Abstract
AbThset ariamc otf the present study was to determine heavy metal reference levels for exposure and risk assessment studies on a local scale. We measured lead (Pb), chromium (Cr), zinc (Zn), copper (Cu) and cadmium (Cd) content in edible tissues and organs of wild boars harvested in different areas of the Province of Viterbo, Central Italy. The average levels of cadmium recorded in 75 wild boars were 0.085, 0.079 and 1.052 mg Cd kg−1 wet weight (w.w.) in the liver, muscle and kidney, respectively. The majority of the muscle samples and some of the liver samples contained levels of heavy metal that were over the legal limit [EU Maximum Residue Levels (MRLs)] for pigs. Our data are similar to or lower than the values reported in most of the available literature. For Pb concentration, the average values recorded were 0.318, 0.126 and 0.298 mg kg−1 w.w. in the liver, muscle and kidney, respectively. The samples that were non-compliant with regulatory limits (MRLs) for pigs were registered only for muscle. Available data on the presence of Pb content in game meat report lower values than ours, most likely because the area around the bullet path was avoided while sampling. The average values of total Cr were 0.141, 0.139 and 0.097 mg kg−1 w.w. in the liver, muscle and kidney, respectively. For Zn, the mean values were 49.76, 53.21 and 32.46 mg kg−1 w.w. in the liver, muscle and kidney, respectively. Cu content was 46.12, 12.20 and 5.64 mg Cu kg−1 w.w. in the liver, muscle and kidney, respectively. The results obtained have been validated on the basis of the scarce and inconsistent Italian literature available and on international studies.
Introduction
Heavy metals are ubiquitous in soil, water and air. Their transfer to the food chain is an important environmental issue that could represent a risk to human health. Concern over the contamination of game meat with heavy metals is mainly related to toxic elements that accumulate in tissues, such as cadmium (Cd) (CitationBeiglböck et al., 2001), lead (Pb) (CitationKarita et al., 2000), and the metalloid arsenic (As) (CitationMaňkovská and Steinnes, 1995). Some other metals, such as zinc (Zn), copper (Cu) and chromium (Cr), are microelements or trace elements with biochemical and physiological functions (CitationDosi, 2000). Some of these metals act as functional constituents of one-third of known enzymes. Chromium, which is thought to be an essential element, can lead to intoxication phenomena when organisms are exposed to high concentrations or are exposed to the element for a very long period of time. More generally, the balance between the toxic and useful concentration is variable from element to element and from organism to organism (CitationTripathi et al., 1997). Several reports indicate that game meat can be an important source of trace metals because of its increasing availability, mainly due to increased hunting activity (CitationRamanzin et al., 2010). Statistics concerning wild boar harvesting and meat availability in Italy are provided in , in which the present data are compared with data from the 1998–1999 hunting season. The total culling of ungulates in Italy may be estimated at more than 230,000 heads per year. Wild boar harvesting represents approximately 160,000 heads; however, the data available for this species are incomplete, and figures are most likely underestimated. As reported by CitationRamanzin et al. (2010), wild boar contributes to 80% of the total ungulate meat availability in Italy, with over 5000 tons of carcasses (). Wild boar is almost the only source of ungulate meat in the Northern and Southern Apennines, and accounts for 75% of the total meat produced in the Western Alps (CitationRamanzin et al., 2010). Over the last ten years, the total wild boar harvest has increased by approximately 70%, and it is expected that the culling rate will increase in some regions because of crop damage (CitationAmici et al., 2012).
Table 1 Statistics of wild boar harvesting (heads) and meat (tons) availability in different areas of Italy.
There are often significant regional differences in heavy metal content in the organs and tissues of wild ungulates (CitationAastrup et al., 2000). Single metals are higher in animals living near mining sites or other sources of pollution (CitationMaňkovská and Steinnes, 1995; CitationReglero et al., 2009; CitationPokorny et al., 2009). The accumulation of Cd and Pb increases with age (CitationFalandysz et al., 2005; CitationHunt et al., 2009). A primary source of Pb contamination originates from residual metal elements in bullets (CitationFalandysz et al., 2005). Very high values have been registered in the muscle area surrounding the bullet pathway. Pb represents an important concern for game meat consumption, and the accurate trimming of the meat around wounds and bullet pathways is recommended (CitationDoborowolska and Melosik, 2008; CitationHunt et al., 2009). Some muscle, liver and kidney samples contained concentrations of heavy metal that were over European or national legal limits (CitationSwiergosz et al., 1993; CitationSantiago et al., 1998; CitationZaccaroni et al., 2003; CitationFalandysz et al., 2005; CitationMandas, 2005; CitationLazarus et al., 2008); however, the average values recorded in the organs and tissues were under the legal limits. Some authors believe the health risk for game meat consumers to be negligible (CitationLazarus et al., 2008), even in highly polluted areas (CitationPokorny et al., 2009). However, there have been no specific exposure assessment studies, especially on a local scale, both for the general population and for particular categories (CitationDanieli et al., 2012). In general terms, many studies on the heavy metal content of edible tissues of wild boar refer to opportunistic sampling (CitationZaccaroni et al., 2003; CitationMandas et al., 2005) and are used to provide evidence of local sources of pollution (CitationSwiergosz et al., 1993; CitationFalandysz, et al. 2005; CitationPokorny et al., 2009). These studies often include a small number of samples or small sampling areas (CitationSwiergosz et al., 1993) or consider only one edible organ (CitationGuazzetti et al., 2001; CitationOrusa et al., 2004). There has been no systematic collection of data, and this means that it has been impossible to conduct reliable exposure assessment studies. Collecting useful data on contamination of wild animal tissue by heavy metals is the first step in determining the intake of these elements, both in the overall population and in specific groups, such as hunters and their families.
The aims of the present study were: i) to highlight the content of copper, zinc, lead, cadmium and chromium in the edible tissues and organs of wild boars harvested in 6 hunting areas of the Province of Viterbo; and ii) to describe the distribution pattern and reference levels of heavy metals in the edible tissues of wild boar to be used for local scale exposure assessment studies.
Materials and methods
Study area
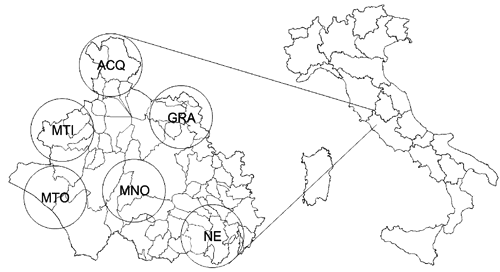
The study area is represented by 6 wild boar game management districts within the administrative boundaries of the Province of Viterbo, covering an area of 3614 km2 (Region of Lazio, Central Italy) ().
Meteorological records for the area during the 7-year period from January 2004 to December 2010 show a range in monthly mean air temperature between 5.9°C in January and 23.8°C in July. Average annual rainfall was 848 mm, distributed over 203 days with a minimum in the summer and a peak in autumn. The landscape is highly fragmented, especially at altitudes below 600 m, where cultivated fields are interspersed with woodlands and scrublands, with an increase in woodlands and scrublands going inland from the coast to the hill areas. Along the coast, the fields are planted with vegetables. The hinterland plain can be irrigated and summer crops, such as maize and sunflowers, predominate. These are very often sown after the autumn-winter cereals and forages. At the higher altitudes, orchards (vineyards, olive groves, chestnut and hazelnut) and woodlands dominate the landscape. The forests are mainly composed of turkey oak (Quercus cerris), downy oak (Quercus pubescens), hop hornbeam (Ostrya carpinifolia), manna ash (Fraxinus ornus), european chestnut (Castanea sativa), and several shrub and bush species (Rosa canina, Arbutus unedo, Erica arborea, Juniperus spp., Cytisus spp., Rosmarinus officinalis). Most of the forests are coppiced, while timber forests are more frequently to be found in protected areas. The A. Volta electrical power plant (in the southwest of the area) and some authorized landfills are located in the province outside the 6 hunting districts. The area is crossed by the Fiora and Paglia rivers that rise from Monte Amiata where cinnabar mines and geothermal phenomena are to be found (CitationBargagli et al., 1991).
Wild boar population status
The particular organization of the agricultural and forest lands in the study area provides abundant feed for the wild boar population throughout the year: cereals such as wheat, oats and barley in the spring, maize in the summer, chestnuts and hazelnuts in autumn, and acorns in winter. Furthermore, drive-hunting teams provide high-energy supplemental feeding, especially during autumn and winter, to ensure the wild boars stay within their own hunting areas. Consequently, the wild boar population in the Province of Viterbo has increased dramatically over the last fifteen years. At the moment, the wild boar population can be considered stable, although some fluctuations have been observed, and the official harvesting range is between 5000 to 7000 heads per year. The main factor that limits an increase in population is hunting. Hunters are active three times a week from the 1st November to the 31st January. Parks, restocking areas, oases and military areas cover approximately 11% of the Province’s agro-forestry surface area. Private and free hunting areas cover 13% and 76% of the territory, respectively. Wild boar hunting teams cover approximately 350 km2 (i.e. 13% of the free hunting areas).
Organ and tissue sampling
Wild boars were shot by hunters belonging to 6 different hunting districts within the Province of Viterbo, Central Italy: Acquapendente (ACQ), Monti di Castro (MTI), Montalto di Castro (MTO), Monte Romano (MNO), Graffignano (GRA) and Nepi (NE) (). A total of 75 adult animals were culled, aged between 2 and 5 years. The carcasses were randomly selected from the annual hunting bag. Sex was recorded at sampling (38 males, 37 females). Muscle and offal (the liver and kidneys) were collected, individually packed in polyethylene bags, and transferred to the laboratory using refrigerated bags within a few hours. During sampling operations, special care was taken to avoid tissues near the bullet entry or fragmentation (CitationHunt et al., 2009). The muscle, liver and kidney samples were frozen (−20°C) and stored until analysis.
Elemental analysis
Finely ground samples (0.5 and 0.6 g for the meat and offal, respectively) were dispersed in H2O2 (30% for trace analysis) and/or HNO3 (65% for trace analysis) (Sigma-Aldrich S.r.l., Milan - Italy) and then submitted to a specific microwave digestion cycle () using a MLS 1200 microwave laboratory system (Milestone Inc., Shelton - USA). At the end of the mineralization procedure, all the samples were quantitatively recovered in polyethylene tubes and diluted to 10 mL using ultrapure water (specific conductivity 0.055 µS/cm) (Sartorius Stedim, Firenze - Italy). The samples were properly diluted with 1.0% (v/v) HNO3 before analysis.
Table 2 Microwave digestion condition optimised for wild boar meat, liver and kidney.
Analysis of Cd, Pb, Cr, Zn and Cu was performed using a Varian SpectrAA 30 atomic absorption spectrophotometer equipped with a Zeeman graphite furnace (Varian, Palo Alto – USA). Chemical (or matrix) interference was minimized by using platform atomization techniques with the aid of appropriate matrix modifiers: 1.0% (w/v) NH4H2PO4 for Cd and 1.0% (v/v) H3PO4 for Pb, while no modifier was added for the Cr, Zn and Cu analysis. Five calibration standards for Cd (1.56–25.00 µg/mL), Pb (3.13–50.00 mg/mL), Cr (0.79–12.50 µg/mL), Zn (15.63–250.00 µg/mL) and Cu (12.50–200.00 µg/mL) were obtained by diluting certified standards (1.000±0.002 g/L) (Merk, Darmstadt, Germany) with 1.0% (v/v) HNO3. The certified reference material (CitationEuropean Commission, 1998) was treated in the same manner as the wild boar samples, to verify the accuracy of the analytical method. Recoveries for Cd, Pb, Cr, Zn and Cu were then calculated using the certified values as references. The purity of the reagents and water, and the level of cleanness of the laboratory glassware, MW liners and plastics were monitored by including a Reagent Laboratory Blank (RLB) for every MW mineralization run (CitationEPA, 1998). The concentration of all the elements analyzed were expressed in mg/kg w.w. based on the following equation:
where
[El]sample is the concentration of element in the tissue sample;
ABSλ the absorbance recorded at 228.8 nm for Cd, 283.3 nm for Pb, 357.9 nm for Cr, 307.6 nm for Zn and 327.4 nm for Cu; Intcc (in AU) and Slopecc (in mL µg−1) the intercept and slope, respectively, of the calibration curve developed for each element;
DF diluting factor applied;
Vmin final volume of mineralized samples (mL);
Wsample sample weight in grams.
The detection limits (LOD) and quantification limits (LOQ) were also determined for each element following the DIN procedure n. 2345 (DIN, 1994).
Performance values of the method were: LOD = 0.003 mg kg−1, LOQ = 0.009 mg kg−1, Rec% (mean±s.d.) = 87.1 ± 0.6 for cadmium; LOD = 0.010 mg kg−1, LOQ = 0.030 mg kg−1, Rec% = 94.1 ± 1.8 for lead;
LOD = 0.012 mg kg−1, LOQ = 0.037 mg kg−1,
Rec% = 91.1 ± 2.4 for chromium;
LOD = 0.060 mg kg−1, LOQ = 0.189 mg kg−1,
Rec% = 89.8 ± 1.4 for zinc;
LOD = 0.045 mg kg−1, LOQ = 0.140 mg kg−1,
Rec% = 93.3 ± 1.3 for copper.
The data presented in this study were not corrected for recoveries.
Statistical analysis
The concentration data obtained using the spectrophotometric readings were stored using MS Excel 2002® (Microsoft Corporation, Redmond – USA) spreadsheets. The statistical analysis and graphic representation of the data were performed using STATISTICA 6.0 (StatSoft Inc., Tulsa - USA) software package. Data distributions were tested using Shapiro-Wilks statistics (CitationShapiro et al., 1968) and were non-compliant with parametric statistics; they were, therefore, log transformed before further analysis. A similar transformation has been used in other papers on the accumulation of heavy metals (CitationGuazzetti et al., 2001). The factorial analysis of the distribution and areas for contamination of the tissue was conducted using a GLM-type approach. The statistical model included the harvesting area and the tissue, as well as their interaction as main factors. The significance of the differences was tested using the F statistic; within the relevant factors, the differences were evaluated using the Fisher LSD test (Lowest Significance Differences). A residual probability value of 5% (P=0.05) was adopted as the minimum level of significance.
Results and discussion
Data distribution and statistics for the liver, kidney and muscle samples are presented in . Overall, left-censorship in the analytical data set was virtually absent. A very low, left-censored rate was only recorded for chromium in the liver (<5%; data not shown). With the exception of the chromium levels in the muscle, all the metal concentration data were adequately fitted by log-normal distributions (Kolmogorov-Smirnov and Pearson chi-square test, P>0.1).
Table 3 Data distribution and statistics of cadmium, lead, chromium, copper and zinc (mg kg−1 wet weight, unless otherwise stated) in wild boar liver, muscle and kidney (overall values).
Cadmium
The average levels of Cd () recorded in the muscle and kidney were 0.079 and 1.052 mg kg−1 w.w., respectively. These mean values are quite high if compared with the European Minimum Risk Levels (MRLs) set by the European Commission Regulation 420/2011 (European Commission, 2011) for meat and offal of farmed pigs (0.05 and 1.00 mg Cd kg−1 w.w. for muscle and kidney, respectively). From a total of 75 muscle samples tested, 75% contained a Cd value above the limit (1st Q.le = 0.056 mg Cd kg−1 w.w.). For a total of 65 kidney samples, 48% contained Cd values over the EU MRL (median 0.975; 3rd Q.le 1.267 mg Cd kg−1 w.w.). The median was slightly lower than the mean for all the tissues (), and a modest asymmetry of the data distribution was recorded. Over 60% variability in cadmium contamination in the three tissue types was observed (Relative Standard Deviation, RSD % = 72.7, 62.1 and 65.0 for the liver, muscle and kidney, respectively). The cadmium content in the kidney tissue was higher (P<0.01) than in the liver and muscle tissues, while no differences were observed between the liver and muscle (). For all the tissues analyzed, samples belonging to wild boars shot in the ACQ hunting area showed greater cadmium contamination than the samples from MNO and MTO. A comparison with all the other areas showed that the kidney samples harvested from the GRA area were less contaminated (P<0.01). If we consider the EU MRLs established for the meat and offal of farmed pigs, the kidney samples collected in ACQ contained the highest number of samples above the EU MRL (1.0 mg kg−1 w.w.), with an average value of 1.706 mg Cd kg−1 w.w. In addition, both the median and the 1st Q.le values were higher than 1.0 mg Cd kg−1 w.w. More than 75% of the samples were non-compliant with the MRL set by European Commission Regulation 420/2011 (European Commission, 2011). In contrast, kidney samples from the MTO and GRA areas contained a mean value of cadmium below the limit (0.792 and 0.594 mg Cd kg−1 w.w., respectively), with less than 25% non-conformity of samples (3rd Q.le equal to 0.975 and 0.886 mg Cd kg−1 w.w. for the MTO and GRA areas, respectively). All the muscle samples from NE were contaminated with Cd above the EU MRL of 0.05 mg kg−1 w.w. established for pork.
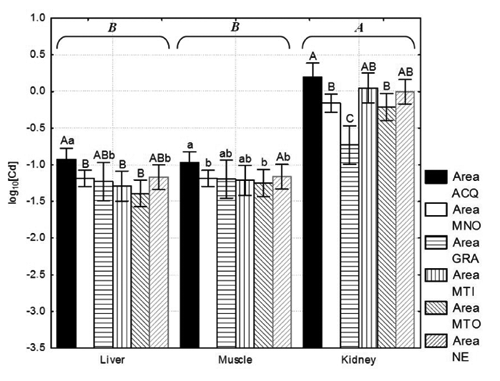
The mean values recorded in the liver samples from the ACQ and MTI areas were significantly higher than those for the other 4 areas; however, these results are comparable with those observed in Sardinian wild boars by CitationMandas (2005) who reported mean values ranging between 0.38 and 0.58 mg Cd kg−1 w.w.; these were lower than the mean value of 0.55 mg Cd kg−1 w.w. reported by CitationGasparik et al. (2012). For muscle, particularly high values were recorded in the samples from ACQ (P<0.05) (). A similar value was recorded for the renal tissue for which, even for the MTI harvesting area, the levels of accumulation were significantly higher than those observed in the other areas (P<0.05) (). There was no difference between data collected on the presence of cadmium in wild boar muscle in the present paper and those values reported in the literature (range 0.001–0.355 mg Cd kg−1 w.w.) (CitationRudy, 2010; CitationTaggart et al., 2011). Nevertheless, we observed lower values than those reported by CitationHernández et al. (1985) for cadmium in muscle (geometric means of 0.07 vs 0.12 mg Cd kg−1 w.w.) or in liver (geometric means of 0.067 vs 0.45 mg Cd kg−1 w.w.). Our data also show lower values than those reported by CitationGasparik et al. (2012) who reported 0.16 and 3.22 mg Cd kg−1 w.w. as the mean values for muscle and kidney, respectively, and those reported by CitationWolkers et al. (1994) who reported a median cadmium concentration of 2.05 mg kg−1 d.w. (dry weight) in liver samples of wild boar aged 1.5–5 years. Adopting a conversion factor of 3.4, the cadmium concentration reported by CitationWolkers et al. (1994) corresponds to 0.60 mg kg−1 w.w., which is 10 times greater than the median (0.067 mg kg−1 w.w.)
observed in our study. Other authors have indicated that such variations in cadmium levels can be found among wild boars hunted in different areas, even within the same country (CitationSwiergosz et al. 1993). However, this figure most likely reflects the high levels of environmental pollution, as also highlighted by CitationGasparik et al. (2012).
Lead
The average lead content in muscle (0.126 mg Pb kg−1 w.w.) was over the regulatory limit (0.1 mg Pb kg−1 w.w.), with a frequency of non-compliant data over 75% (1st Q.le 0.109 mg Pb kg−1 w.w.) (). In liver, the highest value was slightly higher than the EU MRL for pigs (0.5 mg Pb kg−1 w.w.) and non-compliant content was only observed in one sample (). As a general consideration, there were no significant differences in the lead content in the liver and kidneys but values for both tissue types were higher than in muscle (P<0.01), and the observed variability was significantly smaller than that recorded for cadmium, rarely exceeding 30% RDS.
A consideration of the sampling area could explain the observed differences in the levels of accumulation recorded. However, the lack of scientific studies into the level of anthropogenic pollution and chemical composition of the soil means the causes for this variability cannot be clarified. Higher concentrations were observed for lead in the samples from the MTI area, while the lowest values were recorded in the NE area (P<0.01) (). The similar values observed for Pb accumulation in the liver and kidney are in contrast to data reported by CitationMartelli (2005) who investigated Pb accumulation in wild boar from 2 areas in Tuscany with a different degree of anthropogenic disturbance. He reported high accumulation levels in liver (exceeding regulatory limits) but did not observe high values in kidney. CitationPiskorová et al. (2003) observed more lead in kidney than in liver (mean concentrations 0.39 mg and 0.24 mg Pb kg−1, respectively) for wild boars hunted in the Slovak Republic. CitationMartelli (2005) observed similar lead concentrations in the liver (0.302–0.674 mg Pb kg−1 w.w.) and kidney (0.401 to 0.774 mg Pb kg−1 w.w.) of wild boars reared near an industrialized area.

The mean occurrence of Pb in the liver was approximately 0.320 mg kg−1 w.w. for all the areas of sampling but differences were without statistical significance (P>0.05). The mean value we have registered in liver was close to that obtained by CitationMandas (2005) in 3 areas of Sardinia in animals aged 3–5 years (0.11 and 0.28 mg Pb kg−1 w.w.). In our study, no differences in the levels of lead concentration of muscle between the areas were recorded. Nevertheless, it should be noted that the area of ACQ showed particularly high right-end values (2 samples with contamination over 0.20 mg Pb kg−1 w.w.). In the kidney samples, high values of Pb contamination were recorded for the MTI area, and significantly lower values were recorded for the samples belonging to the NE area (P<0.01) (). In wild boars reared in pens near an industrial complex, CitationMartelli (2005) observed a significant presence of lead in muscles and other tissues (0.302–0.674 mg Pb kg−1 w.w. in liver, 0.401–0.774 mg Pb kg−1 w.w. in kidney, and 0.022–0.082 mg Pb kg−1 w.w. in muscle). The high variability of the lead concentration in wild boar muscle and liver has been reported in several studies (CitationSwiergosz et al. 1993). CitationWolkers et al. (1994) reported a median concentration of 0.917 mg Pb kg−1 d.w. (0.270 mg Pb kg−1w.w.) in the liver of wild boars shot in The Netherlands. These authors also observed that the meat of farmed wild boars had a significantly lower lead concentration (median 0.082 mg kg−1w.w.) than that of free-living animals. Finally, a recent survey performed in Croatia (CitationBilandzic et al., 2009) noted that lead levels in wild boar meat may vary. The mean meat contamination from 7 Croatian hunting areas ranged from 0.028 to 0.150 mg Pb kg−1 w.w., with pooled data spanning the 0.001–1.01 mg kg−1 w.w. range (CitationBilandzic et al., 2009). In conclusion, our results for lead fall within the range of values observed in the majority of the literature consulted, with the exception of the studies by CitationMorales et al. (2011) and CitationTaggart et al. (2011). These authors reported that the high lead levels could be related to lead dispersion in the animal body due to bullet fragmentation (CitationHunt et al., 2009). The problem of Pb contamination due to bullets has received great attention in the literature and the possible impact on human health should not be underestimated.
Chromium
The median values for the total chromium in the wild boar liver were slightly higher (0.123 mg kg−1w.w.) than in the muscle (0.109 mg kg−1w.w.), with a proportionally slight difference in the mean values (). The average and median chromium contents of the kidney were slightly lower than those of the muscle and liver, although differences did not reach significance. Great variability was seen in all tissues with an RDS range of between 61.3% and 77.9% (). Among tissues or organs, the preferential accumulation of chromium appears to be significantly lower in the kidney compared with the liver and muscle tissue (), although no differences were observed. There was very little difference in the presence of chromium in the kidney of wild boar () between different study areas (P>0.05) although, on average, those coming from the MTI area were more contaminated. A certain uniformity was also observed for muscle (), although the highest values were recorded in tissue samples from animals harvested in ACQ and MNO. A similar trend towards uniformity was also observed in kidney samples ().
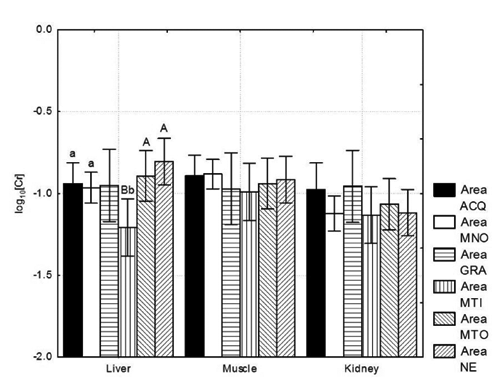
Chromium could play different roles in the living organism and is a contaminant that can be ingested via the diet (CitationGunderson, 1995); however, it is also a trace element essential for many biochemical processes. Currently, there is no EU limit for chromium in animal food products. For this reason, only a few studies have reported the chromium content in either small or large game animals. CitationSwiergosz et al. (1993) observed a mean chromium level in wild boar liver of 0.705 mg kg−1w.w. This result is most likely caused by environmental pollution, and it is 5 times greater than the level we observed in the same organ (0.134 mg Cr kg−1w.w.). CitationWlostowski et al. (2006) observed that wild animals, although living in unpolluted habitats, contain higher amounts of trace elements in their tissues and organs compared with farmed animals. However, we cannot ultimately affirm or exclude that our data might reflect low-to-moderate environmental pollution because no reference values in wild boars hunted in unpolluted areas are available.
Seňczuk (1990), indicated that physiological levels of chromium in wild boar liver and kidney should be within the range of 0.015–0.220 mg Cr kg−1 d.w. (approximately 0.004–0.064 mg Cr kg−1 w.w.). For muscle tissue, CitationBratakos et al. (2002) reported that the chromium content of pork ranged from 0.05 to 0.14 mg Cr kg−1 w.w. Because chromium supplementation is not allowed in animal feed in the EU (CitationMantovani et al. 2009), these values may be considered a reference level for chromium in pork, even though higher levels should be expected in feral pigs or wild boars living in the same areas (CitationWlostowski et al., 2006). In our study, the chromium level in wild boar muscle, liver and kidney ranged from undetectable to 0.692 mg kg−1 w.w. This range is 4 times wider than that recorded by CitationBratakos et al. (2002) in pigs. The values we observed also fall in the range reported by CitationPiskorová et al. (2003) for the muscle (0.02–0.49 mg kg−1 w.w.) of wild boars harvested in a polluted region of the Slovak Republic. In Italy, CitationMartelli (2005) reported similar values for a small number of animals. A general consideration of the available literature leads us to conclude that the high values reported in our data are most likely due to the accumulation of chromium related to the aging of the animals and/or a moderate load of chromium from the environment. However, it must be underlined that the relationship between chromium accumulation and aging is unclear (CitationGamberg et al. 2005), and further investigation is needed to ascertain whether our data reflect a moderate environmental load of this element.
As previously indicated, chromium is also an essential nutrient for animals, and an adequate ingestion level (35 µg day−1 for a 60 kg adult) has been recommended by the Institute of Medicine of the U.S. National Academy of Science (CitationIOM, 2000). Nevertheless, a high intake can be toxic (CitationAnderson, 1997; CitationBielicka et al. 2005). A higher range of intake levels (from 50 to 200 µg day−1 person−1) was assessed by CitationAnderson (1997). The scarce number of toxicological reports has prevented international health organizations (US-FNB, 2001; UK-EVM, 2003; SCF, 2003) from establishing limits for ingestion of chromium and its compounds. Currently, upper limits of 250 mg Cr day−1 person−1 (CitationWHO, 1996) and the oral MRLs of 5 µg CrVI day−1 kg−1 b.w. (body weight) for a brief exposure period (15–365 days) and 1 µg CrVI day−1 kg−1 b.w. for exposure for periods of over one year (CitationATSDR, 2008) should be adopted. Uncertainty also arises from the scarce data on chromium speciation in feed (CitationBoon et al. 2012). In fact, chromiumIII is most likely the predominant form occurring in feed because it is the most stable (CitationEPA, 1998, CitationIOM, 2000; CitationLevina and Lay, 2008). In addition, chromiumIII is gastrointestinally absorbed at 0.4–2.5% (CitationIOM, 2000). Currently, no chromium deficiency has been reported in human populations (CitationStallings and Vincent, 2006), and the measure of its essentiality is still controversial (CitationStearns 2000; CitationLevina et al. 2003; CitationStallings and Vincent 2006). In contrast, there is a large volume of literature that discusses the genotoxic effect in animals and humans (CitationSnow, 1991; CitationBridgewater et al., 1994) due to the accumulation and excessive intake of chromium.
Zinc
Similar mean values were registered in the liver and muscle for zinc, and those for the kidney are significantly lower (P<0.01) (; ). A similar trend was also registered for the median, maximum and minimum values. Variability for the different tissues (SDR) ranged between 15.2% and 26.3%. The content of zinc in the liver was significantly higher in GRA compared with MNO (P<0.01) and all the other areas (P<0.05) (). In muscle, the highest value was registered in MNO compared with ACQ (P<0.01) and GRA (P<0.05).
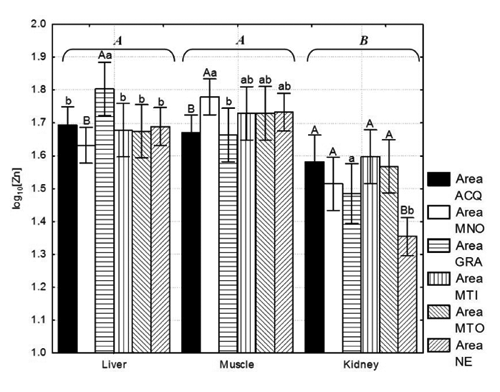
There was a high variability between the different study areas in the accumulation of zinc in the kidney () and significant differences were registered when a comparison was made between the NE hunting area and the other areas (P<0.01), excluding MNO (P<0.05).
The suggestion that zinc accumulation in the liver and muscle is similar, and higher than that in the kidney, already suggested above, was therefore confirmed. Sporadic cases of contamination by zinc over 70 mg kg−1w.w. were recorded in samples of liver harvested from ACQ, MNO and GRA. Considering the kidney samples analyzed, the average contamination was variable in samples from ACQ, MTI and MTO, approximately 40 mg Zn kg−1 w.w. in GRA, decreased to 30 mg Zn kg−1 w.w. in MNO, and decreased further to approximately 20 mg Zn kg−1 w.w. for the samples from NE (P<0.01).
CitationFalandysz (1994) reported average wild boar muscle values of 28–37 mg Zn kg−1w.w. with a range oscillating between 4.3 and 130 mg Zn kg−1w.w. CitationGasparik et al. (2012) reported a mean value of 28.20 mg Zn kg−1 w.w.. These average data are lower than those reported in our study; however, they have a wider range (). CitationFalandysz (1994) reported mean values of 37 to 48 mg kg−1 w.w. and 30 to 31 mg kg−1 w.w. in liver and kidney, respectively, that were similar to those of our study and with a similar range of variation. Similar mean values were also reported by CitationSwiergosz et al. (1993): 114–143 mg Zn kg−1 d.w. and 82–104 mg Zn kg−1 d.w. in muscle and kidney, respectively. CitationGasparik et al. (2012) reported 28.20 and 20.98 mg Zn kg−1 w.w. in the same organs, respectively. Literature data on zinc from Italy are limited; the only data are reported by CitationZaccaroni et al. (2003) with an average of 94.76 mg Zn kg−1 d.w. in kidney. Average and maximum values recorded for zinc levels muscle do not appear to cause particular concern if compared with the acceptable exposure doses in humans (CitationGupta and Gupta, 1998). However, the high values of zinc in the liver mean it is not recommended for human consumption. Careful consideration should be given to the fact that the role of zinc in humans exposed to a cadmium load is still unclear, as highlighted by Brzóska and Moniuszko-Jakoniuk (2001). Although the accumulation of cadmium in some human tissues and organs has been proven if zinc-deficient diets are administered, further research on this topic is needed (Brzoska and Moniuszko-Jakoniuk, 2001). Our data confirm this hypothesis given that the zinc content in the liver was high in MNO and low in ACQ and vice versa for cadmium ().
Copper
There were significant differences in copper content of different tissues (P<0.01) showing a clear accumulation order (): liver > muscle > kidney. In liver, highly significant differences were observed between samples from the NE and GRA hunting areas (P<0.01); however, significant differences were also observed between other sampling areas (P<0.05).
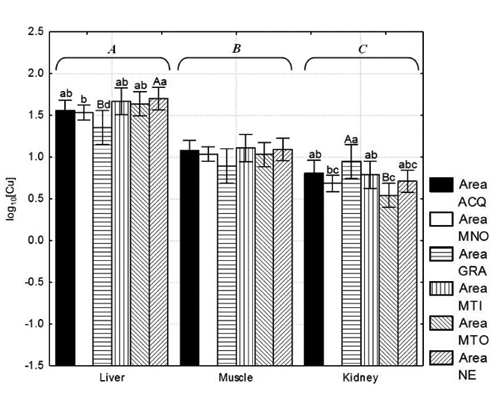
Particularly high values of Cu accumulation in wild boar liver were recorded for the ACQ area where 2 samples exceeded 100 mg kg−1 w.w. Different results were obtained for kidney tissue, as the highest value was observed in GRA and the lowest in NE (P<0.01). Significant differences were also observed between other sampling areas (P<0.05). No differences were observed between muscle samples from different areas. It is interesting to highlight the wide range of copper content, mainly in liver (54.5% SDR).
CitationSwiergosz et al. (1993) reported mean values from 6.4 to 7.4 mg Cu kg−1 d.w. and from 17.2 to 24.5 mg Cu kg−1 d.w., respectively, in the muscle and kidney of wild boars shot in Poland. In Italy, CitationZaccaroni et al. (2003) reported 30 and 21.2 mg Cu kg−1 d.w. in kidney and liver tissue, respectively. CitationWolkers et al. (1994) reported 15–20 mg Cu kg−1 d.w. and 17–18 mg Cu kg−1 d.w. in liver and kidney tissue, respectively, with a very high variability (over 60% SDR). CitationGasparik et al. (2012) reported 1.61, 3.41 and 3.80 mg Cu kg−1 w.w. These are similar to our data for kidney tissue; however, the muscle and liver contents in our study were higher than those recorded in the aforementioned studies. A possible explanation for these differences in the mean level of copper accumulation may be due to the particular geo-environmental context in which our wild boars lived. The soil of the Province of Viterbo is prevalently of volcanic origin. Some metals that occur in the soil and vegetables, such as copper and zinc, are strictly linked to volcanic activity (CitationBargagli et al., 1991), and, most likely, the high value of copper observed, especially in the wild boar liver, may have an alimentary exposure due to the naturally high level of this metal in the wild boar diet constituents. Absorbed copper in the portal blood binds to albumin and transcuprein (CitationWeiss and Linder, 1985) for transport to the liver, which is the major storage organ for metallothionein-bound Cu (CitationVan Pamel et al., 2010)
Average and maximum values of copper concentration were recorded for muscle, although the high values do not appear to cause particular concern if compared with the exposure doses in humans (CitationGupta and Gupta, 1998). However, as also indicated for zinc, the high values in the liver mean it is not recommended for human consumption.
Conclusions
Game meat can be an important source of trace metals because of the increasing availability of this food, especially in hunting communities. This study demonstrates that for the trace elements considered, our data fall in the range observed by other authors, except for copper content in muscle and liver.
The average level of cadmium recorded in the wild boar muscle, liver and kidney were often higher than the EU MRLs for pigs (75% and 50% of non-compliant samples for muscle and kidney, respectively). The mean values recorded in the liver are comparable with those reported in Sardinia and lower than those recorded in the Slovak Republic. There was no difference between the data collected on cadmium occurrence in wild boar muscle and that available in the literature, but these values are generally lower than those recorded in other European countries.
The average lead concentration in muscle was greater than the regulatory limit for pork, with over 75% non-compliance. For the liver, the mean value was less than the regulatory limit, and only one sample was non-compliant with this limit. Similar values of lead content were observed in the liver and kidney but this was higher than in muscle, and the observed variability was low, rarely exceeding 30%. The mean values observed in liver were close to the values recorded in Sardinia. It should be noted that the maximum lead values in different tissues is 2–4 fold higher than the mean, as the areas near the bullet pathway were avoided while sampling. In those studies, high lead levels could be related to lead dispersion into the animal body due to bullet fragmentation. Although very important, the problem of lead dispersion along the bullet pathway is not the focus of the present paper.
Currently, there is no EU limit for chromium in animal food products. For this reason, only a few studies have reported on the chromium content in either small or large game animals. Some authors observed higher chromium levels than those found in the present study; however, result is most likely caused by the environmental pollution of the area, even though to date no reference values in wild boars hunted in unpolluted areas are available. Considering the available literature, it is most likely that our levels of contamination are high because of chromium accumulation due to aging and/or to a moderate environmental chromium load from the environment. Although chromium is an essential element, its genotoxic role has been proven, and attention should be given to its intake. Zinc content in the wild boar liver, muscle and kidney differed among the areas investigated. These values were lower than those registered in other countries but similar to the only report from Italy. The role of zinc in humans should be carefully considered, as the accumulation of cadmium in some human tissues and organs was proved when zinc-deficient diets were administered.
The copper content in the wild boar liver was higher than in the other tissues, and a significant difference was also observed between the content in the muscle and liver. The order of accumulation in these tissues differed from that reported in other studies and content was generally higher. These contrasting results highlight the need for further studies on this element. Nevertheless, the average and maximum values recorded in muscle do not appear to raise particular concern if compared with the exposure doses in humans. However, as also indicated for zinc, the high values in the liver mean it is not recommended for human consumption.
Acknowledgments:
this work was financially supported by the Province of Viterbo, Assessorato Agricoltura Caccia e Pesca, and by the University of Tuscia (RSA 2009–2010).
The authors thank Dr. A. Sabatini for the technical support for laboratory procedures.
The hunters participating in the survey are gratefully acknowledged for their collaboration.
References
- AastrupP. RigetF. DietzR. AsmundG. 2000 Lead, zinc, cadmium, mercury, selenium and copper in Greenland caribou and reindeer (Rangifer tarandus) Sci. Total Environ 245 149 159
- Agency for Toxic Substances and Disease Registry 2008 Draft toxicological profile for chromium. U.S Department of Health and Human Services, Public Health Service Atlanta, GA, USA Available from: http://www.atsdr.cdc.gov/toxprofiles/tp7.pdf
- AmiciA. SerraniF. RossiC.M. PrimiR. 2012 Increase in crop damage caused by wild boar (Sus scrofa L.): the “refuge effect” Agron. Sustain. Dev 32 683 692
- AndersonR.A. 1997 Chromium as an essential nutrient for humans Regul. Toxicol. Pharm 26 S35 S41
- BagdonR.E. HazenR.E. 1991 Skin permeation and cutaneous hypersensitivity as a basis for making risk assessment of chromium as soil contaminant Environ. Health Persp 92 111 119
- BargagliR. BarghigianiC. SiegelB.Z. SiegelS.M. 1991 Trace metal anomalies soils and vegetation on two active island volcanoes: Stromboli and Vulcano (Italy) Sci. Total Environ 102 209 222
- BeiglböckC. SteineckT. TataruchF. RufT. 2001 Environmental cadmium induces histopathological changes in kidneys of roe deer Environ. Toxicol. Chem 21 1811 1816
- BielickaA. BojanowskaI. WiśniewskiA. 2005 Two faces of chromium - pollutant and bioelement Pol. J. Environ. Stud 14 5 10
- BilandžiN. SedakM. VratariD. PeriT. ŠimiB. 2009 Lead and cadmium in red deer and wild boar from different hunting grounds in Croatia Sci. Total Environ 407 4243 4247
- BoonP.E. Te BiesebeekJ.D. SioenI. HuybrechtsI. MoschandreasJ. RuprichJ. TurriniA. AzpiriM. BuskL. ChristensenT. KerstingM. LafayL. LiukkonenK.H. PapoutsouS. Serra-MajemL. TraczykI. De HenauwS. Van KlaverenJ.D. 2012 Long-term dietary exposure to chromium in young children living in different European countries Food Addit. Contam. A 29 1701 1715
- BratakosM.S. LazosE.S. BratakosS.M. 2002 Chromium content of selected Greek foods Sci. Total Environ 290 47 58
- BridgewaterL.C. ManningF.C.R. WooE.S. PatiernoS.R. 1994 DNA polymerase arrest by adducted trivalent chromium Mol. Carcinogen 9 122 133
- BrzóskaM.M. Moniuszko-JakoniukJ. 2001 Interactions between cadmium and zinc in the organism Food Chem. Toxicol 39 967 980
- DanieliP.P. SerraniF. PrimiR. PonzettaM. P. RonchiB. AmiciA. 2012 Cadmium, lead and chromium in large game: a local-scale exposure assessment for hunters consuming meat and liver of wild boar Arch. Environ. Con. Tox 63 612 627
- Deutsches Institut fur Normung 1994 Chemical analysis; decision limit; detection limit and determination limit; estimation in case of repeatability; terms, methods, evaluation Norm 3264 DIN Publ. Berlin, Germany
- DobrowolskaA. MelosikM. 2008 Bullet-derived lead in tissues of the wild boar (Sus scrofa) and red deer (Cervus elaphus) Eur. J. Wildlife Res 54 231 235
- DosiA. 2000 Heavy metals in blubber and skin of Mediterranean monk seals, Monachus monachus from the Greek waters Degree Diss. University of North Wales, Bangor Menai Bridge Gwynedd UK
- EPA 1998 Toxicological review of trivalent chromium. U.S Environmental Protection Agency Washington, DC, USA Available from: http://www.epa.gov/iris/toxreviews/0028tr.pdf
- European Commission 1998 The certification of the mass fraction of As, Cd, Cu, Mn, Pb, Se and Zn in bovine liver CRM 185R Available from: http://irmm.jrc.ec.europa.eu/refmat_pdf/BCR-185r_report.pdf
- European Commission 2011 Commission Regulation of 29 April 2011 amending Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs Official Journal, L 111/3, 30/04/2011 3 6
- EPA 1998 Toxicological review of trivalent chromium U.S. Environmental Protection Agency Washington, DC, USA Available from: http://www.epa.gov/iris/toxreviews/0028tr.pdf
- FalandyszJ. 1994 Some toxic and trace metals in big game hunted in the northern part of Poland in 1987–1991 Sci. Total Environ 141 59 73
- FalandyszJ. Szymczyk-KobrzyskaK. BrzostowskiA. ZalewskiK. ZasadowskiA. 2005 Concentrations of heavy metals in the tissues of red deer (Cervus elaphus) from the region of Warmia and Mazury, Poland Food Addit. Contam 22 141 149
- GambergM. PalmerM. RoachP. 2005 Temporal and geographic trends in trace element concentrations in moose from Yukon, Canada Sci. Total Environ 351–352 530 538
- GasparikJ. DobiasM. CapcarovaM. SmehylP. SlameckaJ. BujkoJ. 2012 Concentration of cadmium, mercury, zinc, copper and cobalt in the tissues of wild boar (Sus scrofa) hunted in the western Slovakia J. Environ. Sci. Heal. A 47 1212 1216
- GuazzettiS. ReggioniW. PepinS. NardelliP. 2001 Piano di monitoraggio sanitario dei selvatici nel territorio della Provincia di Reggio Emilia Proc. 27th Nat. Congr. SIPAS Parma, Italy 27 317 322
- GundersonE.L. 1995 Dietary intake of pesticides, selected elements, and other chemicals: FDA Total Diet Study, July 1986–April 1991 J. AOAC Int 78 1353 1363
- GuptaU. C. GuptaS.C. 1998 Trace element toxicity relationships to crop production and livestock and human health: implication for management Commun. Soil Sci. Plan 29 1491 1522
- HernándezL.M. GonzálezM.J. RicoM.C. FernándezM.A. BalujaG. 1985 Presence and biomagnification of organochlorine pollutants and heavy metals in mammals of Doñana National Park (Spain), 1982–1983 J. Environ. Sci. Heal. B 20 633 650
- HuntW.G. WatsonR.T. OaksJ.L. ParishC.N. BurnhamK.K. TuckerR.L. RussellL. BelthoffJ.R. HartG. 2009 Lead bullet fragments in venison from Rifle-Killed deer: potential for human dietary exposure Plos One 4 e5330
- IOM 2000 Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc Food and Nutrition Board, Institute of Medicine National Academy Press Washington, DC, USA Available from: http://www.nap.edu/openbook.php?isbn=0309072794
- KaritaK. ShinozakiT. YanoE. AmariN. 2000 Blood lead levels in copper smelter workers in Japan Ind. Health 38 57 61
- LazarusM. OrctT. BlanusaM. VickovicI. SostarićB. 2008 Toxic and essential metal concentrations in four tissues of red deer (Cervus elaphus) from Baranja, Croatia Food Addit. Contam. A 25 270 283
- LevinaA. CoddR. DillonC.T. LayP.A. 2003 Chromium in biology: toxicology and nutritional aspects Prog. Inorg. Chem 51 145 250
- LevinaA. LayP.A. 2008 Chemical properties and toxicity of chromium(iii) nutritional supplements Chem. Res. Toxicol 21 563 571
- MandasL. 2005 Problemi sanitari della fauna selvatica nelle oasi di protezione faunistica 3 11 Reg. Congr. on Ambiente e sviluppo sostenibile: La nuova provincia del Medio Campidano – tutela e valorizzazione della fauna locale Montevecchio (CA), Italy
- MaňkovskáB. SteinnesE. 1995 Effects of pollutants from an aluminium reduction plant on forest ecosystems Sci. Total Environ 163 11 23
- MantovaniA. FrazzoliC. La RoccaC. 2009 Risk assessment of endocrine-active compounds in feeds Vet. J 182 392 401
- MartelliP. 2005 Controllo ispettivo qualitativo di carni di cinghiale regolarmente macellati nella provincia di Siena Degree Diss. Università di Pisa Italy
- MoralesJ.S.S. RojasR.M. Pérez-RodríguezF. CasasA.A. LópezM.A.A. 2011 Risk assessment of the lead intake by consumption of red deer and wild boar meat in Southern Spain Food Addit. Contam 28 1021 1033
- OrusaR. AbeteM.C. TarascoR. FerrariA. RobettoS. ValvoT. 2004 Residues of cadmium, chromium and lead in wild boars hunted in Aosta Valley during 2003 Proc. 48th Nat. Congr. SISVet Grado (GO), Italy 48 429 430
- PiskorováL'. VasilkováZ. KrupicerI. 2003 Heavy metal residues in tissues of wild boar (Sus scrofa) and red fox (Vulpes vulpes) in the Central Zemplin region of the Slovak Republic Czech J. Anim. Sci 48 134 138
- PokornyB. JelenkoI. KierdorfU. KierdorfH. 2009 Roe deer antlers as historical bioindicators of lead pollution in the vicinity of a lead smelter, Slovenia Water Air Soil Poll 203 317 324
- RamanzinM. AmiciA. CasoliC. EspositoL. LupiP. MarsicoG. MattielloS. OlivieriO. PonzettaM. P. RussoC. Trabalza MarinucciM. 2010 Meat from wild ungulates: ensuring quality and hygiene of an increasing resource Ital. J. Anim. Sci 9 e61
- RegleroM.M. TaggartM.A. Monsalve-GonzalezL. MateoR. 2009 Heavy metal exposure in large game from a lead mining area: Effects on oxidative stress and fatty acid composition in liver Environ. Pollut 157 1388 1395
- RudyM. 2010 Chemical composition of wild boar meat and relationship between age and bioaccumulation of heavy metals in muscle and liver tissue Food Addit. Contam 27 467 472
- SantiagoD. Motas-GuzmanM. RejaA. Maria-MojicaP. RoderoB. Garcia-FernandezA.J. 1998 Lead and cadmium in red deer and wild boar from Sierra Morena Mountains (Andalusia, Spain) B. Environ. Contam. Tox 61 730 737
- Scientific Committee on Food 2003 Opinion of the Scientific Committee on Food on the tolerable upper intake level of trivalent chromium European Commission, Health and Consumer Protection Directorate-General Brussels, Belgium Available from: http://ec.europa.eu/food/fs/sc/scf/out197_en.pdf
- SeňczukW. 1990 Toksykologia PZWL Ed. Warszawa, Poland
- ShapiroS.S. WilksM.B. ChenH.J. 1968 A comparative study of various tests for normality J. Am. Stat. Assoc 63 1343 1372
- SnowE.T. 1991 A possible role for chromium(III) genotoxicity Environ. Health Perspect 92 75 81
- StallingsD. VincentJ.B. 2006 Chromium: a case study in how not to perform nutritional research Curr. Top. Nutraceut. R 4 89 112
- StearnsD.M. 2000 Is chromium a trace essential metal? Biofactors 11 149 162
- SwiergoszR. PerzanowskiK. MakoszU. BitekI. 1993 The incidence of heavy metals and other toxic elements in big game tissues Sci. Total Environ 134 Suppl.1 225 231
- TaggartM.A. RegleroM.M. CamareroP.R. MateoR. 2011 Should legislation regarding maximum Pb and Cd levels in human food also cover large game meat? Environ. Int. 37 18 25
- TripathiR. M. RaghunathR. KrisnamoorthyT.M. 1997 Dietary intake of heavy metals in Bombay city, India Sci. Total Environ 208 149 159
- UK Expert group on Vitamins and Minerals 2003 Safe upper levels for vitamins and minerals Report of the Expert group on Vitamins and Minerals Food Standards Agency London, UK Available from: http://cot.food.gov.uk/pdfs/vitmin2003.pdf
- US Food and Nutrition Board 2001 Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium and zinc National Academy Press Washington, DC, USA Available from: http://www.nap.edu/open-book.php?isbn=0309072794
- Van PaemelM. DierickN. JanssensG. FievezV. De SmetS. 2010 Selected trace and ultratrace elements: biological role, content in feed and requirements in animal nutrition - elements for risk assessment Technical report submitted to EFSA Available from: http://www.efsa.europa.eu/it/supporting/doc/68e.pdf
- WeissK.C. LinderM.C. 1985 Copper transport in rats involving a new plasma protein Am. J. Physiol. Endocrinol. Metab 249 E77 E88
- WHO 1996 Trace elements in human nutrition and health - A report of a re-evaluation of the role of trace elements in human health and nutrition World Health Organisation Geneva, Switzerland Available from: http://whqlibdoc.who.int/publications/1996/9241561734_eng.pdf
- WlostowskiT. BondaE. KrasowskaA. 2006 Free-ranging European bisons accumulate more cadmium in the liver and kidneys than domestic cattle in north-eastern Poland Sci. Total Environ 364 295 300
- WolkersH. WensingT. Groot BruinderinkG.W.T.A. 1994 Heavy metal contamination in organs of red deer (Cervus elaphus) and wild boar (Sus scrofa) and the effect on some trace elements Sci. Total. Environ 144 191 99
- ZaccaroniA. AndreaniG. ZucchiniM. MerendiF. SimoniP. 2003 Heavy metals in wild boar (Sus scrofa) and related lesions Hystrix 14 174 175