Abstract
This study evaluated the effects of doses of exogenous fibrolytic enzymes (enzyme) on in vitro (IVD) and in sacco degradation (ISD) of dry matter (DM) and neutral detergent fibre (NDF) of diets with 70% concentrate (as DM), as well as their effects on growth performance in lambs. A gas production technique was used to determine IVD. Six ruminally cannulated lambs in a replicated 3×3 Latin Square were used to determine ISD. Three diets (treatments) added with enzyme (0, 3 and 6 g enzyme/kg DM) were evaluated for IVD and ISD. For the growth assay, 48 lambs (17.6±2.5 kg of body weight) fed on two diets with 0 or 3 g enzyme/kg DM were used.
There were linear increases of gas production rate as enzyme level in the diets increased. At 48 h of fermentation, there were quadratic increases of IVD as enzyme level increased in the diets. A quadratic change was observed in volatile fatty acids and ammonia N as enzyme was increased in the diet. At 12 h, the highest enzyme level (6 g) increased ISD of DM as compared with the control. There was a quadratic effect on the disappearance rate as enzyme level in the diet increased.
Exogenous fibrolytic enzymes improved IVD degradation and fermentation characteristics as well as ISD rate of DM, but there were not any effect of these enzymes on ISD of NDF of diets and growth performance of finishing lambs.
Introduction
Improving degradation of fibrous and non-fibrous carbohydrates in the rumen is important for feed utilization in ruminants. Exogenous fibrolytic enzymes have improved feed intake (CitationJalilvand et al., 2007; CitationKrueger et al., 2008), which could be attributed to increased ruminal fibre digestion (CitationEun and Beauchemin, 2008), but the mechanism of this increment is not understood. The use of fibrolytic enzymes as feed additives to improve degradation of fibre has been studied under in vitro, in sacco and in vivo conditions, but the responses have been highly variable. Several factors such as enzyme doses (CitationColombatto et al., 2007) and type of diet (CitationPinos-Rodriguez et al., 2008) could affect the fibrolytic activity of exogenous enzymes (CitationBeauchemin et al., 2003). Indeed, fibrolytic enzymes increased degradation of substrates, but it depends on proportion of concentrate in the diet (CitationGiraldo et al., 2008) and enzyme doses (CitationJalilvand et al., 2008). Besides, the optimal level of enzyme could depend on the diet, indicating the need to determine the optimum application rate of enzyme preparation for individual feeds (CitationYang et al., 1999). Therefore, the objectives of this study were to evaluate the effects of an exogenous fibrolytic enzyme mixture on in vitro degradation, in sacco disappearance of a diet with 70% concentrate, as well as its effects on growth performance of lambs.
Materials and methods
This experiment was conducted under the supervision and approval of the Academic Committee of Instituto de Recursos Genéticos y Productividad of the Campus Montecillo, Colegio de Postgraduados, according to regulations established by the Animal Protection Law, enacted by the State of México in México. Three diets (treatments) with three levels of fibrolytic enzymes: 1) 0 g enzymes (control); 2) low level (3 g enzyme/kg DM); 3) high level (6 g enzyme/kg DM), were formulated for lambs. Diets were 70% concentrate and 30% forage (). The dry matter (DM) of feeds was determined by oven drying at 65°C to a constant weight. Samples were ground with a Wiley Mill fitted with a 1 mm screen (Arthur H. Thomas, Philadelphia, PA, USA) for chemical analysis or a 2 mm screen for in vitro degradation and in sacco ruminal disappearance determination. Hundred grams of sample were frozen at 4°C, and then analyzed for DM, nitrogen (N), acid detergent fibre (ADF), ash (CitationAOAC, 1995), and neutral detergent fibre (NDF; CitationVan Soest et al., 1991).
Table 1 Ingredients and chemical composition of experimental diets.
The enzyme product (Fibrozyme, Alltech Inc., Nicholasville, KY, USA) was extensively characterized by CitationRanilla et al. (2008), who detected that at pH 6.5 and 39°C, 1 g of enzyme preparation liberated 583 Amol of xylose per min from oat spelt xylan and 163 Amol per min of glucose from carboxymethyl-cellulose. The enzyme was a powder mixture which was mixed daily with the diet.
A manual system was used to measure gas production through in vitro incubation at 39°C, according to CitationTheodorou et al. (1994). The incubations were conducted in glass flasks (125 mL), sealed with butyl rubber stoppers and a screwed plastic cap, containing 90 mL of a the culture medium (CitationMalafaia et al., 1999), 10 mL of the ruminal inoculum and 500 mg of DM of experimental diets (). Rumen inoculum was obtained from lambs fed with each corresponding diet (treatment) used in the ISD trial. The samples were incubated in triplicate for each treatment and time. The gas pressure was obtained by manometric readings (0 to 1 kg/cm2), while the volume was measured by a graduated syringe (10 mL). The determinations were done at 0, 1, 2, 3, 5, 7, 9, 12, 16, 20, 24, 30, 42, 54, 66, 78, 90 and 96 h after the addition of the ruminal inoculum. Immediately after the inoculation of the ruminal inoculum, an initial reading was taken and used to standardize the pressure and discharge the gas volume in all flasks. To quantify the gas production derived from the culture medium and the ruminal inoculum, four flasks were used as a blank. The pressure and volume values were registered and added to the values of the previous readings. Thus, the cumulative pressure and volume of the fermentation gases were obtained.
To evaluate in vitro ruminal fermentation and IVD, on 48 h of fermentation, ruminal fluid samples and DM residuals were collected from two additional glass flasks per treatment. The fluid samples were acidified with 3 M metaphosphoric acid (1:10 dilution), cooled at 4°C for 30 min, and centrifuged (25,000 × g; 4°C; 20 min). Supernatants were removed and frozen. On supernatants, volatile fatty acid (VFA; CitationErwin et al., 1961) with a gas chromatograph (Claurus 500, Perkin Elmer), and ammonia-N concentrations (CitationMcCullough, 1967) with a UV-VIS spectrophotometer (630 nm, CARY I-E, VARIAN), were determined. The solid residues were filtered (Whatman 541). During filtration, in order to minimize the microbial matter attached to residues, the residues were rinsed with acetone until the became clear. Filters and residues were dried for 24 h at 90°C and weighed after cooling in desiccators.
Linear regression analysis was performed with the pressure and volume data and regression coefficients were calculated. The volume of gas corrected by the pressure related to the incubated DM was calculated according to CitationTheodorou et al. (1994). The DM cumulative gas production profiles were evaluated using the logistic model as reported by CitationMalafaia et al. (1999): V(t) = VF/1 + exp [2+4R (L−t)], where V represent the total gas produced from the digested fraction at time t, its respective gas production rate R and the duration of the initial gas volume L.
In vitro incubation times were used to fit non-linear regression models using the ‘NLIN’ procedure (CitationSAS, 1999). The experimental design for this study was a completely randomized with three treatments; triplicates (glass flasks) were included to provide the error term and the GLM procedure (CitationSAS, 1999) was used.
In an in situ trial, six Criollo sheep (3.5 years old; 53±6.8 kg body weight) fitted with ruminal cannulas (Bar Diamond Inc., Parma, ID) were used in a double 3×3 Latin square design balanced for residual effects. Lambs were housed in individual metabolic pens in a naturally ventilated barn. Diets (treatments) were offered at 8:00 and 16:00 hours. Lambs had free choice access to diets and water. The experiment comprised 6 periods of 21 days as follows: days 1 to 19, adaptation; days 20 and 21, ruminal in sacco incubations. Eighteen bags (5×7.5 cm; pore 52±7 µ) each with 5 g DM of experimental diets () were placed in the rumen of each lamb at 08:00 and removed (three for each time) at 3, 6, 12, 24, and 48 h. Before insertion into the rumen, three additional bags per sample were manually washed with water (39°C) for 20 min, and the solubility was calculated. In sacco disappearances of DM and NDF of diets were calculated using the values determined before and after ruminal incubation of bags.
In sacco ruminal kinetics of DM and NDF were calculated using the Gompertz model reported by CitationSusmel et al. (1999) as: dis(t) = a + (b exp[(−C) exp(−Dt)]), where: dis is the disappearance (g/kg) from the bag at time t (h); a is the ruminally soluble DM fraction (g/kg); b is the insoluble, but potentially disappearing fraction (g/kg); C is the fractional disappearance rate of a + b; and D is a parameter to measure rate of disappearance. According to the Gompertz model, the fractional rate of disappearance varies as a function of time, and the average value (i.e., a constant comparable to the exponential rate of disappearance) is derived as: c= D/C.
In sacco incubation times were used to fit non-linear regression models using the ‘NLIN‘ procedure (CitationSAS, 1999). The experimental design for IVD was a completely randomized with three treatments; triplicates (glass flasks) were included to provide the error term and the GLM procedure (CitationSAS, 1999) was used. The experimental design for ISD was a replicated 3×3 Latin Square including square (fixed, 1 df), sheep within square (random, 4 df), period (random, 2 df), diet (fixed, 2 df), and error (8 df).
In a performance trial, forty eight lambs [(17.6±2.5 body weight (BW)] were devided into groups of three animals, as per their initial BW. Within each block an animal was assigned randomly to one of three dietary treatments. The enzyme was mixed daily with the feed stuffs immediately before the morning feeding (08:00). Lambs had free access to diets and water. Feed refusals were recovered and weighed daily. Body weight was recorded each 21 d during 84 days, and average daily gain (ADG) was calculated. Individual dry matter intake (DMI) and ADG were used to calculate feed efficiency (DMI/ADG).
The growth assay was analyzed as a randomized complete block design with pens as blocking factors. Because interactions of treatment × period were not significant, ADG, DMI, and feed conversion data were averaged (84 days).
Those data were analyzed using Proc Mixed (CitationSAS, 1999). The model included block (random, 15 df) and enzyme level (fixed, 2 df), and period (fixed, 3 df). The effect of enzyme on initial BW, final BW and total gain were evaluated using the same model, except those cases in which an average value was used. The covariance structure that resulted in the lowest Akaike’s information was ARH(1).
In all studies (in vitro, in sacco and in vivo), polynomial effects (linear and quadratic) were used to evaluate the effects of increasing exogenous fibrolytic enzymes in the diets.
Results
Ingredients and chemical composition of experimental diets are shown in . Parameters of gas production kinetic of DM at 0 to 96 h of fermentation, as wells as values for IVD, VFA, and ammonia N at 48 h fermentation are shown in . Enzyme did not affect total gas produced (V). There were linear increases for duration of initial duration of initial gas volume (L) and gas production rate (R), as the enzyme level was increased in the diet.
Table 2 Effect of fibrolytic enzymes on kinetics of ruminal in vitro gas production of dry Matter fermentation.
At 48 h fermentation, a quadratic increment of IVD was detected as enzyme level in the diet increased. At the same fermentation time, enzymes did not affect total VFA and acetate concentrations (mmol/L), but propionate and butyrate were affected quadratically as enzyme level increased in the diets. Thus, the highest concentration of propionate was with the low level (3 g) of enzyme, and the highest concentration of butyrate was with control diet (0 g enzyme). As enzyme level in the diet increased, there was a linear reduction on in vitro ammonia N concentrations.
Enzymes did not affect ISD at 3, 6, 24 and 48 h of incubation, but at 12 h 6 g enzyme/kg DM increased ISD as compared to 0 and 3 g enzyme (). Kinetics of ISD for both, DM and NDF, are shown in . For DM, enzymes did not affect its soluble (a), potentially disappearance (b) and total disappearance (a + b); however, a quadratic increase of disappearance rate was found as the enzyme in the diet increased. For NDF, enzymes did not affect the parameter of ISD kinetics.
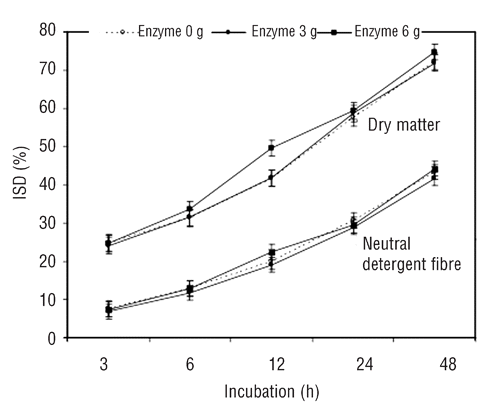
Table 3 Effect of fibrolytic enzymes on kinetics of ruminal in sacco disappearance.
Initial and final BW, total gain, ADG, DMI, and feed conversion (DMI/ADG) are shown in . Enzymes (3 g/kg DM) did not have any effects on these variables evaluated in lambs fed a 70% concentrate diet.
Table 4 Effect of fibrolytic enzymes in lambs.
Discussion
Level of enzyme did not affect total gas production (GP), that is, total fermentable material was not increase. CitationColombatto et al. (2003b) and CitationJalilvand et al. (2008) evaluated two levels of two enzyme products on GP and concluded that final GP values of forages were not increased by enzyme addition. The fitted GP data showed that increasing enzyme levels in the diets caused a linear increase on gas production rates. Positive responses to enzyme addition level in the rate of GP have been reported (CitationColombatto et al., 2003a). Our findings suggest that the enzyme was able to degrade complex substrates to simpler ones, allowing a faster ruminal colonization and fermentation, as reported by CitationColombatto et al. (2003a). The lack of effects on final GP suggests that the substrates degraded by the enzymes, would have been degraded in the medium anyway, albeit at a later time (CitationColombatto et al., 2007). In contrast, the increments on the lag phase brought about the enzyme, as observed in our experiment, have been discussed also in low-quality roughages (CitationJalilvand et al., 2008; CitationTang et al., 2008). Indeed, CitationForsberg et al. (2000) postulated that addition of an enriched polysaccharidase enzyme results on an immediate attack by microorganisms, provided that there is available carbohydrate to facilitate more rapid microbial growth and shortening the lag time.
At 48 h of fermentation, the IVD of DM was increased linearly as enzyme level increased. Indeed CitationTang et al. (2008) found that exogenous fibrolytic enzymes (5.0 and 7.5 g/kg) increased IVD of DM of forages. In our in vitro study, the enzyme (3 g/kg DM) increased molar proportion of propionate and decreased molar proportion of butyrate as compared the control. Changes in VFA proportions as a direct effect of adding exogenous fibrolytic enzymes have been reported, implying that these enzymes could affect microbial growth and/or shift the metabolic pathways by which specific microbes utilize substrates (CitationEun and Beau-chemin, 2008).
At 12 h, but nor after and later, enzymes increased ISD of DM (). The most active period for a fibrolytic enzymes mixture appears to be during the first 12 h (CitationMoreno et al., 2007). It is possible that no effects would have been observed had the incubation been extended longer, which supports the hypothesis that enzymes stimulate initial phase degradation of substrate, increasing just degradation rate, but not extension of ruminal degradation (CitationPinos-Rodríguez et al., 2002; CitationGiraldo et al., 2008). Although the enzyme increased ISD rate of DM, the other kinetic parameters were not affected, results that do not agree with that reported by CitationPinos-Rodriguez et al. (2008) who found that the same enzyme mixture enhanced potentially disappearance fraction and its disappearance rate of diets and feeds. Effects of fibrolytic enzyme mixtures are not limited to the dietary component to which the enzymes are applied. This would explain why fibrolytic enzymes can effectively improve ISD of DM fraction, besides increasing ISD of fibre components of diets, mostly when enzymes are added to the concentrate fraction of a diet or to high-concentrate diets (CitationBeauchemin et al., 2003). Besides, CitationPinos-Rodríguez et al. (2002) reported that in lambs these enzymes increased apparent digestibility of CP in addition to the NDF apparent digestibility in alfalfa hay.
CitationBeauchemin et al. (2003) indicated that fibrolytic enzymes cause highly variable responses on intake, body weight gain, and feed efficiency in finishing beef cattle fed high-grain diets. No previous evidence was found about the effect of enzymes on BW changes and feed efficiency in lambs fed 70% concentrate diets, but our results indicate that a fibrolytic enzymes mixture did not change BW in lambs. This lack of effect on growth performance could be attributed to the fact that there was not preincubation between the enzyme and the substrate (diet). Thus, a preincubation period is very important (CitationElwakeel et al., 2007; CitationKrueger and Adesogan, 2008) to allow, before feeding, a proper adsorption and binding of the enzyme to substrate, attachment and protection against degradation by rumen proteases (CitationForwood et al., 1990; CitationBeauchemin et al., 2003) and a stable enzyme-feed complex (CitationKung et al., 2000), all of which apparently did not take place in our study.
Conclusions
The results suggest that 6 g of exogenous fibrolytic enzymes improved IVD and ISD of DM of experimental diets, however there was not any positive effects of these enzymes on ISD of NDF of diets as well on growth performance of finishing lambs. Due to this concern, further studies under in vivo conditions are needed to evaluate the factors that can affect the enzyme action to improve fibre digestion and animal performance.
References
- AOAC 1995 Official Methods of Analysis 16th ed. Association of Official Analytical Chemists Arlington, VA, USA
- Beauchemin K.A. Colombatto D. Morgavi D.P. Yang W.Z. 2003 Use of exogenous fibrolytic enzymes to improve feed utilization by ruminants J. Anim. Sci 81 37–47
- Colombatto D. Mould F.L. Bhat M.K. Morgavi D.P. Beauchemin K.A. Owen E. 2003a Influence of fibrolytic enzymes on the hydrolysis and fermentation of pure cellulose and xylan by mixed ruminal micro organisms in vitro J. Anim. Sci 81 1040–1050
- Colombatto D. Mould F.L. Bhat M.K. Owen E. 2003b Use of fibrolytic enzymes to improve the nutritive value of ruminant diets. A biochemical and in vitro rumen degradation assessment Anim. Feed Sci. Tech 107 201–209
- Colombatto D. Mould F.L. Bhat M.K. Owen E. 2007 Influence of exogenous fibrolytic enzyme level and incubation pH on the in vitro ruminal fermentation of alfalfa stems Anim. Feed Sci. Tech 137 150–162
- Elwakeel E.A. Titgemeyer E.C. Johnson B.J. Armendariz C.K. Shirley J.E. 2007 Fibrolytic enzymes to increase the nutritive value of dairy feedstuffs J. Dairy Sci 90 5226–5236
- Erwin E.S. Marco G.J. Emery E.M. 1961 Volatile fatty acid analysis of blood and rumen fluid by gas chromatography J. Dairy Sci 44 1768–1776
- Eun J.S. Beauchemin K.A. 2008 Relationship between enzymatic activities and in vitro degradation of alfalfa hay and corn silage Anim. Feed Sci. Tech 14 53–67
- Forsberg C. Forano E. Chesson A. 2000 Microbial adherence to the plant cell wall and enzymatic hydrolysis Cronje P.B. Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction CABI Publishing Wallingford, UK 79–97
- Forwood J.R. Sleper D.A. Henning J.A. 1990 Topical cellulose application effects on tall fescue digestibility Agron. J 82 909–913
- Giraldo L.A. Tejido M.L. Ranilla M.J. Carro M.D. 2008 Effects of exogenous fibrolytic enzymes on in vitro ruminal fermentation of substrates with different forage:concentrate ratios Anim. Feed Sci. Tech 141 306–325
- Jalilvand G. Naserian A. Odongo N.E. Kebreab E. Valizadeh R. Eftekhar Shahrodi F. France J. 2007 Effects of abomasal infusion of cottonseed oil and dietary enzyme supplementation on dairy goats J. Anim. Feed Sci 16 389–396
- Jalilvand G. Odongo N.E. López S. Naserian A. Valizadeh R. Eftekhar Shahrodi F. Kebreab E. France J. 2008 Effects of different levels of an enzyme mixture on in vitro gas production parameters of contrasting forages Anim. Feed Sci. Tech 146 289–301
- Krueger N.A. Adesogan A.T. 2008 Effects of different mixtures of fibrolytic enzymes on digestion and fermentation of bahiagrass hay Anim. Feed Sci. Tech 145 84–94
- Krueger N.A. Adesogan A.T. Staples C.R. Krueger W.K. Kim S.C. Littell R.C. Sollenberger L.E. 2008 Effect of method of applying fibrolytic enzymes or ammonia to Bermudagrass hay on feed intake, digestion, and growth of beef steers J. Anim. Sci 86 882–889
- Kung L. Jr Treacher R.J. Nauman G.A. Smagala A.M. Endres K.M. Cohen M.A. 2000 The effect of treating forages with fibrolytic enzymes on its nutritive value and lactation performance of dairy cows J. Dairy Sci 83 115–122
- Malafaia P.A.M. Filho S.C.V. Vieira R.A.M. 1999 Kinetic parameters of ruminal degradation estimated with a non-automated system to measure gas production Livest. Prod. Sci 58 65–73
- McCullough H. 1967 The determination of ammonia in whole blood by a direct colorimetric method Clin. Chem. Acta 17 297–304
- Moreno R. Pinos-Rodríguez J.M. González S. Álvarez G. Garcia J.C. Mendoza G. Bárcena R. 2007 Effect of exogenous fibrolytic enzymes on in vitro ruminal degradation of rations for dairy cows Interciencia 32 850–853
- Pinos-Rodríguez J.M. González S.S. Mendoza GD. Bárcena J.R. Cobos M.A. Hernández A. Ortega M.E. 2002 Effect of exogenous fibrolytic enzyme on ruminal fermentation and digestibility of alfalfa and rye-grass hay fed to lambs J. Anim. Sci 80 3016–3020
- Pinos-Rodríguez J.M. Moreno R. González S.S. Robinson P.H. Mendoza G. Álvarez G.A. 2008 Effects of exogenous fibrolytic enzymes on ruminal fermentation and digestibility of total mixed rations fed to lambs Anim. Feed Sci. Tech 142 210–219
- Ranilla M.J. Tejido M.L. Giraldo L.A. Tricárico J.M. Carro M.D. 2008 Effects of an exogenous fibrolytic enzyme preparation on in vitro ruminal fermentation of three forages and their isolated cell walls Anim. Feed Sci. Tech 145 109–121
- SAS 1999 User’s Guide. Version 6.12 SAS Institute Inc Cary, NC, USA
- Susmel P. Spanghero M. Stefanon B. 1999 Interpretation of rumen degradability of concentrate feeds with a Gompertz model Anim. Feed. Sci. Tech 79 223–237
- Tang S.X. Tayo G.O. Tan Z.L. Sun Z.H. Shen L.X. Zhou C.S. Xiao W.J. Ren G.P. Han X.F. Shen S.B. 2008 Effects of yeast culture and fibrolytic enzymes supplementation on in vitro J. Anim. Sci 86 1164–1172
- Theodorou M.K. Williams B.A. Dhanoa M.S. McAllan A.B. France J. 1994 A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds Anim. Feed Sci. Tech 48 185–197
- Van Soest P.J. Robertson J.B. Lewis B.A. 1991 Symposium: carbohydrate methodology, metabolism, and nutritional implications in dairy cattle J. Dairy Sci 74 3583–3597
- Yang W.Z. Beauchemin K.A. Rode L.M. 1999 Effects of an enzyme feed additive on extent of digestion and milk production of lactating dairy cows J. Dairy Sci 82 391–403