Abstract
The aim of the study was to investigate the effect of Ascophyllum nodosum, an edible brown macroalga, on some haematologic parameters of dairy cows. Nineteen clinically healthy Holstein cows, an average 4.3 years old, were divided into two groups for 49 days. Ten cows received control diet (roughages and concentrate) while the concentrate of 9 cows was additionally supplemented with 80 g A. nodosum/cow/day. Average daily milk production (controls 39.6 kg/cow; A. nodosum 40.2 kg/cow), milk protein and fat were not affected by the alga supplementation. Glucose, sorbitol dehydrogenase, haemoglobin, haematocrit and white/red blood cells were evaluated in weekly blood samples. It was seen that A. nodosum increased blood glucose and decreased sorbitol dehydrogenase compared to controls, without any adverse effects on the other examined parameters. Consequently, A. nodosum may be suggested as a functional ingredient in dairy cow nutrition, improving energy utilization and expressing hepatoprotective effect.
Introduction
The relationship between diet and health was initially proposed by Hippocrates about 2500 years ago. Today, there is increased interest among consumers and animal breeders in natural products that can promote health or reduce the risk of disease. Macroalgae or seaweeds can be considered among these functional foods. These algae have been traditionally used in many parts of the world as medicine or food (CitationChandini et al., 2008).
A well known brown macroalgae is Ascophyllum nodosum, classified in Phaeophyceae and containing many nutritional components, such as polysaccharides, fatty acids, polyphenols and peptides (CitationPlaza et al., 2008; CitationTierney et al., 2010). A. nodosum has the highest content of total polysaccharides among brown algae, ranging between 42–70% of dry weight (CitationHoldt and Kraan, 2011). It is rich in unique polysaccharides (phycocolloids), such as alginic acid (28%), fucoidan (11.6%), laminarin (4.5%) and mannitol (7.5%) (CitationO’Sullivan et al., 2010; CitationHoldt and Kraan, 2011). These polysaccharides are not found in terrestrial plants (CitationKhan et al., 2009) and most of them are dietary fibres which cannot be digested by the human organism. Therefore, they could potentially be used as prebiotic functional substances for human and animal health applications (CitationChristaki et al., 2010; CitationO’Sullivan et al., 2010; CitationHoldt and Kraan, 2011).
The lipid content in A. nodosum is low, about 2–7% of dry weight, whereas there are sufficient amounts of n-3 fatty acids (CitationKumari et al., 2010) to offer protection from human cardiovascular diseases (CitationSimopoulos, 2002). A. nodosum has a high concentration of vitamins (A, C, D, E) and minerals (Ca, P, Na and K) (CitationFitzgerald et al., 2011). Protein content varies between 3–15% (CitationFleurence, 2004). In addition, it has been reported to contain acidic amino acids, ranging from 18% to 44% (CitationHarnedy and Fitzgerard, 2011), and peptides that have been proven to have hypotensive effect on the human circulatory system (CitationFitzgerald et al., 2011). Other important components found in A. nodosum are polyphenols, such as phlorotannins, that can account for up to 15% of the algal dry mass and can act as antioxidants and antibacterial compounds (CitationWang et al., 2009; CitationHoldt and Kraan, 2011). Furthermore, it contains carotenoids, especially the photosynthetic pigments like chlorophyll and fucoxanthine, which exhibit antioxidant capacity (CitationLordan et al., 2011).
Traditionally, A. nodosum has been used as soil fertilizer for many varieties of crops in coastal areas all over the world (CitationNorrie and Hiltz, 1999). According to CitationSimmons-Boyce et al. (2009), inclusion of up to 15% of A. nodosum in the diet produces no toxic effects, and in recent years A. nodosum has been utilized as an additive for animal feeding (CitationLordan et al., 2011). Meals or extracts of A. nodosum have been examined as natural feed supplements to improve animal health and performance in lambs (CitationSaker et al., 2004; CitationArcher et al., 2007), cattle (CitationAnderson et al., 2006), weanling pigs (CitationDierick et al., 2009), grower-finisher pigs (CitationGardiner et al., 2008), and chickens (CitationGravett, 2000).
Nevertheless, there is no literature available on the influence of this seaweed on haematologic parameters of dairy cows. These parameters are recognized as a method of documenting overall animal health status (CitationKumar et al., 2003; CitationHagawane et al., 2009). Therefore, the aim of the present study was to determine whether the supplementation of the brown seaweed A. nodosum in the feed of dairy cows had any effect on their milk production and haematologic parameters.
Materials and methods
Animals
The study was carried out on a commercial farm in northern Greece. Nineteen healthy Holstein dairy cows, average age 4.3 years, which had previously completed at least one lactation period, were divided into two groups. The control group (A) had 10 cows and the A. nodosum group (B) had 9 cows. These animals had similar milk productivity (Group A 34.3 kg milk per cow/day; Group B 34.1 kg milk per cow/day) and there was no significant difference in their average body weight (Group A 523.1 kg; Group B 539.8 kg) (P>0.05). The whole experiment was performed under commercial conditions and lasted seven weeks. Animals were clinically examined every week.
Diets
The A. nodosum supplement used in this experiment was bought from Acadian Seaplants Limited, Dartmouth, Canada. According to the manufacturer, its chemical composition was: crude protein 6%, carbohydrates 52%, crude fibre 6%, crude fat 2%, ash 22%, moisture 12%, Ca 1%, P 0.1%, Na 2.4%, Cl 1%.
Cows in the control Group A received a diet without A. nodosum, whereas in Group B a total of 80 g powdered A. nodosum was added daily to the concentrate of each cow. The daily diet consisted of roughages (30 kg corn silage and 2 kg alfalfa, mixed with 2kg molasses) served in the morning (8.00 am) and the evening (8.00 pm) and concentrate. The additional concentrate () was offered individually to each cow twice a day, in the morning (7.00 am) and the evening (7.00 pm), in two equal meals after milking and with consideration given to individual cow milk production. The concentrate was formulated to meet the cows’ nutrient requirements in order to balance the milk production at a rate of 1 kg concentrate for 3.3 litre of milk. All cows had free access to water.
Table 1 Composition of concentrate feed.
The cows were acclimatized to their diet for a period of three weeks, beginning on Day 21 after calving, until Day 42. The first day of the experiment was Day 43 after calving.
Milk production
Milk production was recorded daily throughout the experiment. Milk protein and fat percentage were determined at weekly intervals using a MilkoScan 120 Analyzer (Foss Analytical, Slangerupgade, Denmark).
Haematologic parameters
At the end of every week, blood samples were collected from each cow immediately after the afternoon milking (7.00 pm), by jugular vein puncture, in Vacuette CE tubes (Greiner-Bio One) containing K3 EDTA. Haemoglobin (Hb, mg/dL), packed cell volume (PCV, %), erythrocyte count (TEC, ×106/mm3) and total leukocyte count (TLC, ×103/mm3) were determined in a Micros haematologic analyzer (OT-CT-OS-ABCVet-Ichror, ABX Diagno stics) according to the specific procedure of the manufacturer. Glucose was determined according to CitationBarham and Trinder (1972) using a Gilford Stasar II photometer. Sorbitol dehydrogenase (SDH) was measured according to CitationGerlach and Hiby (1974).
Statistical analysis
SPSS 16.0.1 statistical software (SPSS Inc., Chigaco, IL, USA) was used to analyze experimental data. The general linear model function was used for the analysis of variance (ANOVA). A P level <0.05 was considered significant. Homogeneity of the variances was examined with Levene’s test (CitationLevene, 1960).
Results and discussion
There was no significant difference between the two groups in average milk production or milk protein and fat (P>0.05) during the whole experimental period (). shows the blood glucose concentration in the 7 weekly samplings. Glucose was significantly higher in the A. nodosum group compared to controls in the second (P<0.05), fourth (P<0.01), fifth (P<0.001), sixth (P<0.001) and seventh (P<0.001) weeks, although values remained within normal ranges. Also, SDH was significantly lower for the A. nodosum group compared to controls in the third (P<0.01), fourth (P<0.01), fifth (P<0.01), sixth (P<0.001) and seventh (P<0.001) weeks, although values remained within normal ranges ().
Table 2 Milk production, milk protein and milk fat of cows receiving either 0 g A. nodosum per cow per day (Group A) or 80 g A. nodosum per cow per day (Group B).
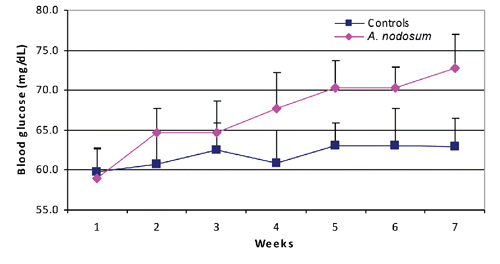
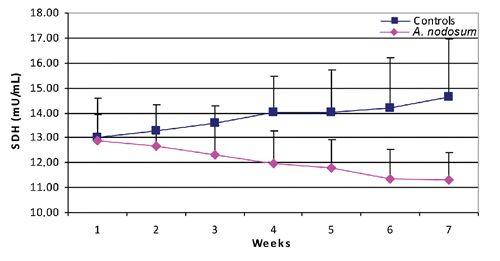
Furthermore, there was no significant difference in haematologic parameters, Hb, PCV, TEC, and TLC, between the two groups in any of the weekly measurements and values stayed within normal ranges (P>0.05) ().
Table 3 Blood Hb, PCV, TEC and TLC of cows receiving either 0 g A. nodosum per cow per day (Group A) or 80 g A. nodosum per cow per day (Group B).
The present research indicates that the dietary addition of A. nodosum to dairy cows increased blood glucose levels compared to controls, and this effect was gradually more pronounced over the duration of the experiment, although values remained within normal ranges (CitationBraun et al., 2007). In previous studies, higher blood glucose levels have been reported when A. nodosum was added to the diets of goats (CitationKannan et al., 2007) or lambs (CitationArcher et al., 2007).
The energy requirements of cows increase after calving and during lactation due to milk production and body maintenance (CitationReynolds, 2005). The blood glucose level is regarded as one of the indicators of energy status in ruminants and a precursor for lactose synthesis in the mammary gland (CitationSchultz, 1968; CitationNafikov and Beitz, 2007; CitationReynolds, 2005). It is known that cows that are high milk producers are only just able to synthesize sufficient glucose and are prone to metabolic disorders.
One possible mechanism by which A. nodosum may operate to increase the blood glucose of dairy cows is that this seaweed may increase the production of propionate in the rumen which finally leads to glucose production (CitationBergman et al., 1974). Propionate represents an important metabolic link between energy intake and liver glucose production, since up to 76% of liver glucose synthesis is produced from propionate (CitationReynolds et al., 1994). Gluconeogenesis is very important in ruminants and even more so in lactating cows (Naficov and Beitz, 2007).
Another possible mechanism could be that the addition of A. nodosum may stimulate the development of intestinal microflora resulting in improved feed digestion and better utilization of feed nutrients (CitationHnisova et al., 2011). Furthermore, it was reported that A. nodosum can limit the growth of potential pathogens, such as E. coli 0157:H7, both in in vitro (CitationWang et al., 2009) and in vivo studies on cattle (CitationBraden et al., 2004). Furthermore, A. nodosum supplementation can enhance immune function and overall animal health in lambs (CitationSaker et al., 2004), and beef steers (CitationSaker et al., 2001; CitationAnderson et al., 2006). This improved immunity seems to be related to the antioxidant content of the seaweed (CitationAllen et al., 2001).
Another significant finding in our experiment is that dietary A. nodosum resulted in a gradual decrease in cow SDH during the lactation period under study, while SDH was gradually increased in the control cows; however, all values remained within reference ranges. SDH is a liver-specific enzyme in large domestic animals which is involved in carbohydrate metabolism. It catalyzes the conversion of fructose to sorbitol in hepatocytes (CitationSoveri et al., 1992; CitationGrucka-Mamczar et al., 2007). The activity of SDH in the blood of healthy animals is low, whereas its elevation above normal range indicates hepatocellular injury. It is considered a sensitive indicator of hepatocellular damage (CitationWiesner et al., 1965) and it is possible that the use of A. nodosum in this study could exert a hepatoprotective effect on the cows.
Good animal utilization of feed nutrients are of primary importance in efforts to increase milk protein and fat content (CitationNeitz and Robertson, 1991). Consequently, it could be expected that A. nodosum supplementation in cow feed may result in increased milk production or changes in milk composition. Some researchers (CitationMcHugh 2003; CitationCvetkovic et al., 2004) who examined the use of A. nodosum meal in dairy cow feeds found that milk production and milk protein content were increased. Nevertheless, in our study, there were no changes in milk production and milk protein and fat contents compared to control cows. Similar findings were also reported by CitationCermak et al. (2011) who examined hydrolyzed A. nodosum in dairy cows and CitationPompeu et al. (2011) who examined A. nodosum on heat stressed dairy cows. It can be hypothesized that the good animal health status and housing conditions in our experiment probably limited the beneficial effect of A. nodosum on milk production. In this experiment, blood Hb, PCV, TEC and TLC of dairy cows were measured in an effort to estimate overall health status. Red blood cells are sensitive to oxidative damage and this makes them an appropriate model for studying oxidative stress (CitationSaker et al., 2004). Also, blood hematocrit and hemoglobin values give information about disorders such as anemia, lack of aminoacids, etc. (CitationNdlovu et al., 2007) while white blood cells are linked to the immune system function (CitationArcher et al., 2007). Since there were no significant differences between controls and cows fed A. nodosum, it can be deduced that erythropoiesis was not impaired by the seaweed under study and immune function was not affected.
Conclusions
In conclusion, A. nodosum supplementation in dairy cow diet increased blood glucose levels, whereas it decreased blood SDH. Furthermore, it had no adverse effect on the examined haematologic parameters: Hb, PCV, TEC, and TLC. Consequently, A. nodosum may be suggested as a functional ingredient in dairy cow nutrition, improving energy utilization and expressing a hepatoprotective effect.
References
- AllenV.G PondK.R SakerK.E FontenotJ.P BagleyC.P IvyR.L EvansR.R SchmidtR.E FikeJ.H ZhangX AyadJ.Y BrownC.P MillerM.F MontgomeryJ.L MahanJ WesterD.B MeltonC 2001 Tasco: Influence of a brown seaweed on antioxidants in forages and livestock-A review J. Anim. Sci 79 E21 E31
- AndersonM.J BlantonJ.RJr GleghornJ KimS.W JohnsonJ.W 2006 Ascophyllum nodosum supplementation strategies that improve overall carcass merit of implanted English crossbred cattle Asian-Aust. J. Anim. Sci 19 1514 1518
- ArcherG.S FriendT.H CaldwellD AmeissK KrawczelP.D 2007 Effect of seaweed Ascophyllum nodosum on lambs during forced walking and transport J. Anim. Sci 85 225 232
- BarhamD TrinderP 1972 Improved colour reagent for determination of blood glucose by oxidase system Analyst 97 142 145
- BergmanE.N BrockmanR.P KaufmanC.F 1974 Glucose metabolism in ruminants: Comparison of whole-body turnover with production by gut, liver and kidneys Fed. Proc 33 1849 1849
- BradenK.W BlantonJ.RJr. AllenV.G PondK.R MillerM.F 2004 Ascophyllum nodosum Supplementation: A preharvest intervention for reducing Escherichia coli O157:H7 and Salmonella spp. in feedlot steers J. Food Prot 67 1824 1828
- BraunU HeusmannB CamenzindD HaessigM 2007 Comparison in blood glucose concentrations after administration of a glucose solution via the jugular vein and portal vein in cows J. Vet. Med. A 54 398 400
- CermakB HnisovaJ PetraskovaE SochM VostooupalB 2011 Influence of chosen stimulants on selected quality ingredients of cow's milk and rumen parameters Anim. Sci. Biotechnol 44 19 23
- ChandiniS.K GanesanP SureshP.V BhaskarN 2008 Seaweeds as source of nutritionally beneficial compounds – A review J. Food. Sci Technol 45 1 13
- ChristakiE KaratziaM Florou-PaneriP 2010 The use of algae in animal nutrition J. Hellenic Vet. Med. Society 61 267 276
- CvetkovicB BroukM.J ShirleyJ.E 2004 Impact of dried seaweed meal on heatstressed lactating dairy cattle Dairy Day (Report of Progress 941) Kansas State University Agricultural Experiment Station and Cooperative Extension Service Available from: http://hdl.handle.net/2097/6732
- DierickN OvynA De SmetS 2009 Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs J. Sci. Food Agric 89 584 594
- FitzgeraldC GallagherE TasdemirD HayesM 2011 Heart health peptides from macroalgae and their potential use in functional foods J. Agric. Food Chem 59 6829 6836
- FleurenceJ 2004 Seaweed proteins YadaR.Y Proteins in Food Processing Woodhead Publishing Limited Cambridge, UK 197 213
- GardinerG CampbellA O'DoertyJ PierceE LynchP LeonardF StantonC RossR LawlorP 2008 Effect of Ascophyllum nodosum extract on growth performance, nutrient digestibility, carcass characteristics and selected intestinal microflora populations of grower-finisher pigs Anim. Feed Sci. Tech 141 259 273
- GerlachU HibyW 1974 Sorbitol dehydrogenase BergmeyerH.U Methods of Enzymatic Analysis Vol. 2 New York Academic Press 512 523
- GravettR.B 2000 The effects of Ascophyllum nodosom on immune function, performance, and carcass characteristics of sheep and cattle Degree Diss Texas Tech University Lubbock, TX, USA
- Grucka-MamczarE BirknerE Zalejska-FiolkaJ MachoyZ KasperczykS BlaszczykI 2007 Influence of extended exposure to sodium fluoride and caffeine on the activity of carbohydrate metabolism enzymes in rat blood serum and liver Res. Report Fluoride 40 62 66
- HagawaneS.D ShindeS.B RajguruD.N 2009 Haematological and blood biochemical profile in lactating buffaloes in and around Parbhani city Vet. World 2 467 469
- HarnedyP.A FitzgeraldR.J 2011 Bioactive proteins, peptides, and amino acids from macroalgae J. Phycol 47 218 232
- HnisovaJ PetraskovaE CermakB SochM FrelichJ 2011 The effect of biostimulative substances on daily milk yield and quality components in cow's milk Anim. Sci. Biotechnol 44 55 58
- HoldtS.L KraanS 2011 Bioactive compounds in seaweed: functional food applications and legislation J. Appl. Phycol 23 371 393
- KannanG TerrillT. H KouakouB GalipalliS 2007 Blood metabolite changes and live weight loss following brown seaweed extract supplementation in goats subjected to stress Small Ruminant Res 73 228 234
- KhanW RayirathU.P SubramanianS JitheshM RayorathP HodgesD.M CritchleyT.A CraigieJ.S NorrieJ PrithivirajB 2009 Seaweed extracts as biostimulants of plant growth and development J. Plant Growth Regul 28 386 399
- KumarB.R MuralidharanM.R RameshV ArunachalamS SivakumarT 2003 Effect of transport stress on blood profile in sheep Indian Vet. J 80 511 514
- KumariP KumarM GuptaV ReddyC.R.K JhaB 2010 Tropical marine macroalgae as potential sources of nutritionally important PUFAs Food Chem 120 740 757
- LeveneH 1960 Robust tests for equality of variances OlkinI GhuryeS.G HoeffdingW MadowW.G MannH.B Contributions to probability and statistics: essays in honor of Harold Hotelling Stanford University Press Palo Alto, CA, USA 278 292
- LordanS RossP.R StantonC 2011 Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases Mar. Drugs 9 1056 1100
- McHughD.J 2003 A guide to the seaweed industry FAO fisheries technical paper 441 FAO Publ. Roma, Italy
- NafikovR.A BeitzD.C 2007 Carbohydrate and lipid metabolism in farm animals. Symposium: History of Nutrition: impact of Research with Cattle, Pigs, and Sheep on Nutritional Concepts J. Nutr 137 702 705
- NdlovuT ChimonyoM OkohA.I MuchenjeV DzamaK RaatsJ.G 2007 Assessing the nutritional status of beef cattle: Current practices and future prospects Afr. J. Biotechnol 6 2727 2734
- NeitzM.H RobertsonN.H 1991 Composition of milk and factors that influence it Bulletin No. 421, Directorate of Agricultural Information Department of Agriculture Publ. Pretoria, South Africa
- NorrieJ HiltzD.A 1999 Seaweed Extract Research and Applications in Agriculture Agro Food Ind. Hi-Tech 10 15 18
- O'SullivanL MurphyB McLoughlinP DugganP LawlorP.G HughesH GardinerG.E 2010 Prebiotics from marine macroalgae for human and animal health applications Mar. Drugs 8 2038 2064
- PlazaM CifuentesA IbanezE 2008 In the search of new functional food ingredients from algae Trends Food Sci. Tech 19 31 39
- PompeuL.B WilliamsJ.E SpiersD.E WeaberR.L EllersieckM.R SargentK. M FeyerabendN.P VelliosH.L EvansF 2011 Effect of Ascophyllum nodosum on alleviation of heat stress in dairy cows Professional Animal Scientist 27 181 189
- ReynoldsC.K 2005 Glucose Balance in Cattle 143 154 Proc. Florida Ruminant Nutrition Conf. Gainesville, FL, USA
- ReynoldsC.K HarmonD.L CevaraM.J 1994 Absorption and delivery of nutrients for milk protein synthesis by portaldrained viscera J. Dairy Sci 77 2787 2808
- SakerK.E AllenV.G FontenotJ.P BagleyC.P IvyR.L EvansR.R WesterD.B 2001 Tasco-Forage: II Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J. Anim. Sci 79 1022 1031
- SakerK.E FikeJ.H VeitH WardD.L 2004 Brown seaweed- (TascoTM) treated conserved forage enhances antioxidant status and immune function in heatstressed wether lambs J. Anim. Physiol. An. N 88 122 130
- SchultzL.H 1968 Ketosis in dairy cow J. Dairy Sci 51 1133 1140
- Simmons-BoyceJ.L PurcellS.L NelsonC.M MacKinnonS.L 2009 Dietary Ascophyllum nodosum increases urinary excretion of tricarboxyl acid cycle intermediates in male Sprague-Dawley rats J. Nutr 139 1487 1494
- SimopoulosA.P 2002 The importance of the ratio of omega-6/omega-3 essential fatty acids Biomed. Pharmacother 56 365 379
- SoveryT SankariS NieminenM 1992 Blood chemistry of reindeer calves (Rangifer tarandus) during the winter season Comp. Biochem. Physiol 102A 191 196
- TierneyM CroftA.K HayesM 2010 A review of antihypertensive and antioxidant activity found in macroalgae Bot. Mar 53 387 408
- WangY XuZ BachS.J McAllisterT.A 2009 Sensitivity of Escherichia coli to seaweed (Aschophyllum nodosum) phlorotannins and terrestrial tannins Asian-Aust. J. Anim. Sci 22 238 245
- WiesnerI.S RawnsleyH.M BrooksF.P SeniorJ.R 1965 Sorbitol dehydrogenase in the diagnosis of liver disease Am. J. Dig. Dis 10 147 151