Abstract
Effects of sugar cane extract (SCE) on the piglet intestinal histology were observed. Twelve castrated male piglets weaned at the age of 26 days were allotted to three groups fed diets containing 0, 0.05 or 0.10% SCE. At the end of feeding experiment, each intestinal segment was taken for light or scanning electron microscopy. Feed intake, body weight gain and feed efficiency did not show a difference among groups. Most of the values for villus height, villus area, cell area and cell mitosis numbers were not different among groups, except for that the villus area of the 0.10% SCE group and the cell area of both SCE groups increased significantly at the jejunum compared to the control (P<0.05). For cell mitosis numbers, the 0.10% SCE group was higher than the 0.05% SCE group at the jejunum. Compared with the majority of flat cells of each intestinal segment in the control, the SCE groups had protuberated cells. In the 0.05% SCE group, deeper cells at the sites of recently exfoliated cells in the duodenum, cell clusters aggregated by protuberated cells in the jejunum and much more protuberant cells in the ileum were observed. These histological intestinal alterations suggest that SCE could raise the functions of intestinal villi and epithelial cells, especially at the 0.05%.
Introduction
The weaning of piglets at three to four weeks of age is thought to be the most critical period in the life of pigs, because the piglets are subjected to stressors such as environmental, nutritional and microbial unbalances (CitationNabuurs, 1998). Weaning induces decreased feed intake, impaired intestinal morphology and function, diarrhea, and decreased growth immediately after weaning (CitationPluske et al., 1995,Citation1997). To solve these problems, antibiotics (CitationKyriakis et al., 1997) and probiotics (CitationTortuero, 1973; CitationHan et al., 1984; CitationKalbande et al., 1992) have been used as feed supplements to promote growth performance and to control disease. The facts that using antibiotics produces resistant microorganisms to antibiotics (CitationNewman et al., 1990) and that some probiotics have no effect on improving body weight gain (CitationWatkins and Kratzer, 1983; CitationWatkins and Kratzer, 1984; CitationMaiolino et al., 1992) suggest that an alternative to using antibiotic and probiotic growth promotants is needed to obtain consistent results. Some dietary ingredients such as organic acid, zinc and copper have also tried to counteract postweaning problems. Dietary organic acids improved the growth performance of weaned piglets (CitationPartanen and Mroz, 1999). Feeding high dietary concentrations of zinc and copper stimulated growth of weanling pigs (CitationHill et al., 2000). As a another growth promotant, sugar cane extract (SCE) might have a possibility to solve postweaning problems, because the SCE has growth-promotion effect (CitationEl-Abasy et al., 2002; CitationEl-Abasy et al., 2004).
SCE is a byproduct of removing glucose, fructose and sucrose from sugar cane juice produced from sugar cane in the raw sugar manufacturing process. SCE has functions such as immuno-stimulation (CitationEl-Abasy et al., 2003) and growth-promotion (CitationEl-Abasy et al., 2002; CitationEl-Abasy et al., 2004). These functions seem to be induced by increased function of intestine, because the dietary SCE caused the hypertrophied intestinal villi and epithelial cells on the villus apical surface in chickens (CitationYamauchi et al., 2006a,Citationb).
The intestine is well known to be direct organ for digestion, absorption and immunity of ingested feed, and intestinal histological alterations were induced by the diets fed to the animals (CitationLanghout et al., 1999; CitationYasar and Forbes, 1999). Increased values of villus height and cell mitosis numbers as well as protuberated cells were found in activated function of intestine (CitationYamauchi et al., 2010). Therefore, intestinal histology is thought to be able to assess the supplemented feed ingredients.
Chickens and pigs are monogastric animals, and have similar intestinal histology. After feeding charcoal powder (including wood vinegar compound liquid), increased intestinal villus height and protuberated cells on the villus apical surface were found both in chickens (CitationSamanya and Yamauchi, 2001) and pigs (CitationMekbungwan et al., 2004). As the hypertrophied intestinal histologies were observed in chickens after feeding dietary SCE (CitationYamauchi et al., 2006a), the present feeding SCE on pigs would also induce almost similar intestinal histological alterations.
In this study, the effects of 0.05 or 0.1% dietary SCE on intestinal histology in piglets were observed using light and scanning electron microscopy to obtain a basal data. In addition, growth performance was compared among groups.
Materials and methods
Sugar cane extract preparation
SCE (169 g/kg CP, 5 g/kg fat, 361 g/kg ash) was produced from sugar cane (Saccharum officinarum L.) in the raw sugar manufacturing process by Shin Mitsui Sugar Co., Ltd. (Tokyo, Japan) as follows: most of sugar components such as glucose, fructose and sucrose from sugar cane juice were separated by an ion exchange column chromatography using synthetic adsorbent to produce SCE. Then this SCE was adsorbed to oilcake of rice bran (on dry matter, 1:4) and dried for dietary supplement ().
Table 1 Feed ingredients and chemical composition of basal commercial pre-starter and starter mush diets of piglets.
Animals and housing
Feeding experiments were carried out two times, in spring (6 piglets) and summer (6 piglets) (total 12 piglets). For each season, 6 commercial crossing castrated male piglets [(Large white x Landrace) x Duroc], were weaned at the age of 26 days with an average body weight of 8 kg. Piglets were allotted to three groups of two animals; a control group was fed the basal diet and the other groups were fed the basal diet supplemented with 0.05 or 0.1% SCE. The two pigs of each treatment were housed in wire pens (1.5 m×1.0 m) under daily lighting regimen of 24 h of light and environmental room temperature. Each pen had a feeder and a water nipple to ensure ad libitum feeding and free water access. A commercial basal diet was pre-starter diet (Just one starter®) for first two weeks, and starter diet (We milk finisher®) for the final two weeks (Toyohashi Feed Mills Co., Ltd., Toyohashi, Japan) (). During the feeding experimental period, feed intake and body weight were measured weekly. At the end of each feeding experiment, piglets (final body weight average was 22.10, 22.65, 23.00 kg for control, 0.05% SCE and 0.10% SCE groups, respectively) were taken to a slaughterhouse; each piglet was anaesthetised with a 15 mL somunopenchil (64.8 mg/ml pentobarbital sodium) (Kyoritsuseiyaku Co. Ltd., Tokyo Japan) by intravenous injection and exsanguinated. All experimental procedure was carried out according to the humane care guideline for the care and use of laboratory animals established by the Mitsui Sugar Co. Ltd.
Tissue sampling
Four pigs from each of the three groups were used for tissue sampling. The abdominal cavity was opened along the midline immediately after the piglets were euthanized. Five-cm sample of the duodenum was taken about 20 cm caudal to the stomach and of the ileum approximately 20 cm proximal to the ileocolonic junction. The middle part of the remaining small intestine was regarded as the jejunum. Each sample was ligated with a thread at both ends and a mixture of 30 ml/L glutaraldehyde and 40 ml/L paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) was injected. Then, each sample was removed from the abdominal cavity, kept in a bottle with the same fixative, and prepared for light and scanning electron microscopy.
Light microscopy
A 2×3 cm segment from each 5-cm intestinal segment was fixed with Bouin’s fixative solution for one week at room temperature, embedded in paraplast and cut into 5 µm cross sections. Every 10th section was collected and stained with hematoxylin-eosin. For villus height measurement, the villi including the lamina propria were chosen and the length from the villus tip to the bottom excluding the intestinal crypt was measured. Two villi were selected under 10×4 magnification for each section. Sixteen values of villus height were counted from 8 sections per piglet, and the average of these values was expressed as the mean villus height for each piglet. To measure villus area, the width of villus was measured at the basal and apical parts. Two villi were selected under 10x4 magnification for each section. Sixteen samples were counted from 8 sections per piglet. The apparent villus area was calculated from the villus height, basal width and apical width. The average of these values was expressed as the mean villus area for each piglet.
To measure one cell area on the 5-µm cross section, the area of the epithelial cell layer was randomly measured in the middle of the villi and the number of cell nuclei within this layer was counted. The area of the epithelial cell layer was then divided by this number. This measurement was employed in one to two fields per section. Sixteen samples were counted from 8 sections per piglet, and the average of these values was expressed as the mean cell area for each piglet.
To measure the cell mitosis number per crypt, five crypts with almost the same size as one microscopic field (10x40 magnification) were randomly selected, the mitosis numbers were counted and then expressed as cell mitosis per one crypt. One to two fields per section were measured and 16 cell mitosis numbers were counted from 8 sections per piglet, and an average of these values was expressed as a mean cell mitosis number for each piglet. These measurements were recorded using an image analyser (Nikon Labophot-2, Nikon Co., Ltd., Tokyo, Japan). Finally, the mean of each intestinal parameter from the respective four piglets was expressed as the mean villus height, villus area, cell area and cell mitosis for one group.
Scanning electron microscopy
A 4×5 mm segment from the 5-cm duodenal segment close to the light microscopic sample was cut, and slit longitudinally along the nonmesenteric side for its entire length. The intestinal contents were washed with 0.01 M phosphate-buffered saline (pH 7.4). The tissue samples were pinned flat to prevent curling and fixed vertically with the mucosal surface facing downwards in a fixative mixture of 3% glutaraldehyde and 4% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) at room temperature for 1 h. The tissue block was further cut into a 4x7 mm rectangle and fixed for an additional 1 h. The pieces were rinsed with 0.1 M sodium cacodylate buffer (pH 7.4) and postfixed with 10 g/L osmium tetroxide in ice-cold buffer for 2 h. The specimens were dried in a critical-point drying apparatus (Hitachi Freeze Dryer, Hitachi Ltd., Tokyo, Japan). The dried specimens were coated with platinum (Hitachi E-1030 Ion Sputter, Hitachi Ltd.) and observed with a scanning electron microscope (Hitachi S-4300SE/N, Hitachi Ltd.).
Statistical analysis
From the viewpoint of animal welfare, three animals are known to be enough for histological observations. In this study, we used four piglets. All data collected for growth performance and light microscopic examination were statistically analyzed by using the one-way analysis of variance (ANOVA), and significant differences between the treatments were determined with Duncan’s multiple range test using the SAS® program (SAS Institute, Inc., Cary, NC, USA). Differences at P<0.05 were considered as significant.
Results
Feed intake (43.15, 44.57, 44.85 kg for control, 0.05% SCE and 0.10% SCE groups, respectively), body weight gain (13.99, 14.42, 14.83 kg for control, 0.05% SCE and 0.10% SCE groups), and feed efficiency (0.324, 0.323, 0.330 for control, 0.05% SCE and 0.10% SCE groups) did not show a difference among groups.
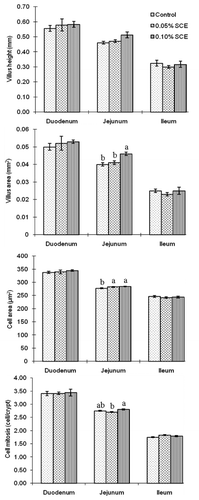
Most of the values for villus height, villus area, cell area and cell mitosis numbers were not different among groups, except for that the villus area of the 0.10% SCE group and the cell area of both SCE groups increased significantly compared to the control (P<0.05) (). In regard to cell mitosis, the 0.10% SCE group was higher numbers than the 0.05% SCE group.
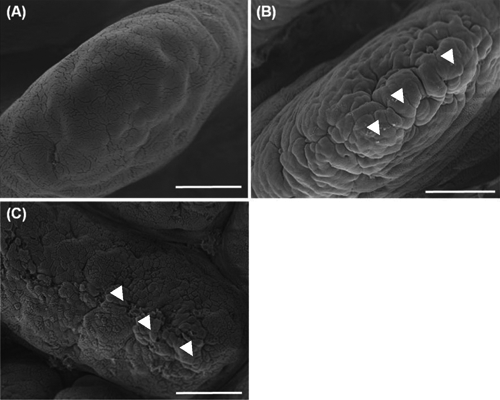
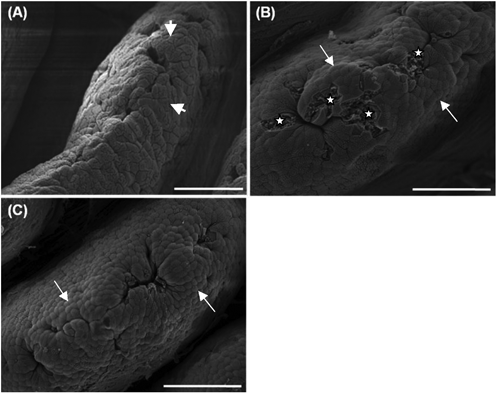
The duodenal apical surface of the control had flat cells ( A, long arrows). In the 0.05% ( B) and the 0.10% ( C) SCE groups, many clearly protuberated cells were found (short arrows). In addition, in the 0.05% SCE group, deeper cells at the sites of recently exfoliated cells (stars) frequently appeared. The jejunal apical surface of the control had flat cells, showing a smooth surface ( A). In the 0.05% ( B) and 0.10% ( C) SCE groups, cell clusters (arrow heads) aggregated by many protuberated cells were shown to have developed in addition to individual protuberated cells. These cell clusters were much more developed in the 0.05% SCE group than in the 0.10% SCE group. The ileal apical surface of the control ( A) had flat cells, but the 0.05% ( B) and 0.10% SCE ( C) groups had many protuberated cells.
Discussion
The main aim of this study was to demonstrate that dietary SCE can induce hypertrophied intestinal villi and epithelial cells. Long intestinal villi mean a greater surface area for nutrient absorption (CitationOnderci et al., 2006). Greater intestinal villus height and increased cell mitosis numbers are indicators that the function of the intestinal villi was hypertrophied (CitationLanghout et al., 1999; CitationYasar and Forbes, 1999). Furthermore, increased villus size was also related to raised cell proliferation in the crypt (CitationLauronen et al., 2000) and provided more surface area for nutrient absorption and thus improved nutrient digestibility (CitationOnderci et al., 2006). In the present study, the villus height, villus area, and cell area of the duodenum and jejunum were slightly improved with increased SCE levels, although no significantly increased. The villus area and cell area of the 0.10% SCE group were significantly higher than those of the control. The cell mitosis numbers for the 0.10% SCE group were much greater than those of the 0.05% SCE group. These results suggest that the intestinal villi might be slightly hypertrophied by dietary SCE, especially in the 0.10% SCE group. On the other hand, epithelial cells on the villus apical surface of the SCE groups developed into protuberated cells, and many more developed into cell clusters in the 0.05% SCE group. Cellular protrusion features demonstrated that cellular functions were activated (CitationYamauchi et al., 2006b). Such cell protrusion features were also seen in chickens (CitationKhambualai et al., 2010) and in pigs (CitationMekbungwan et al., 2008). In addition, cell clusters were observed in chickens fed SCE (CitationKhambualai et al., 2010). These data indicate that epithelial cells on the villus apical surface would be hypertrophied by the dietary SCE, especially in the 0.05% SCE group. It is not clear at present why hypertrophied epithelial cells developed in the SCE group, but it is possibly related to a certain ingredient of SCE. Further study is being carried out to find such a component from SCE. Besides, small particle feed ingredient such as semi-purified pellet diet can easily induce hypertrophic intestinal alteration at epithelial level than villus level (CitationManeewan and Yamauchi, 2003).
Conclusions
The 0.10% SCE, having higher values of light microscopic parameters, may effectively raise the villus function. The 0.05% SCE group, having much more protuberated cells and cell clusters, may effectively activate the cellular function. Using SCE in weaning piglets appears to have an effect on the maintenance of intestinal health.
Acknowledgments:
the authors wish to thank Miss Oraya Khambualai, Mr. Jassada Ruttanavut and Mr. Tossaporn Incharoen, Kagawa University, Kagawa, for their kind help with intestinal sampling.
References
- El-AbasyM MotobuM NakamuraK KogeK OnoderaT VainioO ToivanenP HirotaY 2004 Preventive and therapeutic effects of sugar cane extract on cyclophosphamide-induced immunosuppression in chickens Int. Immuno - pharmacol 4 983 990
- El-AbasyM MotobuM SameshimaT KogeK HirotaY 2003 Adjuvant effects of sugar cane extracts (SCE) in chickens J. Vet. Med 65 117 119
- El-AbasyM MotobuM ShimuraK NaK.J KangC.B KogeK OnoderaT HirotaY 2002 Immunostimulating and growth-promoting effects of sugar cane extract (SCE) in chickens J. Vet. Med. Sci 64 1061 1063
- HanI.K LeeS.C LeeJ.H LeeK.K LeeJ.C 1984 Studies on the growth promoting effects of probiotics. 1. The effects of Lactobacillus sporogenes on the growing performance and the changes in microbial flora of the feces and intestinal contents of the broiler chicks Korean J. Anim. Sci 26 150 157
- HillG.M CromwellG.L CrenshawT.D DoveC.R EwanR.C KnabeDA LewisA.J LibalG.W MahanD.C ShursonG.C SouthernL.L VeumT.L 2000 Growth promotion effects and plasma changes from feeding high dietary concentrations of zinc and copper to weanling pigs (regional study) J. Anim. Sci 78 1010 1016
- KalbandeV.H GaffarM.A DeshmukhS.V 1992 Effect of probiotic and nitrofurin on performance of growing commercial pullets Indian J. Poultry Sci 27 116 117
- KhambualaiO YamauchiK RuttanavutJ IncharoenT KashimuraJ 2010 Effect of sugar cane extract, commercial probiotic and their mixture on growth performance and intestinal histology in broiler chickens Am. J. Anim. Vet. Sci 5 132 138
- KyriakisS.C TsiloyiannisV.K LekkasS PetridouE VlemmasJ SarrisK 1997 The efficancy of enrofloxacin in-feed medication, by applying different programmes for the control of post-weaning diarrhea syndrome of piglets J. Vet. Med 44 513 521
- LanghoutD.J SchutteJ.B Van LeeuwenP WiebengaJ TammingaS 1999 Effect of dietary high and low methyllated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chickens Brit. Poultry Sci 40 340 347
- LauronenJ PakarinenM.P KuusanmakiP SavilahtiE VentoP PaavonenT HalttunenJ 2000 Intestinal adaptation after massive proximal small bowel resection in the pig Brit. Poultry Sci 41 416 423
- MaiolinoR FiorettiA MennaF MeoC 1992 Research on the efficiency of probiotics in diets for broiler chickens Nutr. Abstr. Rev. B 62 482 482
- ManeewanB YamauchiK 2003 Effects of semi-purified pellet diet on the chicken intestinal villus histology J. Poultry Sci 40 254 266
- MekbungwanA YamauchiK SakaidaT 2004 Intestinal villus histological alterations in piglets fed dietary charcoal powder including wood vinegar compound liquid Anat. Histol. Embryol 33 11 16
- MekbungwanA YamauchiK SakaidaT BuwjoomT 2008 Effects of a charcoal powder-wood vinegar compound solution in piglets for raw pigeon pea seed meal Animal 2–3 366 374
- NabuursM.J.A 1998 Weaning piglets as a model for studying pathophysiology of diarrhea Vet Quart 3 42 45
- NewmanK.E DawsonK.A MoreheadM.C 1990 Antagonistic activities of bacterial isolates from probiotic feed supplements upon pathogenic and rumen bacteria J. Anim. Sci 68 (Suppl.1) 505 (Abstr.)
- OnderciM SahinN SahinK CikimG AydinA OzercanI AydinS 2006 Efficacy of supplementation of α-amylase-producing bacterial culture on the performance, nutrient use and gut morphology of broiler chickens fed a corn-based diet Poultry Sci 85 505 510
- PartanenK.H MrozZ 1999 Organic acids for performance enhancement in pig diets Nutr. Res. Rev 12 117 145
- PluskeJ.R HampsonD.J WilliamsI.H 1997 Factors influencing the structure and function of the small intestine in the weaned pig: a review Livest. Prod. Sci 51 215 236
- PluskeJ.R WilliamsI.H AherneF.X 1995 Nutrition of the neonatal pig VarleyM.A The neonatal pig: development and survival CAB Int. Wallingford, UK
- SamanyaM YamauchiK 2001 Morphological changes of the intestinal villi in chickens fed the dietary charcoal powder including wood vinegar compounds J. Poultry Sci 38 289 301
- TortueroF 1973 Influence of implantation of Lactobacillus acidophilus in chicks on the growth, feed conversion, malabsorption of fats syndrome and intestinal flora Poultry Sci 52 197 203
- WatkinsB.A KratzerF.H 1983 Effect of oral dosing of Lactobacillus strains on gut colonization and liver biotin in broiler chicks Poultry Sci 62 2088 2094
- WatkinsBA KratzerF.H 1984 Drinking water treatment with commercial prepara tion of a concentrated Lactobacillus culture for broiler chickens Poultry Sci 63 1671 1673
- YamauchiK BuwjoomT KogeK EbashiT 2006a Histological alterations of the intestinal villi and epithelial cells in chickens fed dietary sugar cane extract Brit. Poultry Sci 47 544 553
- YamauchiK BuwjoomT KogeK EbashiT 2006b Histological intestinal recovery in chickens refed dietary sugar cane extract Poultry Sci 85 645 651
- YamauchiK IncharoenT YamauchiK 2010 The relationship between intestinal histology and function as shown by compensatory enlargement of remnant villi after midgut resection in chickens Anat. Rec 293 2071 2079
- YasarS ForbesJ.M 1999 Performance and gastro-intestinal response of broiler chicks fed on cereal grain-based foods soaked in water Brit. Poultry Sci 40 65 67