Abstract
This study was conducted to investigate the effectiveness and possible mechanisms of an herbal prescription, Huangqi Maxingshigan decoction (Radix Astragali, Herba Ephedrae, Almond, Gypsum Fibrosum, Radix Glycytthizae) on infectious laryngotracheitis (ILT) in chickens. One hundred and sixty one-day-old chicks were randomly allocated into four groups, housed in isolated cages of 40 birds each: group was Huangqi Maxingshigan decoction treatment group, group II as Ding Chuan San control group, group III as the model group, and group IV as the blank control group. Except for the blank control group, other groups were challenged with infectious laryngotracheitis virus via the intratracheal route on age day 52. Birds in Group I were administered Huangqi Maxingshigan decoction at a concentration of 0.4 mL, or with Ding Chuan San in group II for comparison at 48 h post virus challenge (day 54) when they showed clinical signs, 2 times daily for 5 days consecutively. Then, the superoxide dismutases (SOD) activity and malondialdehyde (MDA) contents in serum, the expression of IFN-γ, IL-4 mRNA in spleen, the sIgA contents in tracheal fluid, the sIgA secreting cells in trachea were determined on day 5, day 15, day 25 post infection. The results showed that Huangqi Maxingshigan decoction could correct Th2-dominant Th1/th2 imbalance through up-regulation of IFN-γ, and down-regulation of IL-4 at the transcriptional level, enhance cellmediated immunity, ease inflammatory responses caused by ILTV. The results also showed that Huangqi Maxingshigan decoction provide an important antioxidant defense in the process of anti-ILT. Furthermore, it could induce the production of sIgA to enhance mucosal immunity.
Introduction
Infectious laryngotracheitis (ILT) is a worldwide contagious respiratory disease in chickens that causes severe economic losses in the poultry industry. Clinically, the disease needs an incubation period of 3–12 days before an acute phase of infection. The disease usually lasts for 1 to 2 weeks. The ILT virus (ILTV) has biological properties of rapid lytic replication in respiratory epithelial tissues and establishing latent infections in sensory neurons, so it was classified as a member of the Alpha-herpesvirinae subfamily of Herpesviridae (CitationZiemann et al., 1998; CitationWilliams et al., 1992). In contrast with most other alphaherpesviruses, ILTV exhibits a very narrow host range almost exclusively restricted to chickens and chicken-derived cells (CitationGuy and Bagust, 2003). In intensive poultry systems, especially layer flocks, conventionally attenuated vaccines are commonly used to control ILTV (CitationDevlin et al., 2008). However, these vaccine strains have a number of limitations, including insufficient attenuation and the ability to revert to high levels of virulence after in vivo passage (CitationGuy et al., 1990, Citation1991; CitationBagust and Johnson, 1995). Furthermore, after the attenuated vaccine strains were used, an asymptomatic latent infection of the sensory neurons occurred and the virus could infect non-immunized chickens under various stress factors. Therefore, the use of attenuated vaccines is often limited (CitationDevlin et al., 2008). The inactivated ILTV vaccines were also not used in a large-scale control of ILT because of the high cost of immunization, short duration of antibody existence, as well as poor cellular immunity (CitationSun et al., 2006). Though antibiotics for secondary infection are often administered, there is no effective medicine in controlling ILT so far. Thus, searching for effective drugs becomes very urgent, especially in developing nations. A large number of studies have shown that many medicinal herbs have activity of anti-virus, anti-inflammatory, and immunoenhancing effects. Investigation on such herbals in controlling viral diseases becomes more and more important in veterinary practice. We demonstrated in earlier studies that Huangqi Maxingshigan decoction, modified from a Chinese herbal medicine formula known as Maxingshigan decoction, documented in the Chinese Veterinary Pharmacopoeia (CitationCommission of Chinese Veterinary Pharmacopoeia, 2005), is more effective in controlling ILT. In the present study, the effectiveness of several Chinese medicinal formulae on ILT was compared through the principles of Chinese medicinal theory; the death rate, the rate of recovery from a primary infection were compared. At the same time, possible mechanisms of the herbals on treating ILT were investigated in order to provide theoretical information for clinical application.
Materials and methods
Preparation of herbal formulas
The formula involved in this study was Huangqi Maxingshigan decoction with 5 herbal medicines (Radix Astragali, Herba Ephedrae, Almond, Gypsum Fibrosum, Radix Glycytthizae). Chinese herbal formula Ding Chuan San, which is documented in the Chinese Veterinary Pharmacopeia (CitationCommission of Chinese Veterinary Pharmacopoeia, 2005), was used as positive medicine control. All the herbs for the formulas were obtained from Anguo Herbal Mart (Baoding, China). The herbal formulas mentioned above were prescribed proportionally, and were extracted in boiling water 10 times the volume of the herbs for 0.5 h and the aqueous extract separated by filtration. The remaining herb residues were decocted the second time and then filtered. The liquid mixture of the two extraction was heated (50–60°C) through rotary evaporation to reduce the water content, and concentrated into 1:1 decoction (CitationLenon œ., 2007), i.e., 1 mL of the concentrated extract was equivalent to 1 g of the raw herb. Then, the herbal preparations were sterilized in sealed plastic bottles.
Virus strains and propagation
The stock virus Wanggang (WG) strain was purchased from China Institute of Veterinary Drug Control (Beijing, China). It was propagated, assayed and harvested from inoculated specified pathogen-free (SPF) chicken embryos. Then, it was passaged for four generations. Each of the ILTV-infected chickens was inoculated intratracheally with 0.3 mL of the strain WG-ILTV E4, containing 105.47 egg lethal dose at 50% level (ELD50) (CitationNielsen et al., 1998).
Preparation of reagents
The superoxide dismutases (SOD) and malondialdehyde (MDA) assay kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The sIgA Elisa Kit was obtained from ADL Co. (Bloomington, MN, USA). The Oligo d(T)18 primer, dNTPs, TranScript RT, 5x RT buffer and Taq DNA polymerase were purchased from Tiangen Biotech Co., Ltd.D (Beijing, China). The SP-9002 HistostainTM-Plus kits from Zhongshan Biotech Co., Ltd. (Beijing, China) consists of Bloking solution, 3% H2O2, Biotin-goat antimouse IgG, Streptavidin peroxidase (SA/HRP). Mouse anti-chicken IgA antibody was from Southern Biotech, Inc., (Birmingham, AL, USA) and Diaminobenzidine (DAB) from Sigma-Aldrich (St. Louis, MD, USA).
Treatment of animals
One hundred and sixty one-day-old chickens were purchased from a commercial chicken farm in Baoding, fed with standard feedstuff and tap water ad libitum. The birds were not vaccinated with any vaccine during the whole study period, and they were randomly allocated into four groups of 40 birds each. Group I was Huangqi Maxingshigan decoction Group. Group II was Ding Chuan San control group, group III as the model group, and group IV as the blank control group. Each group was housed in a separate isolator. Except for the blank control group, birds in other groups were challenged with the virus harvested from inoculated specified pathogen-free (SPF) chicken embryos at 52 days of age at a dose of 0.3 mL. Group I was administered with Huangqi Maxingshigan decoction at a volume of 0.4 mL. Group II was given Ding Chuan San at the same volume as positive control at 48 h post-inoculation (54 d) when they showed clinical signs. Herbal treatment was given 2 times daily for 5 days consecutively. All birds of therapeutic groups were deprived of water for at least two hours before drinking the herbal decoction ().
Table 1 Animal treatment.
Sample collection
At days 5, 15, 25 post infection, 10 randomly selected birds per group were sacrificed. The spleens for determination of cytokines were removed immediately and placed into liquid nitrogen, then stored at −80°C until RNA extraction and other analysis.
In addition, 10 chickens of every group were chosen randomly and blood samples were taken by cardio-puncture on days 5, 15, 25 post infection, and serum for laboratory examination was collected. The SOD activity and MDA contents in serum were measured. Then, the birds were sacrificed, tracheal fluid was taken for determination of sIgA contents, the trachea was removed and fixed in Bouin’s solution for detecting the sIgA secreting cells.
Assay of superoxide dismutases and malondialdehyde, sIgA
The material for assay of SOD and MDA was serum. It was prepared from blood obtained after incubation at 37°C for half an hour, then, centrifuged 3000 rpm for 5 min, and kept at −80°C for the following assays: SOD was measured by xanthine oxidase method, MDA measured by thiobarbituric acid (TBA) reaction according to the manufacturer’s instructions. The material for assay of sIgA was tracheal fluid, and it was measured by ELISA. The assays were carried out according to the manufacturer’s instructions.
The data were assessed by one-way analysis of variance, using the SPSS 13.0 software. A P value of <0.05 was considered statistically significant.
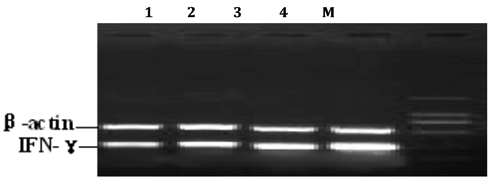
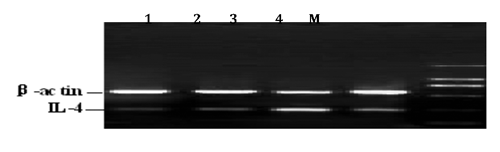
Semi-quantitative RT-PCR for the detection of IFN-γ and IL-4 mRNA
Total RNA from spleen was extracted with RNA Simple Total RNA Kit following the Kit’s instructions. The resulted mRNAs were evaluated, and the quantity and quality were determined by special analysis. Only samples with mRNA purity of about 2 (A260/A280 ratio) or above 2 were used. The specific primers for amplifying the IFN-γ, IL-4 genes were designed using the Primer 5.0 software based on the published cDNA sequence on Genebank (). RNA was reversely transcribed into cDNA with Oligo d(T)18.The RT reaction mixture contained 20 µL :1 µL of Oligo d(T)18 primer, 2 µL of 2.5 mM stock of dNTPs, and 11.5 µL of DNAse-treated RNA in DEPC water were denatured at 70°C for 5 minutes and immediately put on ice for 3 min. The following reagents were added: 4 µL of 5XRT buffer, and 0.5 µL Ribonuclease inhibitor, 1 µL of 50 units/µL of TranScript RT. The contents were mixed gently and briefly centrifuged followed by incubation at 42°C for 50 minutes. TranScript RT was heat inactivated for 5 minutes at 95°C to stop the reaction, and All cDNA samples were stored at −80°C prior to amplification. The resulting cDNA was subjected to polymerase chain reaction (PCR) with respective primers designed from the sequences of two cytokines. PCR conditions were optimized for both IFN-γ and β-actin, and for both IL-4 and β-actin. The reaction volume was 25 µL, including 2.5 µL 10XPCR buffer, 2 µL dNTP, 2 µL IFN-γ or IL-4 sense and anti-sense primer, 2 µL β-actin sense and anti-sense primer, 2 µL cDNA and 0.5 µL Taq DNA polymerase. The thermal cycler profile for IFN-γ and β-actin was as follows: 94°C for 5 min to denature the templates and primers, then denature at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 1 min, total 30 cycles, final extension step was 72°C for 10 min. The amplification condition for IL-4 and β-actin was: 94°C for 5 min to denature the templates and primers, then denature at 94°C for 45 s, annealing at 53°C for 45 s, extension at 72°C for 1 min, total 30 cycles, final extension step was 72°C for 10 min. Different controls were set to monitor thepossible contaminations of genomic and environment DNA both at the stage of RT and PCR. All samples were included in the same run of RT-PCR and repeated at least three times.
Table 2 Primers used for semi-quantitative PCR and PCR conditions for RT-PCR analysis.
The amplified RT-PCR products were subjected to electrophoresis at 200 V on 1% agarose gel for about 25 min, and visualized using ethidium bromide. The net intensities of individual bands were measured using TotalLab v2.01.The ratios of net intensity of IFN-γ or IL-4 to β-actin were used to represent the relative abundance of IFN-γ and IL-4 mRNA expression. The average level of three repeats was used for statistical analysis.
Immunohistochemical examination for IgA secreting cells in trachea
The trachea samples were embedded in paraffin and sections of 5 µm were serially cut and mounted on APES coated glass slides. Twenty sections were prepared for each animal sample. Then the slides were treated with xylene I, II for 7 min each step, then, with 100% ethanol I, II, 95% ethanol, 90% ethanol, 80% ethanol, 70% ethanol in order for 4 min each step. They were rinsed in PBS buffer (0.01M, pH 7.2) for 15 min. The endogenous peroxidase activity was neutralized by 3% H2O2 for 30 min, three times with PBS (0.01M, pH 7.2) for 15 min. The sections were treated with 5% normal goat serum in PBS for 30 min to block non-specific binding and then stained with mouse anti-chicken IgA (1:200) at 4°C over night. The sections were rinsed three times with PBS for 15 min and then incubated with goat anti-mouse IgG at 37°C for 40 min. The sections were rinsed three times with PBS for 15 min; followed by incubation with SA/HRP for 35 min at 37°C and washed. After the sections were rinsed three times in PBS, the reactions were made visible with metalenhanced diaminobenzidine (DAB). All incubations were performed in a moist chamber. Control staining was carried out simultaneously in which the first antibody was replaced with PBS. No specific staining was found in the control.
The sections were observed under Olympus BH-2 microscope, 10 times eyeshot were chosen per section, 10 sections per group, the data of positive cells were calculated by areas. The areas of IgA secreting cells in five different microscope fields was counted using ScanImage software for the statistical analysis of the data. The data were assessed by one-way analysis of variance, using the SPSS software. A P value of <0.05 was considered statistically significant.
Results
Changes of superoxide dismutases in serum
No significant change was observed in SOD activities on day 5 post-inoculation (day 57) between the groups. With the body being challenged by virus and drug action producing, the enzyme activities of SOD were elevated gradually. However, the increased extent of the blank control group (IV) was lower than herbal treated groups and the model group. From day 67 to day 77, the enzyme activities of SOD in herbal treated groups were higher than other groups. Compared with the blank control group (IV), the enzyme activities of SOD were significantly elevated in groups II and IV (P<0.05) ().
Table 3 Effect of modified Maxingshigan decoction on superoxide dismutases activity in ILT chickens (unit: U/L).
Changes of malondialdehyde in serum
MDA contents in serum rose up on day 5 post-inoculation (day 57) because of massive replication of ILTV in vivo. With the effective ingredients releasing and medication time increasing, the MDA contents in serum decreased gradually. The contents in herbal treated group (group I), was lower than the control group (P<0.05). On day 77, the MDA contents in serum were significantly decreased compared with Ding Chuan San treated group (group II), or the model group (III) (P<0.05), as shown in .
Table 4 Effect of modified Maxingshigan decoction on malondialdehyde content in ILT chickens (unit: U/mL).
The IFN-γ mRNA level changes in spleen
The mRNA expression of IFN-γ was decreased significantly when the birds were challenged with ILTV strains and elevated gradually later. However, there was no difference at day 57 or day 67 of age (5 and 15 days post-infection). Changes of IFN-γ mRNA expression in Huangqi Maxingshigan Decoction group paralleled the ones in Ding Chuan San control group at day 67 post-infection. From days 67–77, the expression of IFN-γ mRNA in the therapeutic groups showed a rapid elevation, and a significant difference compared with the blank control group (P<0.05). The trend of increasing in Huangqi Maxingshigan decoction was higher than Ding Chuan San control group. The result revealed that Huangqi Maxingshigan decoction could upregulate the IFN-γ mRNA expression effectively (, ).
Table 5 Analysis results of IFN-γmRNA level in different groups.
The IL-4 mRNA level changes in spleen
Five days post-infection, IL-4 mRNA expression in groups challenged with ILTV showed a rapid increase compared with the blank control group. From day 67 on, the mRNA levels of IL-4 gene in all groups except the blank control group begun to decrease slowly, but no differences were observed among all groups (P>0.05), and the descent format of model group was inferior to those of Huangqi Maxingshigan decoction group and Ding Chuan San control group. At day 25 post infection, IL-4 mRNA expression in the Huangqi Maxingshigan decoction treated group was significantly decreased, and showed great significance compared with the control groups (P<0.05). The results demonstrated that Huangqi Maxingshigan decoction has the function of suppressing the expression of IL-4 gene (, ).
Table 6 Analysis results of IL-4 mRNA level in different groups.
The changes of Th1/th2 level in spleen
The Th1/th2 ratio decreased when the bird challenged with virus. Then, IFN-γ mRNA expression was elevated gradually, and the IL-4 mRNA expression decreased. Th2-dominant Th1/th2 imbalance was disturbed. On days 67 and77, Th1/Th2 level in herbal treated groups showed a rapid increase, and significant different from the control group (P<0.05). Furthermore, variation tendency of Th1/th2 level in spleen paralleled the ones in serum ().
Table 7 Results of IFN-γ/IL-4 mRNA level in different groups.
The dynamic changes of tracheal fluid sIgA antibody
Day 57 was the 5th day after infection, and the virus was still in its latent state in vivo. Therefore there was no significant change in sIgA contents in all groups. With the virus replicating rapidly in vivo and increasingly stimulating to immune systems of the body, sIgA secretion was increased gradually. From day 57 to day 77, sIgA in all groups showed an increasing tendency. As shown in , the increasing altitude of sIgA in group I (Huangqi Maxingshigan decoction) was the highest among herbal treated groups and showed significance with group II (Ding Chuan San group) (P<0.05). At day 77, sIgA in group I was increased by 5.81% compared with that at day 72, and remained increasing onwards, while the groups II and III started to decrease ().
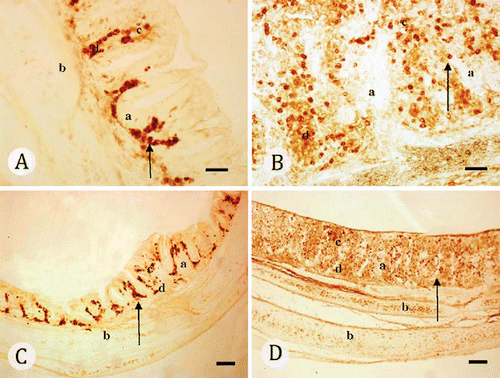
Table 8 Effect of sIgA content in ILT chickens (unit: pg/mL).
The changes of IgA secreting cells in trachea
The data analysis results displayed that, at 5 days post-infection, the sIgA secreting cells in groups challenged with virus were increasing significantly, and showed difference compared with the blank control group (P<0.05). At day 67, the areas of IgA secreting cells in groups challenged with virus continued to increase, but the increasing altitude of groups II and III were lower than Huangqi Maxingshigan decoction group. Group I was significantly higher than the other three groups (P<0.01), and groups II and III showed significant difference with groups I and IV (P<0.01). At day 77, group I remained the increasing strend, and showed significant difference with groups II, III and IV (P<0.01). There exists significance between groups II and III (P<0.05), and the blank control group remains the same (, ).
Table 9 Results of IgA secreting cells in different groups.
Discussion
In this experiment, when the chickens were challenged with infectious laryngotracheitis virus for 2 days (on day 57), the virus was still in latent state in vivo. Thus, the SOD activity and MDA contents of serum in all groups showed no differences at day 57 post-infection. From day 57 onwards, rapid virus replicating not only induced the generation of excessive oxygen free radicals, but also damaged the free radical scavenging system. The SOD activity showed a gradual reduction and the MDA content a rapid elevation. The free radical reaction was becoming gradually active, resulting in the injury of cells by the lipid peroxidation reaction, which stimulated the pathological changes (CitationVukobrat-Bijedic, 2003; CitationTahara, 2004; CitationCorrea, 2004). With the active ingredients of the drug releasing gradually, SOD activity in serum was enhanced constantly. The results showed that Huangqi Maxingshigan decoction effectively enhanced the SOD activity of serum in chickens infected with ILTV, and extended the lifetime of the birds. Meanwhile, the herbal formula could reduce the MDA content, playing a certain role in the antioxidant process. The authors suggested that this might be due to the apparent presence of its main herbs Astragalus and Glycyrrhiza, which possess strong antioxidant properties. In this study Astragalus was added to the formula Maxingshigan decoction. Astragalus contains saponins, total flavonoids, and quercetin that act as the main pharmacological active ingredients of antioxidants, among which the saponins and total flavonoids have been reported to be better in scavenging free radicals, and the pavilion is the most famous antioxidant in flavonoids, having a better synergic when combined with licorice (CitationTang et al., 2003). It is believed that a Chinese herbal formulae consisting of several types of medicinal herbs could exert synergistic effects. On one hand, this may be related with flavonoids, which could terminate the free radical reaction chain through capture of hydrogen and generating free radical intermediates; or enhance the antioxidant enzymes activity in vivo; or as a sequestration mixture of metal ions, block the generation of free radicals in Fenton system (CitationXu, 2006). On the other hand, this may be due to its activity of inhibiting enzyme activity which would lead to the generation of free radical by Astragalus and licorice, demonstrating the antioxidant activity, such as quercetin, playing an active role in anti-oxidation by inhibiting xanthine oxidase and cytochrome P-450, glycyrrhetinic acid in licorice playing a role in anti-lipid peroxidation through inhibiting the hydroxylase. Moreover, some Chinese herbal medicines rich in trace elements also can enhance the activity of enzyme systems. For example, Astragalus contains selenium, which can increase the levels of selenium in serum. Selenium is involved in the composition of glutathione peroxidase and has a stronger role in the reduction of lipid peroxidation in vivo, thereby it could protect cell membrane integrity and normal function. Our findings indicated that Huangqi Maxingshigan decoction containing several herbs that not only demonstrated free radical scavenging activity, enhancing the antioxidant capacity in ILTV chickens, but also amplified the therapeutic efficacies, leading to maximal therapeutic efficacy with minimal adverse effects from the aspect of scavenging free radicals. In conclusion, our studies suggest that Huangqi Maxingshigan decoction is an effective protective agent for ILT in the aspect of Chinese herbal formula-mediated antioxidation.
RT-PCR was a method used for semi-quantitative assay of mRNA expression in recent years (CitationUngere et al., 1993), and foregone sequence or standard template must be known for reference. In this study, the signal from β-actin RT-PCR products in each sample was used as an internal control; β-actin is a housekeeping gene which is stable in the tissue, and multiple of PCR amplification would not change the relative relationship between β-actin and cytokines (CitationMcNeel and Mersmann, 1999). Relative quantification would be valued according to the grayscale band ratio of the target and endogenous reference (β-actin) gene. Therefore, values for the target and endogenous reference (β-actin) genes were determined to calculate the relative transcription of the target mRNA against β-actin mRNA. In this experiment, neither bands of genomic DNA nor of foreign-source pollution were produced, when ddH2O and RNA samples used as control instead of RT products was amplified. Furthermore, the amplification product of the target and the ones of primers remained the same.
Generally, the IFN-γ gene is in the inhibitory state, and will happen to transcript only after activated by induction or stimulation. Virus, bacteria and some Chinese herbal medicines can induce the expression of IFN-γ gene. When virus or Chinese herbal medicine acted on the cell membrane, IFN-γ gene was released from inhibition, synthetic mRNA was produced, and mature IFN-γ was secreted into the extracellular environment. Studies confirmed that Th1 cytokine IFN-γ has the virus-inhibiting activity, inducing cells to produce a variety of enzymes, and anti-virus propagation by interfering with gene transcription of virus or translation of viral proteins as well as some unknown ways (CitationKarupiah et al., 1993; CitationWei et al., 1995). In addition, IFN-γ is a kind of characteristic cytokines secreted by Type 1 helper T (Th1) cells, and therefore is an important symbol to measure the level of cell-mediated immunity (CitationHuang et al., 2003). IL-4 is closely related with inflammatory responses. When immune inflammation happened, IL-4 was produced in abundance to make inflammatory cells in lymph circulation and blood infiltrating and accumulating in pathological tissues (CitationVallance et al., 2007). Our results showed that Huangqi Maxingshigan decoction can effectively inhibit the expression of IL-4 gene in spleen of ILTV chickens, while increase the expressions of IFN-γ gene, alleviate respiratory tract inflammation. The study also confirmed once again, inflammatory reactions in respiratory tract caused by ILTV was closely related to increased secretion of IL-4 and Th2 hyperthyroidism.
Generally, the capacity of Th0 differentiation to Th1 and Th2 is very weak, and both of them were in a state of equilibrium. However, Th1 or Th2 cytokines increased abnormally when this balance was broken under pathological conditions, which aggravated the Th1/Th2 imbalance, eventually leading to the occurrence and development of Thl or Th2-type disease (CitationWu et al., 2001). IFN-γ and IL-4 are the most representative cytokines that reflect Thl/Th2 immune responses patterns (CitationLi et al., 2002). Many studies have shown that Th1/Th2 imbalance is particularly important in the process of inflammatory and remodeling, so regulating the Th1/Th2 imbalance could alleviate respiratory passage inflammation. Therefore, this study selected IFN-γ, IL-4 as the symbol to stand for the Th1/Th2 level in serum and spleen, and studied the effect of Huangqi Maxingshigan decoction on immune function in ILTV chickens. The results showed that there exists a down-regulation of IFN-γ and Th2-dominant Th1/Th2 imbalance when chickens were infected with ILTV. The study revealed that Huangqi Maxingshigan decoction could alleviate respiratory passage inflammation of ILTV chickens, probably by down-regulating the expression of Th2 cytokines mRNA such as IL-4, inducing Th1 immune response to correct the Th1/Th2 imbalance of Th2 dominant immune responses (CitationHopfesnpirger et al., 2001). All cytokines composed of a large network. Occurrence, development and rehabilitation of any disease are not attribute to a single cytokine. Th1 cytokines, such as IL-2 may play an important role in the network of immune regulation, stimulate the secretion of IFN-γ, anti-infectious diseases, and so on. The general immune response was regulated by IL-2 (CitationWang et al., 2006). The previous studies have shown that, IL-2 as a T cell growth factor, on the one hand, can improve the level of humoral immunity. On the other hand, it also can promote CD4+ T-cell-assisted CD8+ T-cell killing effect, enhance NK cell activity and improve the level of cell-mediated immunity (CitationLi et al., 2005). IFN-γ will induce expression of IL-2 receptor gene. In contrast, the binding of IL-2 and IL-2R can be able to activate production of IFN-γ induced by these cells (CitationSchultz et al., 2004), promoting T cell proliferation and enhancement of the immune response. Both of them are the key components of cell-mediated immune response, play an important role in the entire immune response (CitationYang et al., 2005).
In many avian infectious diseases, cellular immune responses play a dominant role in immunological protection (CitationLillehoj et al., 1993). Earlier studies have found that bursectomized chickens in testosterone treatment and cyclophosphamide treatment in which the bursa of fabricius were ablated and the ability to synthesize specific antibody in response to antigenic challenge destroyed, failed to produce either humoral or mucosal antibodies to ILT virus following challenge, even though there was some evidence of lymphoid repopulation of the treated bursae. Similarly, bursectomized chickens had neither IgA- nor IgG-producing cells in the trachea after challenge with ILT virus. However, the bursectomized chickens recovered from a primary infection with ILT virus at a rate similar to that of the intact chickens. More interesting, yet, was the finding that the absence of mucosal antibody did not impair the ability of challenged-bursectomized chickens to resist a challenge infection with virulent ILT virus, and pathogens may be able to establish infections before other specific and non-specific protective mechanisms are invoked to limit the disease. These evidences suggest that secretory IgA, and presumably other mucosal antibodies, can protect the host by reacting with bacteria or viruses and preventing them from attaching to or colonizing mucosal surfaces.
Secretory IgA is a crucial component of firstline immune mechanisms at mucosal surfaces and has many anti-inflammatory functions. sIgA produced by plasma cells is transported by a secretory component (SC)-mediated process through the mucosal epithelial cells into the places where they afford protection by neutralizing or other wise preventing the attachment of viruses to the mucosal epithelium, thus allowing luminal clearance of these potentially pathogenic agents. The IgA secreting cells in the tracheal tissues are the important effect molecules to protect mucosal surfaces, and the changes of IgA secreting cells in tracheal are one of the standards that to estimate tracheal mucosal immunity. In the present study, the IgA of the tracheal mucosa were localized by using immunohistochemical methods, and the sIgA-secreting cells were calculated .The results showed, Huangqi Maxingshigan decoction could increase the sIgA contents in serum and promote the differentiation of mIgA-positive B cells into plasma cells to increase the amount of sIgA secretion in the mucosa of upper respiratory tract, furthermore, the result detected by immunohistochemistry is more significant than that by ELISA. The result also indicated, Huangqi Maxingshigan decoction is an important switch factor for B cells to produce IgA molecules, and can induce specific mucosal IgA antibodies and systemic immunity in the upper respiratory tract. In addition, the results showed that, there exists a certain relationship between the dynamic changes of sIgA and the balance of Th1/Th2, Huangqi Maxingshigan decoction can effectively enhance mucosal immune function of ILT chickens, it is probably due to its ability of upregulating the expression of sIgA regulatory factor effectively.
Conclusions
The study showed that the Chinese herbal medicine Huangqi Maxingshigan decoction could up-regulate IFN-γ, down-regulate IL-4 at the transcriptional level, enhance cell-mediated immunity, and alleviate inflammatory responses caused by ILTV. From the study it can be concluded that Huangqi Maxingshigan decoction is a potentially alternative therapy clinically for treating infectious laryngotracheitis.
Acknowledgments:
this study was financially supported by the Ministry of Science and Technology of China (No. 2011BAD34B02).
References
- BagustT.J. JohnsonM.A. 1995 Avian infectious laryngotracheitis: virus-host interactions in relation to prospects for eradication Avian Pathol 24 373 391
- Commission of Chinese Veterinary Pharmacopoeia 2005 Veterinary Pharmacopoeia of the People's Republic of China Chemical Industry Press Beijing, China
- CorreaP. 2004 The biological model of gastric carcinogenesis IARC Sci. Publ 157 301 310
- DevlinJ.M. BrowningG.F. GilkersonJ.R. FentonS.P. HartleyC.A. 2008 Comparison of the safety and protective efficacy of vaccination with glycoprotein-G-deficient infectious laryngotracheitis virus delivered via eye-drop, drinking water or aerosol Avian Pathol 37 83 88
- GuyJ.S. BagustT.J. 2003 Laryngotracheitis SaifY.M. BarnesH.J. GlissonJ.R. FadlyA.M. McDougaldL.R. SwayneD.E. Diseases of Poultry 11th ed Iowa State Press Ames, IA, USA 121 134
- GuyJ.S. BarnesH.J. MorganL.M. 1990 Virulence of infectious laryngotracheitis viruses: comparison of modified-live vaccine viruses and North Carolina field isolates Avian Dis 34 106 113
- GuyJ.S. BarnesH.J. SmithL.G. 1991 Increased virulence of modified-live infectious laryngotracheitis vaccine virus following bird-to-bird passage Avian Dis 35 348 355
- HopfenspirgerM.T. ParrS.K. HoppR.J. TownleyR.G. AgrawalD.K. 2001 Mycobacterial Antigens Attenuate Later Phase Response, Airway Hyperresponsiveness, and Bronchoalveolar Lavage Eosinophilia in a Mouse Model of Bronchial Asthma Int. Immunopharmacol 1 1743 1751
- HuangJ.L. LongZ.J. WuH.Q. WangT.S. 2003 Effects of Lingguizhugan Decoction on T-lymphocyte Subset and the Activity of Interleukin-2 in Immunosuppressed Mice Induced by Cyclophosphamide Chinese J. Exper. Tradit. Med. Form 9 35 40
- KarupiahG. XieQ.W. BullerR.M. NathanC. DuarteC. MacMickingJ.D. 1993 Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase Science 261 1445 1448
- LenonG.B. LiC.G. XueC.C. ThienF.C. StoryD.F. 2007 Inhibition of release of vasoactive and inflammatory mediators in airway and vascular tissues and macrophages by a Chinese Herbal Medicine formula for allergic rhinitis Evid- Based Compl. Alt 4 209 217
- LiC.Q. XuY.J. ZhongX.N. YangD.L. LiuX.S. XiongW.N. ZhangZ.X. 2002 Changes of cell composition in bronchoalveolar lavage fluid in different Th1/Th2 cell immune response Chinese J. Cell Molecul. Immunol 18 575 577 (in Chinese)
- LiH.M. GuoH.J. LiX.R. WangZ.K. 2005 Influence of Recombinant chicken IL-2 as an immunoenhancer on cell-mediated immunity levels in IBD Chinese J. Vet. Med 41 13 14 (in Chinese)
- LillehojH.S. TroutJ.M. 1993 Coccidia: a review of recent advances on immunity and vaccine development Avian Pathol 22 3 31
- McNeelR.L. MersmannH.J. 1999 Distribution and quantification of β1-, β2-and β3-adrenergic receptor subtype transcripts in porcine tissues J. Anim. Sci 77 611 621
- NielsenO.L. JørgensenP.H. HedemandJ. JenseniusJ.C. KochC. LaursenS.B. 1998 Immunohistochemical investigation of the tissue distribution of mannan-binding lectin in non-infected and virus-infected chickens Immunology 94 122 128
- SchultzU. KaspersB. StaeheliP. 2004 The interferon system of non-mammalian vertebrates Dev. Comp. Immunol 28 499 508
- SunZ.G. LiJ.M. XuY.H. ZhangM.F. 2006 The study of Infectious laryngotracheitis virus vaccine China Anim. Husb. Vet. Med 33 41 44
- TaharaE. 2004 Genetic pathways of two types of gastric cancer IARC Sci. Publ 157 327 349
- TangJ.Z. LuX.X. ChenR.F. 2003 Antioxidant property and extraction of flavonoids in Chinese globeflower Food Sci 24 88 91 (in Chinese)
- UngereM. BohmM. ElceJ.S. ErdmannE. LohseM.J. 1993 Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart Circulation 87 454 463
- VallanceB.A. RadojevicN. HogaboamC.M. DengY. GauldieJ. CollinsS.M. 2007 IL-4 gene transfer to the small bowel serosa leads to intestinal inflammation and smooth muscle hyperresponsiveness Am. J. Physiol-Gastr. L 292 385 394
- Vukobrat-BijedicZ. 2003 Carcinoma of the stomach Med Arh 57 Suppl. 2 81 83
- WangD.Y. ZhangH.Q. 2006 A Survey of Researches on Traditional Chinese Medicine for Immuno-enhancing and Tumor-inhibiting through Promoting the Secretion of IL-2 Progr. Pharmaceut. Sci 30 162 165 (in Chinese)
- WeiX.Q. CharlesI.G. SmithA. UreJ. FengG.J. HuangF.P. XuD. MullerW. MoncadaS. LiewF.Y. 1995 Altered immune responses in mice lacking inducible nitric oxide synthase Nature 375 408 411
- WilliamsR.A. BennettM. BradburyJ.M. GaskellR.M. JonesR.C. JordanF.T. 1992 Demonstration of sites of latency of infectious laryngotracheitis virus using the polymerase chain reaction J. Gen. Virol 73 2415 2420
- WuJ. XuJ. ZhongN.S. 2001 BCG and Thl/Th2 balance of allergic asthma Foreign Med. Sci. Section of Respiratory System 21 151 153 (in Chinese)
- XuG.Y. 2006 Determination of Efficient Components and Antioxidative Activities of Chinese Herbs Degree Diss., Hunan Medical University Changsha, China
- YangF.L. YueH. JiaW.X. 2005 Effect of Chicken Interleukin-2 on Immune Function of Chickens Chinese Poultry 27 17 20
- ZiemannK. MettenleiterT.C. FuchsW. 1998 Infectious laryngotracheitis herpesvirus expresses a related pair of unique nuclear proteins which are encoded by split genes located at the right end of the UL genome region J. Virol 72 6867 6874