish larvae and post-larvae. A cheap and profitable production method consists of rearing fish larvae and post-larvae in net cages placed in lakes or reservoirs with submerged illumination to attract zooplancton at night (CitationŽiliukiené, 2005; CitationŽiliukiené and Žiliukas, 2006). According to the literature (CitationSzczerbowski and Mamcarz, 1984) the attractive effect of light caused an increase in presence of the three major zooplankton groups (cladocerans, copepods and rotifers), both inside and near the cage. With such facilities, developed in Poland during the ‘70s, phototaxis was determined to be the key to concentrate zooplancton into cages, thus consistently improving the nutritional substrate to fish larvae. Rearing of fish larvae and post-larvae in illuminated floating cages provides a large potential for intensifying the fish production in lakes (CitationMamcarz, 1989) and offers a possibility to obtain juveniles fish for stocking purposes. The success of this method is mainly affected by the post-larvae feeding behaviour and by the trophic status of the water body. Applications concerning several fish species have been reported, namely: Coregonus sp. (CitationSzczerbowski and Mamcarz, 1984; CitationMamcarz, 1995; CitationDostatni et al., 1999), Perca fluviatilis (CitationSkrzypczak et al., 1998), Aspius aspius (CitationMamcarz, 2001), Esox lucius (CitationŽiliukiené and Žiliukas, 2006) and Abramis brama (CitationŽiliukiené, 2005).
The goal of this study was to investigate the growth performances of Eurasian perch post-larvae, reared in an illuminated floating cage placed in Varese lake in relation to the zooplancton density, with the intent to obtain fingerlings for a restocking program.
Study area
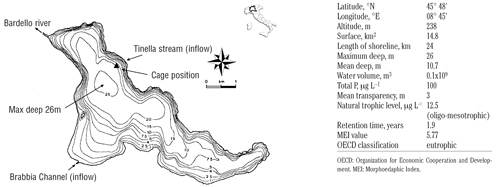
Varese lake is located 45°48’ N, 8°45’ E in a geographic region known as “Insubria” in the North-West Italy. The main morphometric and hydrological characteristics of the lake, 14.8 km2 surface, 26 m maximum deep, 5.77 as morphoedaphic index (MEI), are reported on . The significant demographic increase in the period immediately after the Second World War, together with the extension of the municipal and industrial discharge network without an appropriate water treatment, caused a deep modification of the trophic conditions of the lake. By the end of the ‘60s, with total phosphorous (PT) concentrations estimated to be 440 μg L−1, the lake was classified as hypertrophic (CitationLalumera, 2003; CitationCanziani and Crosa, 2005). In addition to the progressive deterioration of the water quality, the introduction of allochthonous species caused a quantitative and qualitative imbalance of the indigenous fish community composition. After a first drastic decrease among salmonid species, perch (Perca fluviatilis) and pike (Esox lucius) followed as endangered populations, while bleak (Alburnus alburnus alborella), an important forage-fish for Eurasian perch food web, had apparently disappeared from the basin by the end of the ‘80s. All these changes contributed to the final predominance of extremely tolerant fish species, such as rudd (Scardinius erytrophtalmus), common carp (Ciprinus carpio), crucian carp (Carassius carassius), European catfish (Silurus glanis) and catfish (Ictalurus melas). Consequently, nowadays the allochthonous species represent about 40% of the lake fish species (CitationCeccuzzi, 2007).
Materials and methods
A net cage, measuring 2.5 m3 volume and 2.8 m deep, with a mesh size of 1.8 mm was floated on four buoys. The cage was set in the Northern part of the lake (), at approximately 15 m from the shoreline and with the top submerged by 50 cm from the water surface. The cage was illuminated inside by a LED light (24V, 60W) connected to a battery and a solar panel (Solar future© srl) equipped with a timer.
In 2006, the fingerlings selected for the trial were siblings originating from a single egg ribbon (about 40,000 eggs) spawned by wild breeders in Varese lake. The ribbon was collected on 22 April 2006 and was transferred to an experimental hatchery located near the lake bank. In 2007 the siblings were obtained from one egg ribbon spawned by a breeders stock (34 female and 40 male)s caught the previous year in the lake and maintained in an outdoor 3.0 m3 tank.
In both years, the eggs were incubated onto trays at a temperature ranging 14–15°C, until eyes of embryos were visible. Survival rate at this stage, corresponding to two days before hatching, was assessed using a stereoscope. After hatching, a number of 1.000 larvae for each year were transferred in two PVC outdoor tanks, of 150 L volume each. Larvae were fed ad libitum with zooplankton caught in the lake with a plankton net (80 μm mesh size). During the weaning, in relation to the larval stage, different plankton size classes were obtained by sequential sieving through a set of sieves ranging from 120 to 400 μm.
On July 14th 2006 and June 29th 2007, 280 and 300 perch post-larvae, with an initial mean body weight of 0.64±0.09 g and 0.25±0.08 g respectively (P<0.01), were transferred into the cage for 90 days. During this period, zooplankton was collected weekly (every Thursday) in order to assess its dynamics inside and outside the cage. Water samples were collected, with a 1 L volume Ruttner’s bottle, at three different points: inside the cage, 15 cm outside the cage and at a distance of 15 m from the cage, with the latter sample representing the control blank.
The first sampling cycle, programmed to assess the zooplankton dynamics during both dark and light hours, was carried out through 24 hours, without fish in the cage, by collecting a sample every second hours. The collected samples were preserved in the refrigerator at 4°C before the laboratory analyses. Later samplings were conducted weekly during the hours of greater zooplankton concentration in the cage, corresponding to night time from 11:00 p.m. to 3:00 a.m. The zooplankton density was determined by counting under stereoscope, 5 sub-samples of 100 mL each, after filtration on nitrate of cellulose lab filters provided of 3 mm squares, with 47 mm diameter and 3.0 μm porosity (Albet®).
During the weekly samplings, pH, temperature, dissolved oxygen concentration (DO) and saturation percentage were recorded in the water column together with date, time and weather conditions. An oximeter (OXIGUARD® Aquatrade) was used to measure DO (mgL−1), saturation percentage and temperature (°C), while pH values were measured by an Orion 250A+ Thermo Orion© pH-meter. Due to the high periphyton production in Varese lake, the net was cleaned every second day with a high-pressure water jet, in order to avoid mesh fouling. To evaluate growth rate, length (accuracy = ±1 mm) and weight (accuracy = ±1 mg) were detected every 15–20 days in a 20 fry sample. At the end of September, all the fry were harvested, counted and weighed in order to assess survival percentage, as well as the specific growth rate (SGR):
SGR= 100*(Ln (FBW)−Ln (IBW)/ΔT
where: FBW= final body weight (g), IBW= initial body weight (g), ΔT= time (days). At the end of September 2006 and 2007, 50 young-of-the-year (YOY) perch were caught in the lake by a trap positioned near the shoreline. In all specimens, total length (Lt) and standard length (Ls) were measured to an accuracy of ±1 mm and weighed (Wt) to an accuracy of ±1 g. In order to assess the age of the wild samples, scales were taken from above the lateral line and below the insertion of the dorsal fin; in addition, both opercular bones were removed. Opercular bones were cleaned in boiling water and dry stored before the analysis. Scales were cleaned in 8% KOH, washed in distilled water and dry mounted between two glass slides. The fish age was assessed using both the microscopic scalimetric method (CitationBagenal, 1978) and opercula examination as described by CitationLe Cren (1947). Both structures were examined on a stereoscope equipped with a camera (Leica DC-200) in order to photograph the opercular bones and scales of each specimen. Even if the precise hatching date of these specimens was not known, their weight and length were compared with those of reared fish in order to coarsely assess the cage efficiency.
Analysis of variance (ANOVA) was applied to the results. Comparisons among groups for significant differences of mean or rank values were performed after an homogeneity variance test; Sheffe’s test was applied for post-hoc comparisons and statistical calculations were performed with STATISTICA ‘98 (StatSoft®).
Results
shows temperature, oxygen concentration and saturation percentage together with the pH observed during the samplings. The average water temperature in the first five meters of the water column was 22.4±1.03°C in 2006 and 23.3±1.93°C in 2007. From the depth of 5 m to the bottom, that at the cage point was 7 m, the temperature showed a progressive reduction to reach a minimum of 19.0±1.15°C.
Table 1 Chemical and physical water parameters monitored during the samplings in 2006 and 2007.
The average DO concentrations in 2006, was 8.8±1.08 mg L−1 (105.96±19.52%) at the surface and 0.26±0.49 mg L−1 (2.6±6.02%) near the bottom. The same trend was observed in 2007, with an average DO of 9.51±2.04 mg L−1 at the surface (113.00±25.53%), and 0.26±0.49 mg L−1 (3.00±6.02%) near the bottom.
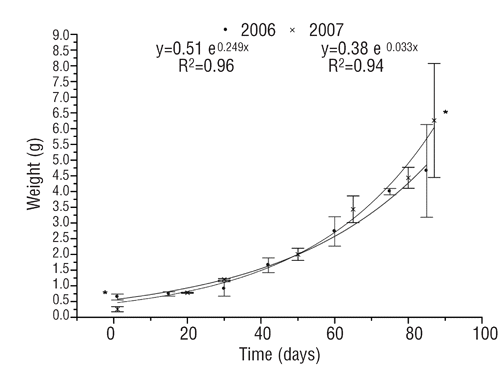
The pH values were more alkaline within the surface and a 4-meter depth (9.08±0.32), while near the bottom (7 m) it was 8.6±0.21. In 2006, the initial mean body weight (IBW) of the post-larvae used for the trial was 0.64±0.09 g, at the end of the 90 days their mean final body weight (FBW) was 4.65±1.47 g, resulting in the calculated SGR equal to 2.04%.
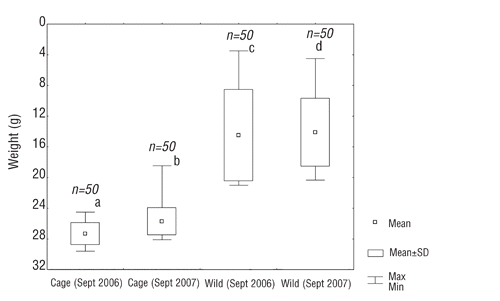
In 2007 the post-larvae IBW was 0.25±0.082 g and at the end of the trial FBW was 6.3±1.69 g (P<0.05), resulting in the calculated SGR 3.42% (). Although the IBW of post-larvae was higher in 2006 than in 2007, at the end of the 90 days experiment, the 2007 fry FBW was significantly higher than in 2006 (P<0.05) (). The final stock survivor percentage was 50.7% in 2006 and 60.7% in 2007. Most of the fry found dead in the cage showed evidence of injuries and signs of attack from their siblings (n=98 in 2006 and n=66 in 2007). The comparison between weight of young-of-year perch (YOY) caught in the lake during September 2006–2007 and the final body weight of the two reared stocks is reported in . There are significant differences (P<0.05) between the reared stocks and the wild perch.
Table 2 Data on perch post-larvae reared in the illuminated cage 2006 and 2007.
shows data of the 24 h monitoring carried out during the first week of trial. The average number of zooplankters per litre inside the cage during the day time was 6.0±3.0 in 2006 and 5.0±4.0 in 2007 while, during the night time, the number of zooplankton organisms was 238±198 zooplankters L−1 in 2006 and 222±147 zooplankters L−1 in 2007. The mean number of zooplankters L−1 assessed in samples taken from the control point was: 17.0±6.0 in 2006 and 10.0±8.0 in 2007 (during 24 h).
Table 3 Zooplankton densities assessed inside the cage and in the control point during the 24 h sampling (2006 and 2007). Different letters indicates significant differences (P<0.05) in number of zooplankters between the sampling points during the night time.
a shows the night zooplankton density observed in the 2006 trial (90 days), with an average value of 322±122 zooplankters L−1. By considering an illuminated water volume of about 250 L, a total number of 80,500 zooplankters were estimated to be present in the cage during the artificial lighting in 2006, corresponding to 268 zooplankters available per fish.
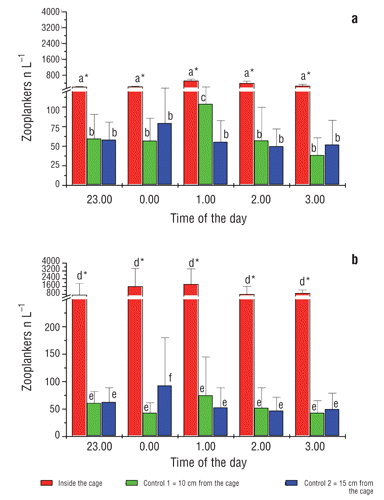
In 2007, the accumulation in the cage during the night was significantly higher (P<0.05) than in 2006, with an average of 945±600 zooplankters L−1, so a total of 240,000 zooplankters, corresponding to 800 zooplankters per fish ( b) was assessed. The number of zooplankters collected at the control point during the 90 days appeared not to be influenced by the light and was similar in both year trials (2006 and 2007), resulting 63.3±50.3 and 61.1±45 zooplankters L−1 respectively (P=0.9).
Zooplankton species found inside the cage were the same as those found in the lake sampling point (control). Cladocera were dominated by Daphnia galeata, Daphnia cucullata, Leptodora Kindtii, Bosmina longirostris and Chydorus sphaericus. Copepoda were dominated by Cyclops vicinus vicinus, Eudiaptomus padanus padanus and Mesocyclops leukarti. Rotifera were dominated by Keratella quadrata, Keratella cochlearis, Testudinella sp., Kellicottia longispina. During the 90 days of rearing, the percentage of the three taxa, inside and outside the cage, varied in relation to their life cycle: on average, cladocerans represented about 40.2%, rotifers 34.6% and copepods 25.2% of the total zooplankters observed.
Discussion
Varese lake can be classified as a warm monomictic lake, with one period of complete water mixing lasting from the end of December to the first half of February. July and August were the months showing the highest epilimnetic temperature and the most stable stratification, with a thermocline situated at approximately at 7.5 m depth. During the thermal stratification period, in the epilimnetic layer the temperature normally exceeded 20°C, reaching maximum values of 25–28°C in July and August, while in the hypolimnetic layer the maximum values ranged within 9− 11°C (CitationZaccara et al., 2007). According to these authors, during the rearing period, the temperature in the first 3 meters of the water column showed an average value of 22.42±1.28°C in 2006 and 23.4±1.94°C in 2007. These values, as reported by CitationFerguson (1958) and CitationHokanson (1977), were near to the physiologic optimum of the species (21–25.4°C).
With respect to water characteristics, perch may survive even at DO concentrations of 2.5 mg L−1 (CitationThorpe, 1977). According to CitationFry (1957), however, oxygen critical level in perch is 7 mg L−1 at 20°C. In the period considered in the present study, from June to September, the average value of DO from −3 to −1 meter depth was always above the critical level, with a slightly over-saturation. DO concentration dropped once during the trial period (2007–08–20) to a value of 6.5 mg L−1 even on the surface, due to an up-welling phenomenon which mixed hypolimnetic and epilimnetic waters up.
Water pH in the basin was always alkaline: average value measured in the first 5 m of the water column was 9.02±0.28 in 2006 and 9.08±0.32 in 2007. The higher pH values observed in the water surface (about 9.3 in both years) is likely due to the frequent algae blooms in the euphotic zone and to the lithological composition of the hydrographic basin of Varese lake, mainly composed of carbonatic rocks (CitationLalumera, 2003). In the epilimnion of many eutrophic lakes, water pH may exceed the value of 9.0 and occasionally approach 10.0 (CitationAyles et al., 1976). In contrast, the biodegradation of organic substances in the hypolimnion can causes a reduction of oxygen concentration and a carbon dioxide accumulation (CO2), corresponding to a pH reduction below the neutrality level. According to CitationCanziani and Crosa (2005), average pH of Varese lake water column is 7.8, reaching a minimum of 7.4 in hypolimnion and a maximum of 9.3 in the epilimnion. During the sampling period no significant differences (P=0.09) were observed among the pH values observed during the day or night time.
Information about the effects of a long-term exposure to high pH water on ion balance, ammonia excretion and survivor in freshwater fish is mainly limited to salmonids such as rainbow trout (Oncorhynchus mikiss). Few studies have investigated the long-term effects of exposure of perch to high pH. CitationScott et al. (2005) observed that perch in Slapton Ley (hypertrophic basin) is able to withstand such high pH values, including values up to 10.54. In an enclosure experiment, perch survived at pH 10.0 but died when water pH reach a value of 11.0 (CitationBeklioglu and Moss, 1995), suggesting that pH values in Varese lake were often near the upper limit for perch survival. Several laboratory studies have shown that rainbow trout can survive in high pH water for a considerable period of time (15 days at pH 10.0 and 28 days at pH 9.5), due to adjustments to branchial ion movements, acid-base balance, ammonia excretion and production (CitationWilkie et al., 1996), which may be similar to mechanisms employed by perch in Slapton Lay and Varese lake. In consideration of the trophic state of lake waters, the cage was positioned in the first three meters of water column from the surface, in order to avoid any problem due to anoxic phenomena in the hypolimnion.
During the 24h samplings (2006 and 2007), zooplankton inside the cage was more concentrated during the night than the day time (P<0.05), due to attractive conditions of artificial lighting. Therefore, perch post-larvae found more available feed during the night than the day time, but were not exposed to predation even if some cannibalism was observed. Similarly to the observations of CitationŽiliukienè (2005), the feeding intensity of the diet cycle of larvae reared in cages differed from what remarked in plain water. CitationCraig (1978) reported perch active feeding during dawn and dusk and an evident decrease in feeding intensity at night in the Windermere lake (52° 21’ N, 2° 56’ W) North-West England. The diet cycle of perch post-larvae reared in illuminated cage conditions yielded the opposite results. According to CitationMamcarz (2001) and CitationSkrzypczak et al. (1998), in our trial the number of zooplankters inside the cage was significantly higher during the night than in the day time. Although the zooplankton density in the control point was similar in both years (in average 63.3±50.3 zooplankters L−1 in 2006 and 61.1±45 zooplankters L−1), the mean number of zooplankton organisms accumulated in the cage during the night was significantly lower (P<0.05) in 2006 (322±122 zooplankters L−1) than in 2007 (945±600 zooplankters L−1). The greater zooplankton accumulation observed in 2007 is likely due to an improved cage management with more constant and careful cleaning of the cage net.
The final survival percentage was relatively high in both years: 50.7% in 2006 and 60.7% in 2007. Similar results were found in the Maroz Lake (53° 32’ N, 20° 24’ E) Northern-Poland, by CitationMamcarz (2001) 57.5% for asp (Aspius aspius), a cyprinid characterized by feeding habits and behaviour similar to those of perch. The lower zooplankton presence in the cage during the day may have induced aggressive behaviour and cannibalism among the reared individuals. Survival percentage in the cage is strictly connected to the dietary habits and the aggressiveness of the reared species. Whenever non-ichthyophagous species are placed into the cage, the survival percentage increases and can reach 90% (without diseases or parasites), as observed with coregonids by CitationRösch and Eckmann (1986). Other issues may affect fish performances within the cages, for example they are unable to follow the zooplankton migration as wild fish do. Moreover, a competition may occur with wild zooplankton feeders moving in proximity of the cage. Also parasitism may affect fish performances within the confined water volume (CitationChampingueulle and Beltran, 1996).
As reported previously, the higher fry growth rate observed on 2007 could be due to a greater zooplankton accumulation in the cage, as a result of better net cleaning. In both year trials, growth rate was higher during the second half of the rearing period (August and beginning of September), while relatively lower growth rates were observed during the first weeks. This fact was probably due to the temperature fluctuations caused by bad weather conditions observed in both years.
In our trials, perch reared in a confined environment with natural food, showed an apparent lower growth rate than in the wild; in fact in September 2006 and 2007 YOY captured in the lake, showed an average body weight of 15.90±4.25 g (2006) and 17.86±4.47 g (2007), that resulted significantly higher (P<0.05) than the ones observed at the end of each year. Generally, Eurasian perch growth is plastic and depends on complex interactions with water temperature (CitationLe Cren, 1958; CitationCoble, 1966), population density (CitationRask, 1983), food density and availability (CitationCraig, 1978; CitationRask 1983). In Varese lake, the species can grow faster than other pre-alpine lake perch populations (CitationCeccuzzi, 2007), such as in Como lake at the same latitude (45° 49’ N, 9° 9’ E). Still at the end of the first year of life, males and females of Varese Lake are, on the average, respectively 6 cm and 6.8 cm longer than perch of Como lake (CitationNegri, 1999) (). According to CitationTolonen et al. (2003), one of the probable reasons for this high growth rate could be the higher primary and secondary water productivity of Varese lake, with the consequently higher presence of zooplankton organisms, chironomids (larvae and pupae) and nekton juvenile (i.e.Scardinius erytrophtalmus). CitationHartmann (1974) observed that in the Bodensee (Germany), both perch growth rate and population density have increased in relation to the trophic condition of the lake. This author suggested that the increased density of invertebrate organisms provided a better food supply and hence a better fish growth rate. Wild perch starts feeding on chironomids larvae and pupae when their length reaches 15 mm. The mean length of preys in perch larvae stomachs is always greater than the mean length in the plankton community, showing that as they grow, perch larvae eat the largest prey available in order to meet their increasing energy demands (CitationGuma’a, 1978).
Table 4 Comparison of perch females and males total length between Como lake (CitationNegri, 1999) and Varese lake (CitationCeccuzzi, 2007).
Perch switching to predatory feeding with an evidently increased reward, leads to energy saving and a more optimal energy balance. Even if the zooplancton density in the cage was high during the night time, long-term feeding on zooplankton results in energy overuse, which cannot be compensated by energy obtained from food. Perch post-larvae and fry reared in cages exhibited some difficulties to switch their feeding behaviour from zooplankton to larger rewarding food (e.g., insect larvae, nekton juvenile) (CitationŽiliukiene and Žiliukas, 2006). This tendency may have induced a cannibalistic behaviour among the confined fish. A number of studies reported that in perch, intra-cohort cannibalism is an important issue that can induce losses exceeding 50% of the initial reared stock (CitationBaras et al., 2003; CitationKestemont et al., 2003; CitationMandiki et al., 2007).
Conclusions
Our results demonstrate that the rearing of perch in illuminated floating cages enables the possibility of producing fry for restocking programs. Floating cage perch farming is possible in temperate zones only during the June-October period. Indeed, when the water temperature drops below 15°C, perch growth is prevented. Further studies are required to determine the optimal densities, the behavioural and feeding aspects of fish growth. Physical and chemical conditions of the basin waters, particularly its temperature (average value 23.4°C), are within optimal parameters reported in literature for perch. Stratification and low dissolved oxygen concentrations in the deep water of Varese lake during the summer, make it advisable to ensure the cage is positioned above the thermocline (i.e. not deeper than 3 m in the water column).
Acknowledgments:
the authors wish to thank the Angling Fishing Association APD Tinella ‘72 for the active collaboration and Varese Lake Professional Fishermen Cooperative. They are also most grateful to Mattia Dellatorre for his technical and laboratory assistance. This study was supported by a grant from Regione Lombardia (PERLAVAR-07 project) with a contribution of Provincia di Varese and Varese Europea Association.
References
- AylesG.B. LarkG.B. BaricaJ. KlingH. 1976 Seasonal mortality of rainbow trout (Salmo gairdneri) planted in small eutrophic lakes of Central Canada J. Fish. Res. Board Can 33 647–655
- BagenalT.B. 1978 Aspects of fish fecundity Gerking S.D. Ecology of freshwater fish production Blackwell Scientific Publications Oxford, UK 75–101
- BarasE. KestemontP. MélardC. 2003 Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions Aquaculture 219 241–255
- BekliogluM. MossB. 1995 The impact of pH on interactions among phytoplankton algae, zooplankton and perch (Perca fluviatilis) in a shallow, fertile lake Freshwater Biol 33 497–509
- CanzianiA. CrosaG. 2005 Raccolta e valutazione della documentazione esistente sul lago di Varese, sugli interventi attuati e sull'evoluzione del suo stato trofico Internal report Provincia di Varese Press Varese, Italy 1–55
- CeccuzziP. 2007 Biology and Ecology of Eurasian perch (Perca fluviatilis) in Varese Lake PhD Thesis Università dell'Insubria Varese, Italy
- ChampinguelleA. BeltranR.R. 1996 Elevage des larves et juvéniles de coregone (Coregonus lavaretus) en cages lacustres éc-lairées pour l'attraction du zooplancton Hydroècol. Appl 1 155–172
- CobleD.W. 1966 Dependence of total annual growth in yellow perch on temperature J. Fish. Res. Board Can 23 15–20
- CraigJ.F. 1978 A study of the food and feeding of perch, Perca fluviatilis L., in Windermere Freshwater Biol 8 56–68
- DostatniD. MamcarzA. KozlowskiJ. PoczyczyńskiP. 1999 Feeding of early and late-hatched withefish in illuminated cages Arch. Pol. Fish 7 281–295
- FergusonR.G. 1958 The preferred temperature of fish and their midsummer distribution in temperate lakes and streams J. Fish. Res. Board Can 15 607–624
- FontaineP. TamazouztL. CapdevilleB. 1996 Growth of the Eurasian perch (Perca fluviatilis L.) reared in floating cages and in water recirculated system: first results J. Appl. Ichthyol 12 181–184
- FryF.E.J. 1957 Aquatic respiration of fish Brown M.E. The Physiology of Fishes I Academic press London, UK 1–63
- GandolfiG. ZerunianS. 1987 I pesci delle acque interne italiane: aggiornamento e considerazioni critiche sulla sistematica e la distribuzione Atti Soc. Ital. Sci. Nat. Museo Civ. Stor. Nat 128 3–56
- Guma'aS.A. 1978 The food and feeding habits of young perch, Perca fluviatilis, in Windermere Freshwater Biol 8 177–187
- HartmannJ. 1974 Der Barsh (Perca fluviatilis) im eutrophierten Bodensee Arch. Hydrobiol 76 269–286
- HokansonK.E.F. 1977 Temperature re-quirements of some percids and adaptations to the seasonal temperature cycle J. Fish. Res. Board Can 34 1524–1550
- KestemontP. JourdanS. HoubartM. MèlardC. PaspatisM. FontaineP. CuvierA. KentouriM. BarasE. 2003 Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: biotic and abiotic influences Aquaculture 227 333–356
- KestemontP. MelardC. FiogbéE. VlavanouR. MassonG. 1996 Nutritional and animal husbandry aspects of rearing early life stages of Eurasian perch (Perca fluviatilis) J. Appl. Ichthyol 12 157–166
- LalumeraG.M. 2003 Ecological changes and biodiversity in Varese Lake. PhD Thesis Università dell'Insubria Varese, Italy
- Le CrenE.D. 1947 The determination of age and growth of the Perch (Perca fluviatilis) from the opercular bone J. Anim. Ecol 16 188–204
- Le CrenE.D. 1958 Observation on the growth of perch (Perca fluviatilis) over twenty-two years with special reference to the effects to temperature changes in population density J. Anim. Ecol 27 287–334
- MamcarzA. 1989 An attempt to assess effects of illuminated cage location on coregonid culture results Acta Ichthyol. Piscat 2 41–54
- MamcarzA. 1995 Rearing of coregonid (Coregonus sp.) larvae in illuminated floating cages: a review Arch. Hydrobiol. Spec. Issues Adv. Limnol 46 287–292
- MamcarzA. 2001 Preliminary results of larval and fry asp (Aspius aspius L.) rearing in the illuminated cages European Aquacul-ture Society (ed.) Larvi '01-Fish and Shellfish Larviculture Symposium Special Publication No. 30 Oostende, Belgium 328–331
- MandikiS.N.M. BabiakI. KrolJ. RasoloJ.F.R. KestemontP. 2007 How initial predator-prey ratio affects intra-cohort cannibalism and growth in Eurasian perch Perca fluviatilis L. larvae and juveniles under controlled conditions Aquaculture 268 149–155
- NegriA 1999 Biologia ed Ecologia del persico reale (P. fluviatilis) nel Lago di Como Home page address: http://www.provincia.como.it.
- RaskM. 1983 Differences in growth of perch (Perca fluviatilis L.) in two small forest lakes Hydrobiologia 101 139–144
- RöschR. EckmannR. 1986 Survival and growth of pre-fed Coregonus lavaretus L., held in illuminated net cages Aquaculture 52 245–252
- SchmittouH.R. 1970 The culture of channel catfish, Icatlurus punctatus, in cages suspended in ponds Proc. Annual Conf. South-eastern Assoc. of Game and Fish Commission-ers New Orleans, LA, USA 23 226–244
- ScottD.W. LucasM.C. WilsonR.W. 2005 The effect of high ion balance, nitrogen excretion and behaviour in freshwater fish from an eutrophic lake: a laboratory and field study Aquat. Tox 73 31–43
- SkrzypczakA. MamcarzA. KujawaR. KucharczykD. Furgala-SelezniovG. 1998 Feeding habits of larval Eurasian perch Perca fluviatilis (Percidae) Ital. J. Zool 65 243–245
- SzczerbowskiJ.A. MamcarzA. 1984 Rearing of coregonid fishes (Coregonidae) in illuminated lake cages. II Environmental conditions during fish rearing Aquaculture 40 147–161
- ThorpeJ. 1977 Synopsis of Biological Data on the Perch: Perca fluviatilis ( Linnaeus, 1758) and Perca flavescens (Mitchell, 1814) FAO Fisheries Synopsis FAO ed. Roma, Italy
- TolonenA. LappalainenJ. PulliainenE. 2003 Seasonal growth and years calss variations of perch near the northern limits of its distribution range J. Fish Biol 63 176–186
- WatsonL. 2008 The European market for perch (Perca fluviatilis) Percid Fish Culture 10–16 Proc. Congr. From Research to Pro-duction Namur, Belgium
- WilkieM.P. SimmonsH.E. WoodC.M. 1996 Physiological adaptations of rainbow trout to chronically elevated water pH (pH = 9.5) J. Exp. Zool 274 1–14
- ZaccaraS. CanzianiA. RoellaV. CrosaG. 2007 A northern Italian shallow lake as a case-study for eutrophication control Limnology 8 155–160
- ŽiliukienéV. 2005 The diet of Abramis brama (L.) larvae reared in illuminated cages J. Appl. Ichthyol 21 406–409
- ŽiliukienéV. ŽiliukasV. 2006 Feeding of early larval Esox lucius L reared in illuminated cages. Aquaculture 258 378–387