Abstract
The objective of this work was to study the effect of small doses of naloxone (Nx) on plasma prolactin (PRL) and testosterone levels, and to correlate hormone changes induced by the opioid antagonist with sexual exhaustion in adult male rabbits. Two groups of 8 adult New Zealand White male rabbits were divided into 2 groups: Group 1 (n=8) was treated with daily intramuscular injections of 0.5 mg of naloxone at 08:00 and at 20:00 hours for 15 days. Group 2 was treated with saline injections. Copulatory behaviour was similar on the first day and during seven days of treatment with Nx in both groups; mounts and copulations averaged 8.1 to 7.21. After 15 days of treatment with Nx, a significant increase in mount/copulation events was observed in naloxone treated rabbits as compared with controls (11.1 vs 7.6) (P<0.001). Mounts and copulations in the Nx treated rabbits was significantly higher than control rabbits (P<0.001) (9.4 vs 7.6) seven days after treatment with Nx was discontinued. During the first week of treatment, plasma testosterone levels in Nx treated rabbits were similar to those of the control group. At the end of the first week of treatment with Nx, there was a continuous increase in testosterone levels and high levels of the androgen were still detectable seven days after naloxone treatment was discontinued. There was a significant overall effect of treatment (P<0.001). It was concluded that endogenous opioids are important modulators of sexual behaviour in rabbits.
Introduction
This work is a follow up of previous studies carried out on the opioid antagonist naloxone (Nx). It has been observed that small doses of Nx are effective in inducing changes both in behaviour and hormone levels. The importance of this study is that these findings (using small doses of naloxone) do not agree with early work carried out using naloxone in doses of 1 and 2 mg/kg to examine the interaction of endogenous opioids in the reproductive physiology of different species. When using high doses of the opioid antagonist, the observed results show many differences between species; either significant changes are seen or there is no observed effect. CitationFitzgerald and Perkins (1994) reported that naloxone (1 mg/kg) did not stimulate courtship behaviour of sexually inactive male rams. Furthermore, when naloxone is administered at a dose of 0.5 mg/kg/h/24 h, CitationCurrie and Rawlings (1989) observed a loss of responsiveness of LH pulse amplitude after the continuous administration of naloxone.
The secretion of hormones related to the reproductive cycles is modulated by the hypothalamic pituitary axis, and this is influenced by hypothalamic peptides secreted in response to internal and external stimuli. Endogenous opioids are peptides that have important functions in reproduction and sexual behaviour (CitationBakker and Baum, 2000). At the hypothalamic level, the µ opioid receptor has been particularly related to gonadotrophin releasing hormone (GnRH) secretion, GnRH is the key neuropeptide controlling reproductive function in all vertebrate species. Endogen and exogen opioid agonists induce a decrease in GnRH secretion. Opioids antagonists facilitate the secretion of GnRH followed by an increase in the plasmatic concentration of luteinizing hormone (LH) and follicle stimulating hormone (FSH) (CitationOrstead and Spies, 1987; CitationBakker and Baum, 2000).
The interaction of opioids with LH accounts for much of the effect of opioids on the secretion of the gonadal steroid hormones, estradiol and testosterone (CitationVuong et al., 2010). Various components of male sexual behaviour, such as mounting and ejaculation, along with others, such as aggression and territorial scent marking, are activated by testosterone (CitationVuong et al., 2010). The administration of small doses of Nx to buck rabbits increased mounts and number of copulations (CitationFuentes et al., 2005). In other species, it was observed that small doses of Nx increased libido and testosterone levels in male goats (CitationFuentes et al., 1998), and decreased plasma prolactin (PRL) concentration in the ewe (CitationFuentes et al., 2007) was due to the effects of Nx related to µ opioid receptors (CitationHalperin et al., 2006).
The effect of low doses of naloxone on hormone levels and behaviour are not immediate; in rams and bucks it was observed that there was no change in testosterone levels during the first seven days of treatment, but after 7–15 days of treatment with the opioid antagonist, significant changes in plasma testosterone were observed (CitationFuentes et al., 1998). Prolactin (PRL) is involved in diverse biological processes; its actions on reproductive processes represent the largest group of functions identified for this hormone. It has been proposed that PRL is involved in ovarian function (CitationHalperin et al., 2006). A previous work reported that plasma PR concentration were lower in high fertility ewes as compared with ewes of low fertility (CitationFuentes, 1986).
In a previous study, we observed that administration of small doses of Nx induced hormone changes in rabbits, but we did not correlate these findings with sexual behaviour. It is important to obtain further information about the interaction of endogenous opioids with sexual behaviour to further understand the general physiology of rabbit reproduction as this will help improve husbandry and reproductive performance of this highly productive species (CitationRommers et al., 2006; CitationFuentes et al., 2004; CitationRibikauskas et al., 2010).
Little is known of the effect of Nx on plasma PRL and testosterone levels in rabbits. Given this, and considering previous studies on the sexual behaviour of male rabbits, we were interested in examining the effect of naloxone administered in small doses on plasma prolactin and testosterone levels, and to correlate sexual exhaustion with naloxone induced hormone changes in New Zealand White male adult rabbits.
Materials and methods
Animals
Two groups of 8 adult New Zealand White male rabbits aged approximately 35 weeks with proven sexual drive and fertility were selected at random from a commercial industrial farm. The animals weighed 3800–4000 g and body condition was excellent. They were housed in the experimental unit of the University Farm. Each male rabbit was housed individually in galvanized cages (90 cm×60 cm×40 cm). Rabbits were housed in an open shed exposed to natural photoperiod conditions (20°50’N 102°46’WL). Average temperature was 20°C; humidity was 45±5%. Water and food (rabbit chow PMI 15.3% CP, 16.5% CF, 1800 Kcal DE) was supplied ad libitum using automatic dispensers.
Fifteen days before the study, all rabbits were anaesthetized with a combination of medetomidine (0.2 mg/kg), ketamine (10 mg/kg) and butorphanol (0.5 mg/kg). When surgery was terminated, atipamezole (1 mg/kg) was administered for a fast recovery (CitationHedenqvist et al., 2002). During anaesthesia, permanent silastic catheters were placed in both external jugular veins, and catheters were driven subcutaneously to the top of the head in between the ears. The catheters were flushed with 50 to 100 µL heparin (1000 units/mL), closed with a plastic syringe luer-lock cap. Due to time restrictions and continuous handling, technicians for each group established a familiarity with the experimental and control rabbits to reduce stress and create conditions in which the experimental procedures could be carried out with confidence and care for the well being of the animals involved.
Blood sampling and hormonal assays
Blood samples were collected at 08:00 and 20:00 hours beginning on the day before treatment was started, and on days 0, 1, 3, 5, 7, 10, 13, 15, 18 and 21 of the experiment: 1.5 or 2 mL of blood were drawn through the indwelling catheter implanted in the jugular vein and placed into 3 mL disposable syringes containing heparin as anticoagulant. These were kept in iced water until centrifuged after which plasma was aspirated and stored at −20°C in screw-topped 3 mL plastic vials until thawed for hormone assay. No discomfort was observed on the part of the rabbits as the blood sample was collected.
Prolactin concentrations were measured by a specific homologous RIA method using AFP-991086 antibody supplied by the National Institutes of Health (NIH, Bethesda, MD, USA). Intra-assay coefficient of variance was less than 5%. Assay sensitivity was 0.120 ng/mL. Testosterone concentrations were determined by radioimmunoassay using a lyophilized aliquot of 250 anti-testosterone-11-BSA serum; intra- and interassay coefficient of variance for this hormone were 6.9 and 12.6%, respectively.
Naloxone treatment
Naloxone hydrochloride was obtained from Sigma Chemicals (Mexico City, Mexico) and dissolved in physiological saline at a concentration of 0.5 mg/mL, in accordance with previous experience using small doses of Nx. Group 1 (n=8) were treated with twice daily intramuscular injections of 0.5 mg of naloxone at 08:00 and at 20:00 hours for a period of 15 days. Group 2 (control) was treated with saline intramuscular injections.
Evaluation of sexual exhaustion
The sexual exhaustion test was used to evaluate the effect of naloxone on hormone levels and to relate them with sexual behaviour (CitationFuentes et al., 2005). Observation of mounts and ejaculations for treated (n=8) and non-treated (n=8) rabbits were carried out at 12:00 hours on each trial day. One technician performed all sampling and behavioral procedures for each animal group to avoid stress and facilitate handling. All male rabbits were tested for sexual exhaustion in the same order during each test.
For each test, 50 sexually receptive females from a commercial unit were selected at random and housed in an adjacent paddock with feeding and watering provided as for males. A fertile sexually receptive female previously tested for lordosis behaviour was introduced to the male’s cage for a period of 4 min. If the male mounted and ejaculated during this period, the female was immediately withdrawn and a new female was introduced in the same cage. The procedure continued until the male was no longer interested in the newly introduced estrous female. When there was no longer a response to the newly introduced female, the male was considered sexually exhausted (CitationFuentes et al., 2005). The sexual exhaustion test was carried out on the first day of treatment and on Days 7 and 14 of naloxone injections. A sexual exhaustion test was carried out seven days after the last naloxone injection. To avoid biostimulation, groups were housed at least 100 metres apart.
Statistical analysis
Sexual behaviour of each group was studied and compared using a Mann-Whitney statistical U-test. Plasma testosterone and prolactin levels were compared using a Student’s t-test. All management and experimental procedures of this work were supervised and approved by the University Ethical Committee for animal experimentation.
Results
Copulatory behaviour on Day 1 and Day 7 of treatment with Nx in both control and treated groups was similar (); mounts and copulations averaged 7–7.5 in both groups.
Table 1 The ffect of low doses of naloxone on copulatory behaviour of adult New Zealand White rabbits. Nx group treated with naloxone, control group treated with saline injections.
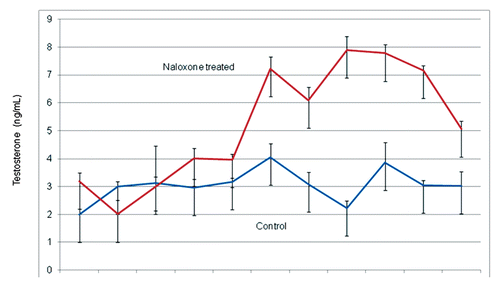
After 15 days of treatment with Nx (3rd trial), a significant increase in mount/copulation events was observed in naloxone treated rabbits as compared with controls (9.7 vs 7.0) (P<0.001). On the 4th trial, seven days after suspension of treatment with Nx, it was observed that mount and copulation events in the Nx treated rabbits was significantly higher than control rabbits (P<0.01) (9.5 vs 7.9). Seven days after of suspension of naloxone medication it was observed that there was a significant increase in mount and ejaculation events in Nx treated rabbits compared with controls. A significant difference was observed when comparing the number of estrous females mounted/ejaculated between groups (P<0.01). Serum testosterone levels were correlated with the effect of Nx treatment, both in concentration and behaviour. During the first week of treatment, plasma testosterone levels in Nx treated rabbits were similar to those of the control group and this agrees with other studies (CitationCastro et al., 2002). However, at the end of the first week of treatment with Nx, testosterone plasma values showed a continuous increase and high levels of the androgen (7 ng/mL) were still detectable seven days after suspension of naloxone treatment ().
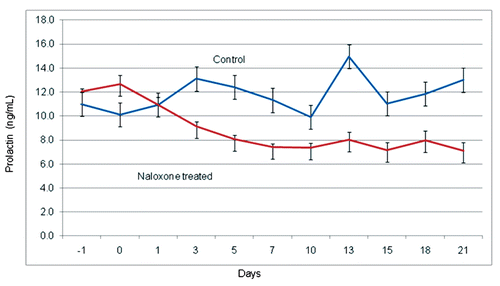
There was a decrease in prolactin levels from Day 1 of naloxone treatment and levels remained low throughout the duration of the experiment (). There was a significant overall effect for treatment (P<0.001) and similar results were observed each day during treatment with Nx (P<0.05).
Discussion
These findings confirm previous observations in other species in which the administration of small doses of naloxone increased libido and testosterone plasma levels in rams, bucks (CitationFuentes et al., 1997; Citation1998) and lactating rats (CitationForsberg et al., 1987). The effect of naloxone on plasma testosterone is interesting, because plasma levels do not change until after the first week of treatment; a finding previously reported in bucks (CitationFuentes et al., 1997). Control rabbits showed a continuous pattern of mounts and ejaculations for the duration of the experiment. It is important to note that to reach sexual exhaustion in Nx treated rabbits, a significant increase in mount/ejaculation events was observed in the 3rd trial, i.e. after 15 days of continuous treatment with Nx. The latter effect shows that acute changes using small doses of Nx are difficult to observe; probably because the effect of the opioid antagonist takes time to interact with µ opioid receptors at the hypothalamic level. Therefore, we could postulate that to elicit physiological changes in behaviour and hormone levels, naloxone should be used and adjusted both in dose and time in order to take effect. In this study, acute changes are transitory while long-term changes using small doses of the opioid antagonist are more sustained, even though medication with naloxone was suspended, a decrease in plasma testosterone and prolactin were still observed. A recent study reported that ultra low doses of naloxone affects the µ receptor at the molecular level; at low doses, affinity for µ opioid receptors increases whereas systematic administration of naloxone (often in high doses which are not selective for µ opiate receptors) tends either to have no effect or to facilitate certain aspects of sexual behaviour (CitationWang et al., 2008). In this study, it was observed that Nx in small doses induces changes in both PRL and testosterone, and that these changes correlate with an increase in sexual activity. When testosterone reaches higher levels, mount and ejaculation events increase.
Prolactin levels decrease in Nx treated rabbits and remain below control levels for the duration of the experiment suggesting that this effect facilitates sexual activity. This finding is in agreement with a recent work in which it was reported that naloxone inhibits PRL release, which is the expected response after the administration of the opioid antagonist (CitationVuong et al., 2010). During mating and sexual behavior, PRL remains unchanged; stimuli associated with mating have no direct influence on the subsequent release of prolactin in rabbits (CitationFuchs et al., 1981). Considering that PRL influences behaviour, it could be suggested that the effect of small doses of naloxone decreases plasma PRL levels and that this facilitated sexual performance in these rabbits.
The use of low doses of naloxone as an opioid antagonist is supported by early research using naloxone in different species. Early studies using naloxone were usually carried out with doses as high as 1–2 mg/kg; a dose that we considered to be extremely high. High doses of the opioid antagonist naloxone produce different levels of distress in humans, ewes and cows; some experimental animals collapsed and died after the bolus injection of the antagonist (CitationAndree, 1980; CitationYang et al., 1988; CitationNanda et al., 1989). Opioid antagonists have a preference for µ-opioid receptors, but at higher doses they antagonize both δ- and κ-opioid receptors. Accordingly, these drugs are considered non-selective opioid receptor antagonists. However, the µ-opioid receptor appeared to play a primary role in mediating the effect of opioids on PRL secretion (CitationVuong et al., 2010)
As previously stated, in this study small doses of naloxone were used in an effort to maintain therapeutic levels and ensure animal well-being (CitationFuentes et al., 2005). Research carried out at the µ opioid receptor level suggests that, when administered in low doses, naloxone has more affinity for µ receptors which are responsible for gonadotrophin secretion. Low doses can reverse the anoestrous condition in lactating ewes, stimulate LH secretion in Soay rams, and facilitate estrous behaviour in ewes (CitationCeli et al., 2007). These observations suggest that previous studies carried out with high doses of Nx should be revisited taking into consideration these and previous findings using small doses of the opioid antagonist.
Future research will examine the possibility of applying these findings to improve sexual performance in males using low doses of orally administered opioid antagonists such as naltrexone.
Conclusions
In conclusion, the administration of small doses of opioid antagonists induce hormone changes and increase sexual performance giving further support to the use of endogenous opioids as important modulators of sexual behaviour in the male rabbit.
References
- AndreeR. 1980 Sudden death following naloxone administration Anesth. Analg 59 782 784
- BakkerJ. BaumM.J. 2000 Neuroendocrine regulation of GnRH release in induced ovulators Front. Neuroendocrin 21 220 262
- CastroA.C. BerndtsonW.E. CardosoF.M. 2002 Plasma and testicular testosterone levels, volume density and number of Leydig cells and spermatogenic efficiency of rabbits Rev. Bras. Pesqui. Med 35 493 498
- CeliP. Walkden-BrownS.W. BlacheD. SzéllA.Z. WilkinsonH.M. MartinG.B. 2007 Twin efficiency for reproductive variables in monozygotic twin sheep Theriogenology 68 663 667
- CurrieW.D. RawlingsN.C. 1989 Fluctuation in responsiveness of LH and lack of responsiveness of FSH to porlonges infusion of morphine and naloxone in the ewe J. Reprod. Fertil 86 359 366
- FitzgeraldJ.A. PerkinsA. 1994 Effect of morphine and naloxone on LH response and sexual behavior of rams (Ovis aries) Domest. Anim. Endocrin 11 271 279
- ForsbergG. EnerothP. SöderstenP. 1987 Naloxone stimulates sexual behaviour in lactating rats J. Endocrinol 113 423 427
- FuchsA.R. CubileL. DawoodM.Y. 1981 Effects of mating on levels of oxytocin and prolactin in the plasma of male and female rabbits J. Endocrinol 90 245 253
- FuentesV.O. 1986 Does Prolactin regulate prolificity in the ewe? Vet. Rec 118 638
- FuentesV.O. FuentesP.I. GarciaA. 1998 The effect of naloxone on plasma concentrations of testosterone in male goats Small Ruminant Res 27 173 176
- FuentesV.O. GonzalesH. SanchezV.M. FuentesP.I. 2007 Effect of small doses of naloxone on the pulsatile secretion of prolactin in the crossbreed ewe during the non breeding season Anim. Reprod. Sci 100 44 50
- FuentesV.O. SanchezV. GonzalezH. FuentesP.I. GarciaA. RosilesR. 1997 La función endócrina del testículo en el carnero criollo mexicano durante las diferentes épocas del año y su control opioidergico durante el anestro J. Vet. Med. A 44 259 263
- FuentesV.O. VillagranC. NavarroJ. 2004 Sexual behavior of male New Zealand White rabbits in an intensive production unit Anim. Reprod. Sci 80 157 162
- FuentesV.O. VillagranC. NavarroJ. FuentesP.I. 2005 Effect of small doses of naloxone on sexual exhaustion in White New Zealand male rabbits Anim Reprod. Sci 90 341 346
- HalperinJ. DeviS.Y. ElizurS. StoccoC. ShehuA. RebourcetD. UntermanT.G. LeslieN.D. LeJ. BinartN. GiboriG. 2006 Prolactin signaling through the short form of its receptor represses forkhead transcription factor FOXO3 and its target gene galt causing a severe ovarian defect Mol. Endocrinol 22 513 522
- HedenqvistP. RoughanB. AntunesL.M. FlecknellP.A. 2002 Anaesthesia in the rabbit: influence of route of administration and the effect of combination with butorphanol Vet. Anaesth. Analg 29 14 19
- NandaA.S. WardW.R. DobsonH. 1989 Opioid modulation of tonic luteinizing hormone release in ovariectomized dairy cows J. Vet. Pharmacol. Ther 12 397 403
- OrsteadK.M. SpiesH.G. 1987 Inhibition of hypothalamic gonadotropin-releasing hormone release by endogenous opioid peptides in the female rabbit Neuroendocrinology 46 14 23
- RibikauskasV. RibikauskienėD. SkurdenienėI. 2010 Effect of housing system (wire cage versus group-housing) and in-house air quality parameters on the behaviour of fattening rabbits World Rabbit Sci 18 243 250
- RommersJ.M. BoitiC. De JongI. BrecchiaG. 2006 Performance and behaviour of rabbit does in a group-housing system with natural mating or artificial insemination Reprod. Nutr. Dev 46 677 687
- VuongC. Van UumS.H.M. O'DellL.E. LutfyK. FriedmanT.C. 2010 The effects of opioids and opioid analogs on animal and human endocrine systems Endocr. Rev 31 98 132
- WangH.Y. FrankfurtM. BurnsL.H. 2008 High-affinity naloxone binding to filamin A prevents mu opioid receptor-gs coupling underlying opioid tolerance and dependence PLoS One 3 e1554
- YangK. HaynesN.B. LammingG.E. BrooksA.N. 1988 Ovarian steroid hormone involvement in endogenous opioid modulation of LH secretion in mature ewes during the breeding and non breeding seasons J. Reprod. Fertil 83 129 139