Abstract
CD34 is a cell surface glycoprotein expressed by hematopoietic progenitors and endothelial cells. It is widely used in the clinic for isolation of human hematopoietic stem cells. In recent years large animals are gaining increasing importance in biomedical research for the study and therapy of human diseases. Sheep has proved to be an useful experimental model for preclinical trials in transplantation procedures. Unfortunately, the lack of specie-specific monoclonal antibodies (MABS) recognizing hemopoietic progenitor cells hampers the use of this animal in experimental hematology. The aim of this paper was to determine whether commercial monoclonal antibodies specific for human CD34 molecule could cross-react with hematopoietic progenitor cells (HPC) present in sheep umbilical cord blood (UCB). Six antihuman CD34 MABS, recognizing the three different epitope classes, were tested in flow cytometry on purified mononuclear cells (MNC) isolated from cord blood of both species. None of the MABS used in this trial seemed to be able to identify HPC from sheep UCB. These data suggest that the panel of monoclonal antibodies used for cross reactivity detection has to be expanded with recently produced reagents. Further studies should be directed towards the production of ovine specific anti CD34 MABS.
Introduction
CD34 is a transmembrane glycoprotein, member of the sialomucin family whose physiological roles are still poorly understood (CitationLanza et al., 2001). At present, CD34 represents the only currently known cell surface antigen able to identify early hematopoietic progenitor cells of all lineages (CitationCivin et al., 1984; CitationKrause et al., 1996). The heavily glycosylated cell surface molecule gives rise to a number of different epitopes (CitationWatt et al., 1987). Monoclonal antibodies have been recognized in three classes according to their epitopes’ recognition and sensitivity to enzymatic cleavage with different glycoproteases (CitationSutherland et al., 1992). The importance of CD34 molecule for experimental hematology is well recognized (CitationGratama et al., 1998), but availability of specific monoclonal antibodies is still limited to few animal species. The surface expression of CD34 antigens is commonly used to discriminate between progenitor cells and mature blood cells in humans (CitationAndrews et al., 1986; CitationBerenson et al., 1991; CitationHe et al., 1992), nonhuman primates (CitationBerenson et al., 1988; CitationIzawa et al., 2004), rodents (CitationBrown et al., 1991; CitationKrause et al., 1994; CitationMorel et al., 1996; CitationGarlanda et al., 1997) and dogs (CitationMcSweeney et al., 1998; CitationNiemeyer et al., 2001).
The use of animal models in preclinical trials is fundamental for estimating the safety and efficacy of innovative treatments such as cell and gene therapy. Large animals, rather than commonly used laboratory rodents, allow a better transposition of biomedical research results into clinical design. Sheep has been largely employed to test efficacy of stem cells transplantation procedures (CitationMenard et al., 2005; CitationMenasche, 2005; CitationNarayan et al., 2006) and it is considered an established animal model for tissue engineering (CitationErggelet et al., 2007; CitationMendelson et al., 2007; CitationSales et al., 2007). At the moment, the lack of monoclonal antibodies recognizing ovine hematopoietic progenitor cells hampered the use of this animal for developing new transplantation procedures useful in hematology disorders therapy. For this reason it is still of considerable value to test MABS defined in other species for cross-reactivity. The aim of this study was to determine by use of flow cytometry analysis whether monoclonal antibodies specific for three different epitopes of human CD34 molecule could identify hemopoietic progenitor cells in sheep umbilical cord blood.
Materials and methods
Samples of UCB was collected from the umbilical vein of five sheep undergoing caesarian section and from five women after vaginal delivery. MNC were isolated in both species using a density gradient centrifugation (Hystopaque-1077; Sigma). Cord blood MNC were analyzed in flow cytometry for cell-surface expression of CD34 using different MABS conjugated with phycoerythrin (PE) recognizing the three different classes of human epitopes: Immu 133 (class I; IgG1, Immunotech, Beckman Coulter, Fullerton, CA, USA), Q-bend/10 (class II; IgG1, Immunotech Beckman Coulter, Fullerton, CA, USA), 563 (class II; IgG1, BD; San Jose, CA, USA), 8G12 (class III; IgG1, BD, San Jose, CA, USA), AC136 (class III; IgG1, Miltenyi Biotec, Bergisch Gladbach, Germany) and 581 (class III; IgG1, Immunotech Beckman Coulter, Fullerton, CA, USA). Immu 133, Qbend/10 and 581 MABS were purchased pooled together in liquid form (CD34 pool). An isotypic control in order to discriminate for non-specific reaction of the tested MABS (Mouse IgG1-PE, Beckman Coulter) was used. An amount of 1×106 cells in a 100 µL final volume of each sample were incubated in dark for 30 minutes on ice, following manufacturers suggestion for antibodies dilution: Immu 133, Q-bend/10, 563, 8G12 and 581 MABS were used at 1:5 dilution while AC136 at a dilution of 1:11. Stained cells were analyzed on a FacsCalibur (BD) flow cytometer. Thirty thousand events were acquired for each samples, and analysis were performed by Cell Quest Pro (BD).
Results and discussion
To our knowledge, this is the first attempt to identify ovine cord blood HPC using human specific anti-CD34 MABS.
In our experimental conditions, none of the tested commercial anti-CD34 MABS was able to recognize sheep cord blood HPC. On the contrary, analysis of human UCB samples, used as control, showed that a percentage of MNC ranging from a low of 0,5% to a high of 2% were CD34 positive, in accordance with data reported by other authors (CitationD’arena et al., 1996; CitationBelvedere et al., 1999; CitationEnde et al., 2001) ().
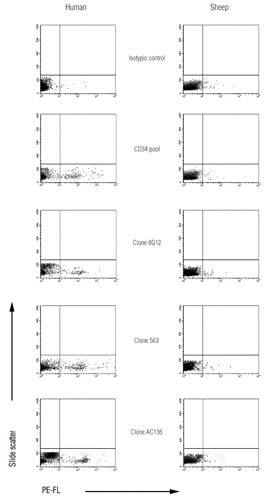
The anti-human CD34 MABS tested in flow cytometry did not prove to be cross-reacting with ovine UCB cells, suggesting that CD34 molecule primary and tertiary structure differs in the two species. Indeed, neither the use of MABS directed to class I and II linear epitopes, nor MABS directed to class III conformational epitopes resulted in a positive reaction.
The choice to test human-specific products was mainly determined by the availability of several anti-human MABS clones directed against the three different CD34 epitopes. However, the possibility that commercially available MABS produced for other species could cross-react with ovine cells should be considered. Recently, CitationSakurai et al. (2006) reported the production of two monoclonal antibodies against bovine CD34. It might be expected that anti-CD34 MABS raised against bovine antigen cross-reacted with ovine cells. Unfortunately, we were not able to test these bovine specific MABS since they still were not commercially available.
Conclusions
In conclusion, our findings suggest that the number of monoclonal antibodies for cross-reactivity detection need to be extended with those of recent production and that further studies should be directed toward the production of specific ovine anti-CD34 MABS.
References
- AndrewsR.G. SingerJ.W. BernsteinI.D. 1986 Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors Blood 67 842–845
- BelvedereO. FeruglioC. MalangoneW. BonoraM.L. DoniniA. DoroteaL. TonuttiE. RinaldiC. PittinoM. BaccaraniM. Del FrateG. BiffoniF. SalaP. HilbertD.M. DegrassiA. 1999 Phenotypic characterization of immunomagnetically purified umbilical cord blood CD34+ cells Blood Cell. Mol. Dis 25 141–146
- BerensonR.J. AndrewsR.G. BensingerW.I. KalamaszD. KnitterG. BucknerC.D. BernsteinI.D. 1988 Antigen CD34+ marrow cells engraft lethally irradiated baboons J. Clin. Invest 81 951–955
- BerensonR.J. BensingerW.I. HillR.S. AndrewsR.G. Garcia-LopezJ. KalamaszD.F. StillB.J. SpitzerG. BucknerC.D. BernsteinI.D. ThomasE.D. 1991 Engraftment after infusion of CD34+ marrow cells in patients with breast cancer or neuroblastoma Blood 77 1717–1722
- BrownJ. GreavesM.F. MolgaardH.V. 1991 The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts Int. Immunol 3 175–184
- CivinC.I. StraussL.C. BrovallC. FacklerM.J. SchwartzJ.F. ShaperJ.H. 1984 Antigenic analysis of hematopoiesis III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-la cells J. Immunol 133 157–165
- D'ArenaG. MustoP. CascavillaN. Di GiorgioG. ZendoliF. CarotenutoM. 1996 Human umbilical cord blood, immunophenotypic heterogeneity of CD34+ hematopoietic progenitor cells Haematologica 81 404–409
- EndeN. LuS. AlcidM.G. Ruifeng ChenR. MackR. 2001 Pooled umbilical cord blood as a possible universal donor for marrow reconstitution and use in nuclear accidents Life Sci 69 1531–1539
- ErggeletC. NeumannbK. EndresbM. HaberstrohcK. SittingercM. KapsC. 2007 Regeneration of ovine articular cartilage defects by cell-free polymer-based implants Biomaterials 28 5570–5580
- GarlandaC. BerthierR. GarinJ. StoppacacciaroA. RucoL. VittetD. GulinoD. MatteucciC. MantovaniA. VecchiA. DejanaE. 1997 Characterization of MEC 14.7, a new monoclonal antibody recognizing mouse CD34, a useful reagent for identifying and characterizing blood vessels and hematopoietic precursors Eur. J. Cell. Biol 73 368–377
- GratamaJ.W. OrfaoA. BarnettD. BrandoB. HuberA. JanossyG. JohnsenH.E. KeeneyM. MartiG.E. PreijersF. RotheG. SerkeS. SutherlandD.R. Van der SchootC.E. SchmitzG. PapaS. 1998 Flow cytometric enumeration of CD34+ hematopoietic stem and progenitor cells Cytometry 34 128–142
- HeX.Y. AntaoV.P. BasilaD. MarxJ.C. DavisB.R. 1992 Isolation and molecular characterization of the human CD34 gene Blood 79 2296–3302
- IzawaK. TaniK. NakazakiY. HibinoH. SugiyamaH. KawasakiA. SasakiE. NishiokaC. IshiiH. SodaY. YagitaH. TaniokaY. TojiA. AsanoS. 2004 Hematopoietic activity of common marmoset CD34 cells isolated by a novel monoclonal antibody MA24 Exp. Hematol 32 843–851
- KrauseD.S. FacklerM.J. CivinCI. MayW.S. 1996 CD34, Structure, biology, and clinical utility Blood 87 1–13
- KrauseD.S. ItoT. FacklerM.J. SmithO.M. CollectorM.I. SharkisS.J. MayW.S. 1994 Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells Blood 84 691–701
- LanzaF. HealyL. SutherlandD.R. 2001 Structural and functional features of the CD34 antigen, an update J. Biol. Reg. Homeos. Ag 15 1–13
- McSweeneyP.A. RouleauK.A. WallaceP.M. BrunoB. AndrewsmR.G. Krizanac-BengezL. SandmaierB.M. StorbR. WaynerE. NashR.A. 1998 Characterization of monoclonal antibodies that recognize canine CD34 Blood 91 1977–1986
- MenardC. HagegeA.A. AgbulutO. BarroM. MorichettiM.C. BrasseletC. BelA. MessasE. BisseryA. BrunevalP. DesnosM. PuceatM. MenascheP. 2005 Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium, a preclinical study Lancet 366 1005–1112
- MenascheP. 2005 The potential of embryonic stem cells to treat heart disease Curr. Opin. Mol. Ther 7 293–299
- MendelsonK. AikawaE. BretA. MettlerB.A. SalesV. MartinD. MayerJ.E. SchoenF.J. 2007 Healing and remodeling of bioengineered pulmonary artery patches implanted in sheep Cardiovasc. Pathol 16 277–282
- MorelF. SzilvassyS.J. TravisM. ChenB. GalyA. 1996 Primitive hematopoietic cells in murine bone marrow express the CD34 antigen Blood 88 3774–3784
- NarayanA.D. ChaseJ.L. LewisR.L. TianX. KaufmanD.S. ThomsonJ.A. ZanjaniE.D. 2006 Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients Blood 107 2180–2183
- NiemeyerG. P. HudsonJ. BridgmanR. SpanoJ. NashR.A. LothropC.D. 2001 Isolation and characterization of canine hematopoietic progenitor cells Exp. Hematol 29 686–693
- SakuraiM. FurusawaT. IkedaM. HikonoH. ShimizubS. GotohaH. KobayashicE. MomotaniE. 2006 Anti-bovine CD34 monoclonal antibody reveals polymorphisms within coding region of the CD34 gene Exp. Hematol 34 905–913
- SalesV.L. MettlerB.A. Lopez-IlasacaM. JohnsonJ.A. MayerJ.E. 2007 Endothelial progenitor and mesenchymal stem cell-derived cells persist in tissue-engineered patch in vivo: application of green and red fluorescent protein-expressing retroviral vector Tissue Eng 13 525–535
- SutherlandD.R. MarshJ.C. DavidsonJ. BakerM.A. KeatingA. MellorsA. 1992 Differential sensitivity of CD34 epitopes to cleavage by Pasteurella haemolytica glycoprotease, implications for purification of CD34-positive progenitor cells Exp. Hematol 20 590–599
- WattS.M. KarhiK. GatterK. FurleyA.J. KatzF.E. HealyL.E. AltassL.J. BradleyN.J. SutherlandD.R. LevinskyR. GreavesM.F. 1987 Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells Leukemia 1 417–426