Abstract
Fundamentally, in land based mediterranean aquaculture, two techniques are applied to supply water with oxygen: paddling water aeration and application of pure oxygen. The two oxygenation techniques result in quite different PO2 regimens and, consequently, different fish growth performance and gill morphology.
Data exist showing a reduction in total respiratory surface (RSA) and increasing gas diffusion distance (GDD) in gills of sea bass (Dicentrarchus labrax, L.) farmed under elevated PO2 regimens. That such a modification might have an effect on the ion regulation has been defined elsewhere as osmorespiratory compromise.
In this study, European sea bass previously acclimatized to two PO2 regimens, mild hypoxia and mild hyperoxia (70–80% and 130–140% of the saturation value, respectively), were challenged for 1 hour with hypo-osmotic plus manipulation stress in two separate trials. During the first trial, when only Na+ loss was determined, the ion efflux during the first 5 min resulted in a rate of 163.72±31 and 112.23±87 nmol g−1 min−1 from hypoxia and hyperoxia sea bass groups, respectively, and, if sustained, would approach 15.3 and 11.2% per hour of the total body Na+, respectively. During the second trial, in which both Na+ and Cl− loss were determined, after 60 min the Na+ loss was shown to be 76.86±12 and 179.28±32 nmol g−1 min−1 for the fish previously acclimatized to hyperoxia and hypoxia regimens, respectively, whereas for Cl− this loss was 62.02±11 and 157.28±28 nmol g−1 min−1, respectively. Our data are compatible with the hypothesis of an osmotic advantage of sea bass exposed to an elevated PO2 regimen, achievable with application of pure oxygen, instead of simple water aeration.
Introduction
European sea bass (Dicentrarchus labrax, L.) is a largely euryhaline species that can be found in truly fresh water, brackish water, normal sea water, or very highly saline water (up to 90 ppt) and it is one of the most important species for Mediterranean aquaculture. Several different technologies are used to farm this species and include extensive farming in large brackish water ponds (Valliculture) and coastal lagoons and intensive farming in landbased raceways and coastal pen cages (CitationBarnabé, 1990; CitationCataudella and Bronzi, 2001). Water-recirculation technology was developed to farm sea bass in inland areas with artificially salted water within the range of 8 to 20 ppt.
The oxygenation techniques applied in landbased intensive farming of this species include either paddling water aerators or dissolving pure oxygen in water with on-purpose-made machines (CitationSaroglia, 2001). It is very well known by stakeholders that with paddling water aerators, the PO2 regimens range among values corresponding to 30–85% of the saturation values, whereas with the application of pure oxygen the regulation often ensure values ranging from 110 to 140% of the saturation values (CitationBarnabé, 1990). Important differences in production quality have been reported as a result of the water oxygenation technology applied (CitationIandoli et al., 1995) and significant differences in sea bass gill morphology have been described as a result of the water PO2 regimens (CitationSaroglia et al., 2000, Citation2002).
Fish gills are responsible for several physiological functions that require anatomical and physiological compromises (CitationRandall and Daxboeck, 1984; CitationOlson, 1991; CitationPerry and McDonald, 1993). The rate of gas transfer through fish gills is regulated by Fick’s diffusion equation, as reported by CitationJobling (1994), and the basic conflict between gas exchange and ion regulation in the gills of freshwater fishes has been described by CitationRandall et al. (1972). The latter authors indicated that a large, permeable gill membrane is required for efficient gas transfer but that a small, impemeable epithelium is needed to minimize diffusive ion losses. These authors demonstrated that rainbow trout accelerate Na+ loss with an increase in oxygen consumption during exercise, which they attributed mainly to an increased functional surface area (FSA) of the gills during activity.
As suggested by CitationNilsson (1986), the balance between ‘need of oxygen’ and ‘need of osmotic regulation’ has been defined as osmorespiratory compromise. There are two major scenarios in which the osmorespiratory compromise may interfere with osmotic homeostasis: when oxygen demand is high, and gill perfusion must be increased to favour gas exchange at the expense of ion regulation; and when the epithelium must be thickened to defend ion balance at the expense of gas exchange. Within a species, changes in environmental conditions, such as elevated temperature or salinity, and exercise, serve to unmask the nature and degree of the osmoregulatory compromise (CitationSardella and Brauner, 2007). In general, elevated metabolism associated with high temperature, result in a partial loss of osmoregulatory control and in other cases, exposure to condition requiring an upregulation of osmoregulatory characteristic of the gills (e.g., exposure to dilute water) can result in morphological changes in the gill that impair gas exchange (CitationHenriksson et al., 2008).
The oxygen demand and PO2 fluctuation could be compensated by adapting the total respiratory surface (RSA), as well as Functional Respiratory surface (FSA), and gas diffusion distance (GDD) in water. In fact, a positive correlation was found in sea bass (D. labrax, L.) between environmental oxygen and both GDD and RSA (CitationSaroglia et al., 2000, Citation2002, Citation2007). Sea bass reared under hyperoxia exibited significantly higher GDD than fish reared under normal oxygen concentrations. This also occurred when improvement in O2 solubility was due to a seasonal water temperature decrease. In their conclusions, CitationSaroglia et al. (2002), suggested that the application of pure oxygen in intensive aquaculture represents an energy advantage for osmoregulatory mechanisms.
However, such sophisticated compensatory mechanisms likely require energy, too. The increase in FSA causes a rise in water loss and ionic gain through the gills in fishes in a hypertonic environment such as salt water (CitationEvans, 1993). Fishes respond to this by increasing their water intake, which requires an increase in energy in order to excrete the excess ions through the gills. In contrast, a higher ion loss occurs when fish are acclimated to a hypotonic environment such as freshwater and respond with adequate mechanisms consisting in an enlarged water uptake and elimination of excess water and ions via the kidney. So far, a demonstration of the effects that the PO2 regimen may have on fish osmotic control is lacking. The aim of this study was to verify the hypothesis that an increase in PO2 actually provides an advantage for ion regulation in fish.
Materials and methods
First acclimatization facilities
All the trials were conducted on European sea bass (Dicentrarchus labrax, L.) obtained from the commercial hatchery Nuova Azzurro in Civitavecchia (Rome) and farmed for an acclimatization period (3 to 6 months) at low density that increased with fish growth from 6 to 10 kg/m3. The fish were kept in 4 m3 fiberglass tanks connected to a water recirculation system providing about 24 water refillings per day, at a water temperature of 20±1°C, salinity 10 g/L, dissolved oxygen (PO2) 95–105% of the saturation value, total gas pressure ≤100%, free CO2<15 mg/L, N-NH4+<0.03 mg/L, N-NO3-<20 mg/L, pH 7.2, and alkalinity 140–200 mg/L CaCO3. The photoperiod was constant at 10L:14D and the fish were fed twice a day with extruded commercial marine fish food (Hendrix s.p.a., Nutreco group, Verona, Italy). When needed, fish were randomly sampled and transferred to adequate tanks, where experimental trials were conducted.
First trial
The first trial consisted in measuring the rate of the Na+ loss at different times, when sea bass acclimatized to different PO2 levels were exposed to a hypo-osmotic challenge. For this purpose, 40 fish ranging from 300 to 400g individual weight, divided in two groups of 20 each and acclimatized as above, were transferred to two 250-L fiberglass tanks with an initial density of <30 kg.m−3. The environmental conditions were the same as previously described, with the exception of PO2, which was either 70–80% (hypoxia treatment) or 130–140% (hyperoxia treatment). In the latter case, total gas pressure ranged from 98 to 102% of the saturation (Novatec® 3000 tensionometer). The desired PO2 conditions were ensured by blowing pure oxygen in the system, at the pipe entering the pump. Feeding regime consisted of Nutreco Perla® extruded pellets, delivered with an automatic tape-feeder at a maintenance ratio. After 60 days, during which no fish died, nine fish from each of the two PO2 conditions were randomly caught and submitted, at the same temperature, to hypo-osmotic stress in Na+- and Cl−-free hard freshwater made by adding CaCl2 to de-ionized water with the pH buffered to 7.2 (<0.01 mmol L−1 Na+ and Cl−, 0.25 mmol L−1 Ca2+, pH 7.5, hardness 10 mg/L as CaCO3) and normoxic PO2, basically according to the protocol of CitationGonzalez and McDonald (1994). For the challenge, fish skin was previously rinsed with deionized water and thereafter 9 fish were randomly put into 3 jars sizing ≈20 L volume, 3 fishes per jar. The jars were filled with exactly 5 L of water. A 100-mL water sample was collected from each jar immediately after introducing the fish in order to determine the Na+ molarity in the water at time t0. During the osmotic challenge, fishes were exposed to a manipulation treatment as well, by manually stirring the jars, in order to magnify the ion losses by increasing blood catecholamines and metabolism. After 5, 10, 30, and 60 min, the same volume of water was sampled to determine Na+ molarity. In computing the results of the analyses, the residual water volume was corrected for the sampled amount. At the end of the trial each group of three fishes was weighed individually to calculate Na+ efflux per minute and per gram of fish. The average weight of fishes was 393.33±5 and 415.67±7.1 g among the hyperoxia and hypoxia populations, respectively (differences at P=0.33 were not significant).
Second trial
The second trial consisted in the assessment of Na+ and Cl− efflux in sea bass acclimatized under two PO2 regimens as in the first trial when exposed to a hypo-osmotic challenge for 60 min. For this purpose, two groups of 40 fishes each acclimatized as above, with an initial body mass ranging from 45 to 55 g, were randomly selected to be transferred to two 250-L fiberglass tanks where environmental conditions were the same as in the first trial, including the PO2 regimens. After 60 days, during which no fish died, 10 fishes from each of the two PO2 conditions were randomly caught and submitted, at the same temperature, to hypo-osmotic stress. Freshwater was prepared as in the first trial; however, after washing and drying, each fish was separately introduced into a jar filled with exactly 2 L water for the challenge. A manipulation treatment as well was performed, by manually stirring the jars, in order to magnify the ion loss by increasing blood catecolamines and metabolism. After 60 min of treatment, the fishes were sampled and weighed individually; the average weight was 64.52±13 and 62.48±12g among the hyperoxia and hypoxia populations, respectively (differences at P=0.78 were not significant). In three fishes per PO2 population, blood samples (about 150 µL) were promptly collected from the caudal vein with a heparinized needle and glass microtube (microsampler AVL®, Roswell, GA, USA) and immediately analyzed with a blood gas analyzer (AVL®, mod. OPTI 2) to determine Na+, Cl−, K+, pH, PO2, PCO2, TCO2, HCO3, Tot. Hb, and % hematocrit. From the 1.9 L of residual water in each jar, a 100-mL sample was collected for ion analyses.
Water analyses
All water samples were immediately stored at −20°C until the analyses. Ionic molar concentrations were determined by ionic chromatography with DIONEX DX500-DX600 and the total amount of Na+ and Cl− in the water was measured with CHROMELEON BIONEX 1999 software, version 6.11, build 490. The ion loss from the fishes in 60 min was calculated for each jar as the difference between the water ion content at the end and at the beginning of the test and dividing the obtained value by the body mass. The average ion loss, expressed as nmol g−1 min−1, was calculated for each fish population.
Statistical evaluations
Results were statistically evaluated, with the SigmaStat® programme, by one-way analysis of variance (ANOVA) followed by Tuckey’s pairwise comparison of the means.
Results and discussion
The Na+ loss at different time intervals during a 60-min hypo-osmotic challenge is reported in and . The ion efflux during the first trial was shown to be, in a 60 min, 3.74 µmol g−1 h−1 and 2.06 µmol g−1 h−1 for fish previously acclimatized under conditions of hypoxia and hyperoxia, respectively (). Therefore, fishes acclimatized at low PO2 lost Na+ 1.8 times faster than fishes acclimatized under hyperoxia. CitationGonzalez and McDonald (1992) reported for rainbow trout, immediately after epinephrine infusion, a Na+ loss that, if sustained, would approach 21% of total body Na+ per hour. In our study, Na+ efflux resulted in a rate of 163.72 and 112.23 nmol g−1 min−1, from the hypoxia and hyperoxia groups, respectively, during the first 5 min of the trial (); if sustained, this would approach 15.3 and 11.2% of total body Na+, in the same order of magnitude (assuming a total body Na+ as 0.06 mmol g−1, unpublished data). Actually, in 1 hour, the total loss approached 6.2% and 3.4% of total body Na+. Again, this is in the order of magnitude of the 3% of the total body Na+ per hour reported by CitationGonzalez and McDonald (1992) for rainbow trout during exercise.
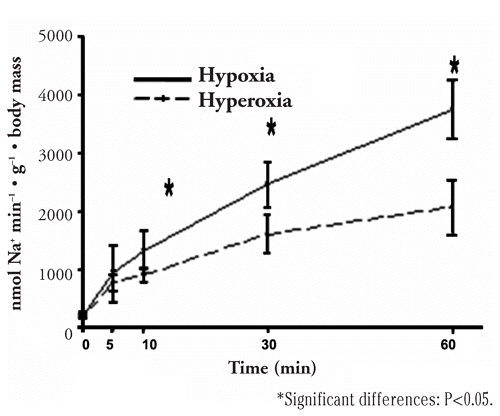
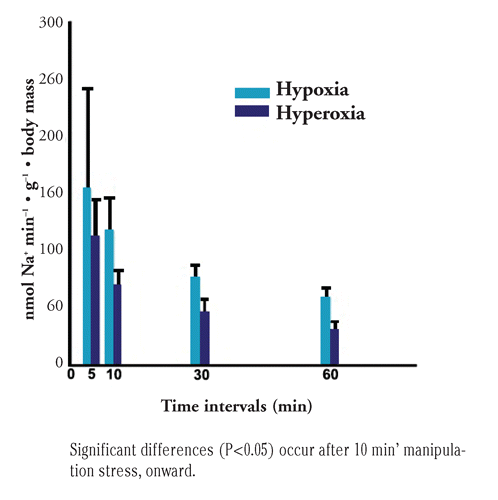
As reported in , a 60-min ion loss from the second trial resulted in a rate of 76.86±12 and 179.28±32 nmol g−1 min−1 1 for Na+ in the fish populations previously acclimatized to the hyperoxia and hypoxia regimens, respectively, approaching in 1 hour 9.6% and 18.4% of total body Na+. For Cl− this loss was 62.02±11 and 157.28±28 nmol g−1 min−1. According to statistical analyses, the rate differences among populations are highly significant for both ions œœ (P<0.001). The fact that the loss rates from the two trials do not correspond exactly can be mainly explained by the fact that different sizes may result in a different ion loosing (inversely proportional with sizes as it is resting metabolism) and in the second trial sizes were smaller than in the first one. Also, we could assume that the associated manipulation stress could not be perfectly repeated in the two trials.
Table 1 TRIAL 2: Na+ and Cl− release in water by sea bass acclimatized at two different PO2 conditions, during a 60-min osmotic challenge. Ion concentrations in water are reported at the beginning of the test and after 60 min; water jar volumes were initially 2 L.
The focus of the present study being on the relationship between the previous PO2 acclimatization regimen and ionic loss at the gills, we have actually measured flux across the whole animal. Even though the ionic loss through the skin may be considered negligible, the kidneys contribute to ionic loss (CitationMcDonald and Wood, 1981). However, it has been estimated in rainbow trout during exercise that on an hourly basis urinary Na+ excretion is unlikely to exceed 1.0 nmol g−1 min−1 (CitationGonzalez and McDonald, 1992), which is more than 100 times less than the Na+ efflux found in this experiment. This confirms that the ionic loss was occurring predominantly across the gills. We also measured increased concentrations of Na+ and Cl− in the jars, instead of the real ionic flow; therefore, the results should be interpreted as a balance between the influx and the efflux from fish to water. Nevertheless, the water utilized for the challenge was almost Na+ and Cl− free, so we may reasonably assume the Na+ and Cl− influx to be negligible.CitationGonzalez and McDonald (1992) previously described the same experimental procedure; however, they used freshwater fishes to avoid the problem of salinity transfer. In our case, sea bass that were previously acclimatized to 10 g/L salinity were transferred to freshwater and that may have resulted in an even more severe stress; nevertheless, the range of ionic loss recorded in sea bass showed the same order of magnitude of the findings by previous authors.
Evidence of a PO2 -controlled osmo-regulatory advantage
This study shows an important relationship between PO2 and the efflux of Na+ and Cl− in fishes exposed to a hypo-osmotic challenge together with manipulation stress; the Na+ and Cl− efflux was elevated 1.9- and 2.5-fold, respectively, in fishes previously acclimatized at the low PO2 conditions. This can be explained by the studies of CitationSaroglia et al. (2000) and CitationSaroglia et al. (2002) in which the experimental conditions, including the level of oxygen and exposure duration, were comparable to ours. They reported that hyperoxia is associated with discrete changes in morphology of the gill, including reduced RSA and increased GDD in the gills of sea bass; the reduced attitude to exchange gases with water (O2 and CO2) also caused a reduction in the ion efflux rate. The manipulation stress increased the catecholamine levels, as this condition probably magnifies the ion efflux by increasing intralamellar pressure; as also interpreted by CitationGonzalez and McDonald (1992), injecting epinephrine in rainbow trout achieved the same effect.
Exposure to hyperoxia, as well as hypercapnia, is reported to elevate the partial pressure of CO2 in the blood (PCO2), resulting in a respiratory acidosis (CitationHôbe et al., 1984, cit. in CitationBrauner et al., 2000). The respiratory acidosis is compensated within 24–96 h, predominantly through an increase in plasma HCO3- and an associated reduction in plasma Cl− (CitationWood and Jackson, 1980; CitationWheatly et al., 1984).CitationBrauner (1999), as well as CitationBrauner et al. (2000), reported on a dramatic change in acid-base equivalent exchanges across the gills during hyperoxia exposure in freshwater fishes and also found that hyperoxia impaired hypo-osmoregulation following seawater transfer of Atlantic salmon (Salmo salar) smolts. Actually, the latter authors exposed fishes to elevated hyperoxia for a short period, during the transport only, whereas in our study the long exposure of sea bass to mild hyperoxic conditions in brackish water could be expected to result in a physiological equilibrium. Nevertheless, our blood analyses were performed after only 1 hour of the successive hypo-osmotic plus manipulation stress; thus, new compensation dynamics might have started. No significant differences were recorded in the blood Na+ and Cl− among sea bass from the two experimental groups; nevertheless, a tentative trend was observed for reduced Cl− in the fish exposed to hyperoxia, associated with a relatively significant hypercapnia, higher oxygen concentration, and lower pH, whereas no changes were seen in HCO3- and total CO2. Again, in spite no significant differences were reported, a tentative trend for lower tot. Hb and Htc% values in the experimental group maintained under hyperoxia is reported in
Table 2 TRIAL 2: Trend on concerning blood parameters in D. labrax, previously acclimatized at two different PO2 conditions, after A 60-min osmotic challenge.
It can be also argued that the different oxygen concentrations may cause an acid-base disturbance, affecting blood pH and gill morphology. In this regard, CitationCecchini and Caputo (2003) published a study on the acid-base balance in sea bass in relation to water oxygen concentration. The study was carried out for 5 weeks under controlled conditions, the PO2 conditions being assayed at 64, 97, 150, and 250% of the saturation values and at a salinity and temperature similar to those in our experiments. In spite the authors included a quantitatively minor error in calculating the average values of the pH, it is evident from their data that no significant differences in sodium concentrations and blood PO2 were existing, whereas the blood pH was only significantly reduced by the hypoxia condition and not in the range from “normoxia” to the highest hyperoxia experienced. In contrast, plasma bicarbonate and PCO2 increased with increasing environmental PO2. Nevertheless, the differences in the reported acid-base values only affected the PO2 range to a low degree, corresponding to our environmental conditions. Although we cannot completely exclude a limited overlapping of the acid-base conditions on the direct effect of PO2 from our data, the differences in the ion regulation are a consequence of the water oxygenation technology applied.
Energy aspects
The overall picture of osmotic regulation for both freshwater and saltwater fish species is reasonably clear since the osmoregulatory organs and tissues have been identified: digestive tract, gill, kidney, urinary bladder, and liver as ureotelic regulators. Both passive and active flux of solutes, mainly NaCl plus urea, in ureotelic tissues have been assessed in many species. The chloride cell, or mitochondria-rich cell, in the teleost gill has been shown to play an important role in ionic homeostasis by compensating passive epithelial ion flux through appropriate active ion uptake and excretion (CitationEvans, 1993). Interactive effects of salinity on physiology and behavior also have to be taken into account including, among others, tissue permeability to water and ions, gill ventilation, perfusion, and functional surface area (CitationSwanson, 1998).
The question is: how much of the metabolic oxygen (MO2) is specifically dedicated to regulating the balance between minerals and water?
CitationBoeuf and Pajan (2001) report conflicting information from the literature regarding the metabolic cost of exposure to different salinities. On one hand, a drastic reduction in the metabolic rate of 20–28% at an isotonic salinity relative to that in freshwater and/or saltwater was reported (CitationRao, 1968). Such studies support the hypothesis that the energy cost of osmoregulation is lower in an isosmotic medium, where the gradients between blood and water are minimal, and that these energy savings are substantial enough to increase growth. For instance, it was estimated that osmoregulation might consume as much as 54–68% of the non-swimming metabolic output in two species of tunas (CitationBushnell and Brill, 1992). Even in species with lower metabolic rates, osmoregulation appears to use a high proportion of the available energy (CitationNilsson, 2007), while CitationBoeuf and Pajan (2001) reported that it ranges from 20 to 50% of the total energy expenditure, roughly 100–150 mL h−1 kg−1 O2 depending on the environmental salinity. Theoretically, a gill ideally designed to absorb oxygen for the energy requirements should be highly permeable, with quite a large surface area, leading to an ascending metabolic cost of osmoregulation (CitationGrau et al., 1994). These conflicting demands may represent the major constraint in the overall metabolic capacity of teleostean fishes. All fishes appear to be limited by the need for an ‘osmo-respiratory compromise’ (CitationNilsson, 1986). The energy cost of ionic regulation increases when the salinity is altered away from iso-osmotic conditions, but any attempt to quantify this cost is probably affected by other metabolic processes that respond to changes in salinity (CitationMorgan and Iwama, 1991).
A central process in osmotic regulation is active Na+ transport; a great deal of energy is consumed in moving ions through a Na+ K+-pump on the basolateral side of the transporting epithelia. The ratio of Na+ transported to ATP consumed in this process is generally known for most transport epithelia (CitationBoeuf and Payan, 2001).
It would also be possible to take into account the cost of Cl− extrusion through the gill, involving complex mechanisms (CitationMarshall et al., 1999; CitationChen et al., 2001) present in normal metabolic processes anyway.
In their study, CitationMorgan and Iwama (1999) specify that the energy required by freshwater and saltwater gills only represents a relatively small (4%) portion of the animal?s total energy budget. However, it is important to consider potential problems posed by only using Na+ flux data to calculate associated ATP usage. For instance, it can be questionned whether Na+ transport is 100% efficient. NaCl transport by mitochondria-rich chloride cells in the gills has not yet been fully elucidated. Na+ is not thought to be directly and exclusively excreted by Na+ K+;-ATPase, but rather via the basolateral tubular network, either through diffusion or an active bulk flow mechanism; thus, CitationBoeuf and Pajan (2001) argued that the energy required could be underestimated. Many of the ions transported by ATPases may simply diffuse back out of the basolateral network into body fluids or, if the bulk flow hypothesis is correct, the energy needed to drive it would also have to be estimated. Another element reported by the latter authors is that accepting a low cost for osmoregulation mechanisms would contradict the demonstrated effect of water salinity, especially in the case of saltwater, on the growth of teleosts (CitationImsland et al., 2001).
Evidence exists that elevate PO2 regimen allow fish to save energy (CitationSaroglia et al., 1998b). Moreover, an ‘on farm’ observed improvement in quality (CitationIandoli et al., 1995), increased immunoglobulin concentration (CitationScapigliati et al., 1999), and specific antibody response (CitationSaroglia et al., 1998a), when pure oxygen is applied instead of simple aeration, sound compatible with an energy saving for osmoregulation.
Conclusions
Our data are compatible with the hypothesis of an osmotic advantage of sea bass exposed to an elevated PO2 regimen. With respect to previously reported literature information on the effect of PO2 on fish GDD, FSA and RSA, this could tentatively be compatible with an advantage for osmo-respiratory compromise as well. Such results would imply that a consistent energy advantage would significantly reduce the cost on fish metabolism. Therefore, a correct evaluation of the cost for osmoregulation is envisaged and the question of the actual energy savings provided by elevated PO2 in water is still open.
Acknowledgments:
the authors wish to thank Dr. Cesare Magugliani and Nuova Azzurro hatchery, Civitavecchia (Roma), Italy, for kindly providing sea bass fingerling. This research has been granted by the Ministry of Agricultural Politicies (MiPA), Italy, under the 5th Framework for Fishery and Aquaculture, law n° 41/82.
References
- Barnabé G. 1990 Rearing bass and gilthead bream Barnabé G. Aquaculture vol. 2 Ellis Hortwood New York, NY, USA 647–686
- Boeuf G. Payan P. 2001 How should salinity influence fish growth? Comp. Biochem. Phys. C 130 411–423
- Brauner C.J. 1999 The effect of diet and short duration hyperoxia exposure on seawater transfer in coho salmon smolts (Onco -rhynchus kishutch) Aquaculture 177 257–265
- Brauner C.J. Seidelin M. Madsen S.S. Jensen F.B. 2000 Effect of freshwater hyperoxia and hypercapnia and their influences on subsequent seawater transfer in Atlantic salmon (Salmo salar) smolts Can. J. Fish. Aquat. Sci 57 2054–2064
- Bushnell P.G. Brill R.W. 1992 Oxygen transport and cardiovascular responses in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) exposed to acute hypoxia J. Comp. Physiol. B 162 131–143
- Cataudella S. Bronzi P. 2001 Acquacoltura Responsabile Uniprom-Unimar Press Roma, Italy
- Cecchini S. Caputo A.R. 2003 Acid-base balance in sea bass (Dicentrarchus labrax, L.) in relation to water oxygen concentration Aquacult. Res 34 1069–1073
- Chen J.M. Cutler C. Jacques C. Boeuf G. Denamur E. Lecointre G. Mercier B. Cramb G. Ferec C. 2001 A combined analysis of the cystic fibrosis transmembrane conductance regulator: implications for structure and diseases model Mol. Biol. Evol 18 1771–1778
- Evans D.H. 1993 Osmotic and ionic regulation Evans D.H. The Physiology of Fishes CRC Press Boca Raton, FL, USA 315–341
- Gonzalez R.J. McDonald D.G. 1992 The relationship between oxygen consumption and ion loss in a freshwater fish J. Exp. Biol 163 317–332
- Gonzalez R.J. McDonald D.G. 1994 The relationship between oxygen uptake and ion loss in fish from diverse habitats J. Exp. Biol 190 95–108
- Grau E.G. III Richman N.H. Borski R.J. 1994 Osmoreception and a simple endocrine reflex of the prolactin cell of tilapia Oreochromis mossambicus Davey K.G. Peter R.E. Tobe S.S.. Perspectives in Comparative Endocrinology National Research Council of Canada ed. Ottawa, Canada 251–256
- Henriksson P. Mandic M. Richards J.G. 2008 The osmoregulatory compromise in sculpin: impaired gas exchange is associated with freshwater tolerance Physiol. Biochem. Zool 81 310–319
- Hobe H. Wood CM. Wheatly MG. 1984 The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. I. Extra- and intracellular acid-base status Respir. Physiol 55 139–154
- Iandoli C. Alaio E. Corbari L. Saroglia M. 1995 Management and quality product: a case study 199–200 Proc. Eur. Conf. EAS Oostende, Belgium
- Imsland A.K. Foss A. Gunnarsson S. Berntssen M.H.G. FitzGerald R. Bonga S.W. Ham E.V. Nævdal G. Stefansson S.O. 2001 The interaction of temperature and salinity on growth and food conversion in juvenile turbot (Scophthalmus maximus) Aquaculture 198 353–367
- Jobling M. 1994 Dissolved gases Joblin M. Fish Bioenergetics Chapman and Hall London, UK 231–266
- Marshall W.S. Embherley T.R. Singer T.D. Bryson S.E. McCormick S.D. 1999 Time course of salinity adaptation in a strongly euryhaline estuarine teleost, Fundulus heteroclitus: a multivariable approach J. Exp. Biol 202 1535–1544
- McDonald D.G. Wood C.M. 1981 Branchial and renal acid ion fluxes in the rainbow trout, Salmo gairdneri at low environmental pH J. Exp. Biol 93 101–118
- Morgan J.D. Iwama G.K. 1991 Effects of salinity on growth, metabolism and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall Chinook salmon (Oncorhinchus tshawytscha) Can. J. Fish. Aquat. Sci 48 2083–2094
- Morgan J.D. Iwama G.K. 1999 Energy cost of NaCl transport in isolated gills of cutthroat trout Am. J. Physiol 277 631–639
- Nilsson G.E. 2007 Gill remodeling in fish - a new fashion or an ancient secret? J. Exp. Biol 210 2403–2409
- Nilsson S. 1986 Control of gill blood flow Nilsson S. Holmgren S. Fish Physiology: Recent Advances Croom Helm, London, UK 86–101
- Olson K.R. 1991 Vasculature of the fish gill: anatomical correlates of physiological functions J. Electron. Microsc. Tech 19 389–405
- Perry S.F. McDonald D.G. 1993 Gas Exchange Evans D.H. The Physiology of Fishes CRC Press Boca Raton, FL, USA 251–278
- Randall D.J. Baumgarten D. Malyusz M. 1972 The relationship between gas and ion transfer across the gills of fishes Comp. Biochem. Physiol. A 41 629–637
- Randall D.J. Daxboeck C. 1984 Oxygen and carbon dioxide transfer across fish gills Hoar W.S. Randall D.J. Fish Physiology Vol. 10A Academic Press Orlando, FL, USA 263–314
- Rao G. 1968 Oxygen consumption in rainbow trout (Salmo gairdneri) in relation to activity and salinity Can. J. Zool 46 781–786
- Sardella A.B. Brauner C.J. 2007 The osmo-respiratory compromise in fish: the effects of physiological state and the environmental Fernandes M.N. Rantin F.T. Glass M.L. Kapoor B.G. Fish respiration and environment Science Publishers Enfield, NH, USA 147–165
- Saroglia M. 2001 L'ossigenazione delle acque Cataudella S. Bronzi P. Acquacoltura Responsabile Uniprom-Unimar Press Roma, Italy 375–394
- Saroglia M. Cecchini S. Terova G. Caputo A. De Stradis A. 2000 Influence of environmental temperature and water oxygen concentration on gas diffusion distance in Sea bass (Dicentrarchus labrax, L.) Fish Physiol. Biochem 23 55–58
- Saroglia M. Cecchini S. Terova G. Caricato G. De Stradis A. 1998 Studio su alcuni meccanismi di adattamento del pesce eurialino alle condizioni di iperossigenazione, in relazione all'ottimizzazione zootecnica: primi risultati Biologia Marina Mediterranea 5 1688–1698
- Saroglia M. Knight M. Terova G. Cecchini S. 1998 Ruolo dell'iperossigenazione e degli incrementi nutrizionali nella minimizzazione del rilascio di azoto dagli allevamenti intensivi di spigola (Dicentrarchus labrax, L.) Biologia Marina Mediterranea 5 1669–1674
- Saroglia M. Terova G. De Stradis A. Caputo A. 2002 Morphometric adaptations of sea bass gills to different dissolved oxygen partial pressure J. Fish Biol 60 1423–1430
- Saroglia M. Terova G. Prati M. 2007 Dissolved oxygen and gill morphometry Fernandes M. N. Rantin F.T. Glass M.L. Kapoor B.G. Fish respiration and environment Science Publishers Enfield, NH, USA 167–190
- Scapigliati G. Scalia D. Marras A. Meloni S. Mazzini M. 1999 Immunoglobulin levels in the teleost Sea bass Dicentrarchus labrax (L.) in relation to age, season, and water oxygenation Aquaculture 174 207–212
- Swanson C. 1998 Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos) J. Exp. Biol 201 3355–3366
- Wheatly M.G. Hôbe H. Wood C.M. 1984 The mechanisms of acid-base ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subseguent normoxia. II. The role of kidney Resp. Physiol 55 155–173
- Wood C.M. Jackson E.B. 1980 Blood acid-base regulation during environmental hyperoxia in the rainbow trout (Salmo gairdneri) Resp. Physiol. Neurobi 55 155–173