Abstract
Microbiological quality of raw milk from eight healthy donkeys reared in Campania Region was investigated. A total of 152 samples were analyzed in order to evaluate the milk safety status trough monitoring mesophilic total bacterial count (TBC) at 32°C and 20°C, psychrophilic TBC at 5°C, Enterobacteriaceae, Salmonella spp., Listeria monocytogenes, Staphylococcus aureus and somatic cell count (SCC). The ranges for mesophilic bacteria at 32°C, 20°C and psy- chrophilic bacteria at 5°C were, respectively, 2.80–4.00 Log CFU/mL, 2.84–3.92 Log CFU/mL and 1.27–2.12 Log CFU/ml. Enterobacteriaceae showed a load ranging between 0.68–1.93 Log CFU/mL. No pathogenic bacteria were isolated. Estimated SCC values were always under 50.000 cells/mL. Additionally quantitative changes of bacterial population in raw bulk milk during eight storing days at 8°C and 3°C, were evaluated. Firstly, fresh bulk milk was contaminated by bacteria with a mean TBC at 32°C and 20°C of 2.71 Log CFU/mL and 2.64 Log CFU/mL, respectively, whereas TBC at 5°C and Enterobacteriaceae were not detected. After eight days of storage at 8°C, TBC at 32°C, 20°C and Enterobacteriaceae increased by three Log and TBC at 5°C by five Log. On the other side, after eight days of storage at 3°C no gradual Log increase was detected. Our results showed that donkey milk could be a good healthy ingredient for feeding where good hygienic procedures are applied and storage is kept at temperature lower than 3°C.
Introduction
In recent times in Italy, interest in donkey breeding for milk production has increased due to nutrient composition of its milk, which is very close to breast milk. When human breastfeeding is not possible, the use of replacer must provide best nutritional and healthy needs for infants. Possible breast-milk substitutes include: commercial infant formula, liquid animal milk (cow or goat), powdered animal milk, dehydrated milk. Donkey’s milk is already used as possible milk (CitationPolidori et al., 2009) to replace cow milk in young children affected by cow milk allergy (CitationMonti et al., 2007; CitationVincenzetti et al., 2008). Similarity consists in the lipid fraction, characterized by high level of linoleic and linolenic acid, in the Ca/P ratio and in the low total protein fraction (CitationSalimei et al., 2004), well-represented by whey proteins such as α-lactalbumin (α-LA), β-lactoglobulins (β-LG) and lysozyme (LYS) (CitationFantuz et al., 2001). Lysozyme with other factors may reduce the incidence of gastrointestinal infections in infants (CitationBusinco et al., 2000). It is known as natural antimicrobial agent acting directly on bacterial cell walls (CitationChiavari et al., 2005) and its concentration results two times higher than human milk (CitationChiavari et al., 2005; CitationVincenzetti et al., 2008) and variable during the different stages of donkey lactation (CitationVincenzetti et al., 2008). Lysozyme may be responsible for the low bacterial load reported in literature (CitationSalimei et al., 2004) and for having positive effects on raw milk storage (CitationZhang et al., 2008).
The aims of the present study were therefore to evaluate i) the individual health status of the donkey’s mammary gland during lactation stage through monitoring total bacteria count (TBC), Enterobacteriaceae and somatic cell count (SCC); and ii) the safety status of donkey milk by investigating the presence of pathogens such as Salmonella spp., Listeria monocytogenes and Staphylococcus aureus. Furthermore quantitative changes of bacterial population in raw bulk milk during storage at two different chilling temperatures, 8°C and 3°C respectively, were also evaluated.
Materials and methods
Sampling
This study was carried out within seven months (from April to November 2010) in an approved farm (Regional Centre of Equestrian Increase) in Campania Region. Samples were collected from eight clinically healthy donkeys (five Martina Franca and three Ragusana breed). Animals were about four years old, fed with hay and oat. Milk production yielded on average 775 mL per mare per milking. Sampling comprised two phases. In the first 7- months phase, a total of 152 raw milk samples were collected from animals with a different lactation stage. The foals were physically separated from the dams three hours before sampling. The mammary glands were cleaned with a mixed of water and chlorine solution and then dried. The first two ejections were discarded. A milking machine installed on a wheeled trolley type with a modified sheep cluster (set at vacuum level 40 kPa, pulse ratio 60–40%, pulse rate 120 cycle/min) was used. Samples were transported cooled to the laboratory and pH measurements were made using FiveEasy ™ pHmeter (Mettler-Toledo Inc., Columbus, OH, USA). Bacteriological analyses were carried out within three hours after sampling. In the second phase, quantitative changes of microbial population during storage were investigated. Bulk milk containing mixed-milk obtained from each donkey was aseptically collected and placed into two different sterile laboratory glass bottles (DURAN Group GmbH, Wetheim, D). Bottles containing 800 ml of milk were stored respectively at 8°C and 3°C for eight days. Bacteriological analyses and pH measurements were carried out every day for considered storage time.
Microbiological and somatic cell count analyses
In the first phase enumeration of both TBC at 32°C, 20°C, 5°C and Enterobacteriaceae was investigated by culture method. Thus, individual aliquots (10 mL) of sampled milk from each animal were aseptically placed into different sterile bags and then homogenized in 90 mL of a quarter-strength Ringer Solution (BR0052, Oxoid Ltd., Hampshire, UK). One ml was inoculated onto Plate Count Agar (PCA, CM0325, Oxoid Ltd.) incubated for five days at 32°C, 20°C and for 10 days at 5°C for TBC enumeration and onto Violet Red Bile Glucose Agar (VRBGA, CM1082, Oxoid Ltd.) incubated for 24 h at 37°C for Enterobacteriaceae enumeration.
Analyses for Salmonella spp. were done in accordance with ISO 6579:09.2006 using a two- step enrichment procedure. Briefly, 10 mL of milk from each animal were pre-enriched in 90 mL of Buffered Peptone Water (BPW, CM1049, Oxoid Ltd.) for 24 h at 37°C. Then, one ml of the pre-enriched broth was incubated for 24 h at 37°C in 10 mL of Kauffmann Tetrathionate- Novobiocin Broth (CM1048, Oxoid Ltd.) supplemented with Novobiocin Selective Supplement (SR0181, Oxoid Ltd.) in accordance with the manufacturer’s instructions and 0.1 mL was incubated for 24 h at 41.5°C in 10 mL of Rappaport-Vassiliadis Soya Pepton Broth (CM0866, Oxoid Ltd.). Subsequently one loopful from each enriched broth was spread onto Mannitol Lysine Crystal Violet Brilliant Green Agar (MLCB, CM0783, Oxoid Ltd.) and onto Xylose-Lysine-Desoxycholate Agar (XLD, CM0469, Oxoid Ltd.). Plates were incubated for 24 h at 37°C. Colonies were biochemically tested by using the API 20E System in accordance with the manufacturer’s instructions (bioMérieux SA, Marcy-l’Etoile, France).
Analyses for L. monocytogenes were done in accordance with ISO 11290–1:2004. An amount of 10 ml of donkey milk was incubated in 90 mL of Fraser Broth (CM0895, Oxoid Ltd.) with Half Fraser Supplement (SR0166, Oxoid Ltd.) for 24 h at 30°C. Subsequently, 0.1 mL was incubated in 10 ml of Fraser Broth (CM0895, Oxoid Ltd.) with Fraser Supplement (SR0156, Oxoid Ltd.) for 24 h at 37°C. One loopful was then streaked onto Palcam Agar (CM0877, Oxoid Ltd.) added with Palcam Selective Supplement (SR0150, Oxoid Ltd.) and onto Chromogenic Listeria Agar (CM1084, Oxoid Ltd.) supplemented with Listeria Selective Supplement (SR0226, Oxoid Ltd.) and Listeria Differential Supplement (SR0244, Oxoid Ltd.). Both plates were incubated for 48 h at 37°C. Listeria monocytogenes like-colonies were biochemically tested by using the API Listeria System in accordance with the manufacturer’s instructions (bioMérieux SA). Staphylococcus aureus counting procedure was performed by spreading one ml of milk onto Baird-Parker Agar Base (CM0275, Oxoid Ltd.) supplemented with Egg Yolk Tellurite Emulsion (SR0054, Oxoid Ltd.). Suspicious colonies were tested for biochemical properties using API Staph System in accordance with the manufacturer’s instructions (bioMerieux SA). Estimation of SCC was obtained by flow cytometry (Fossomatic 5000 Foss Electric A/S).
In the second phase, aliquots (10 mL) from each stored bottle (8°C and 3°C, respectively) were analyzed for TBC at 32°C, 20°C, 5°C and for Enterobacteriaceae enumeration by culture method as previously described. Measurements of pH were also investigated. The analysis of variance was followed by Tukey’s multiple comparison tests using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA).
Results and discussion
During the evaluation period (seven months), all tested samples showed pH values on average of 7.08±0.05. Results for microbiological analyses in relation to each animal are shown in to . Results for microbiological analyses in relation to the lactation stage (days post-partum) are shown in to . Limited data are reported from literature on microbiological characteristics of donkey’s milk. In our findings raw milks collected at farm level were slightly contaminated by bacteria with mean values never higher than 4 Log CFU/mL (). Possible reasons for low bacterial load and constant pH values could be on one side the presence of natural concentration of antimicrobial compounds like lactoferin and moreover lysozyme which acts directly on bacteria (CitationChiavari et al., 2005) and contributes to maintain unvarying pH values (CitationCoppola et al., 2002; CitationSalimei et al., 2004; CitationZhang et al., 2008) and on the other side due to the strictly hygienic milking procedures applied during sampling. Our results agree with CitationCoppola et al. (2002) study where bacterial load of 4.6 Log CFU/mL, 1.5 Log CFU/mL and 3.2 Log CFU/mL were proved respectively for mesophilic bacteria, psychrotrophic bacteria and Enteroba - cteriaceae. Moreover in other studies mesophilic bacteria ranges from 1 to 2.39 Log CFU/mL (CitationPilla et al., 2010) and results of 4.46 Log CFU/mL were also obtained by CitationSalimei et al. (2004). All our samples were negative for Salmonella spp., Listeria monocytogenes and Staphylococcus aureus even though pathogen detection in donkey milk is proved in literature: Staphylococcus aureus was detected by CitationPilla et al. (2010) using molecular analyses and Enterobacter sakazakii was isolated by CitationConte and Passantino (2007) using culture method. SCC must be considered as a good system to evaluate the healthy status of the mammary gland as well as the quality of its milk. In this study SCC values tested always under 50.000 cells/mL as in CitationPilla et al. (2010) study. Our findings met values under 100.000 cells/mL reported by CitationBeghelli et al. (2009). No significative differences were evidenced (P<0.05) among the lactation stages for TBC at 32°C and 20°C and Enterobacteriaceae. Significative differences were evidenced for TBC at 5°C ( to ).
Table 1 Total bacterial count at 32°C expressed as Log CFU/mL for each animal.
Table 2 Total bacterial count at 20°C expressed as Log CFU/mL for each animal.
Table 3 Total bacterial count at 5°C expressed as Log CFU/mL for each animal.
Table 4 Enterobacteriaceae expressed as Log CFU/mL for each animal.
Table 5 Total bacterial count at 32°C expressed as Log CFU/mL in relation of lactation stage (days post-partum).
Table 6 Total bacterial count at 20°C expressed as Log CFU/mL in relation of lactation stage (days post-partum).
Table 7 Total bacterial count at 5°C expressed as Log CFU/mL in relation of lactation stage (days post-partum).
Table 8 Enterobacteriaceae expressed as Log CFU/mL in relation of lactation stage (days post-partum)
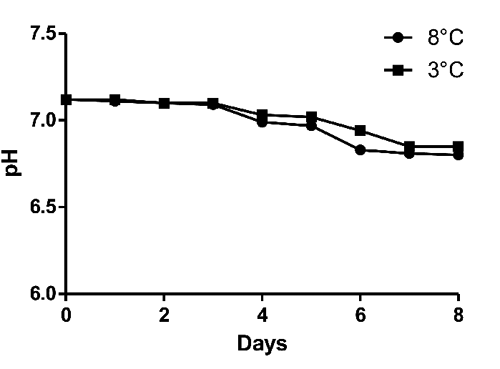
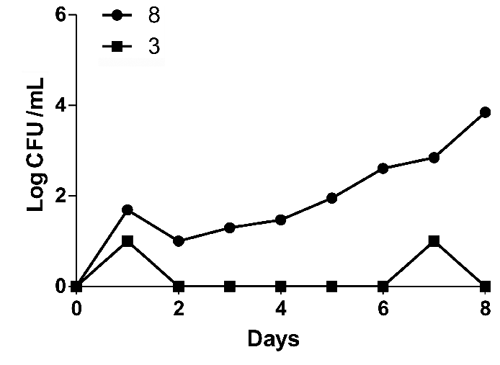
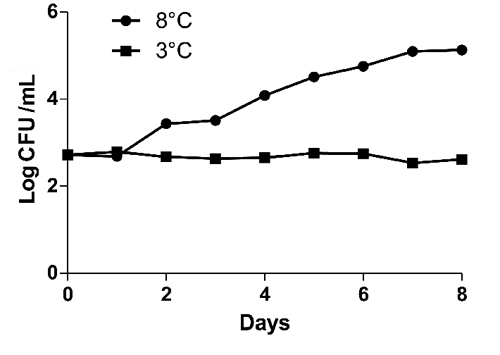
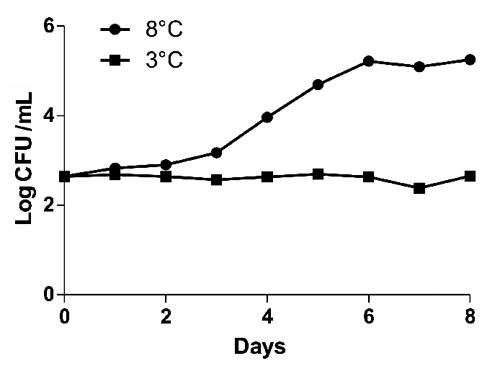
During storage period early pH value was 7.12 (). No significative differences (P<0.05) were evidenced for pH values between two tested storage temperatures. Results for microbiological analyses are shown in to . Immediately after sampling bulk milk was contaminated by bacteria with a mean TBC at 32°C and 20°C of 2.71 and 2.64 Log CFU/mL respectively. TBC at 5°C and Enterobacteriaceae population were not detected. After eight days at 8°C, TBC at 32°C, 20°C and Enterobacteriaceae increased by three Log (, and ) and TBC at 5°C by five Log (). After eight days at 3°C no Log increase was detected for TBC at 32°C, 20°C ( and ) whereas peaks (days 1, 4, 6) were detected for TBC at 5°C () and for Enterobacteriaceae (days 1, 7) (). Our findings are not in agreement with CitationŠari et al. (2012) study, where a gradual Log increase was described for TBC and Enterobacteriaceae in donkey milk stored at 4°C. Significative differences (P<0.05) between 8°C temperature and 3°C temperature were found. According to our results from the 3°C storage experiment keeping milk at refrigerated temperature immediately after milking process might be an effective measure to control bacteria growth and a possible long shelf life can be reached. However, the Enterobacteriaceae detection shows the importance to improve the hygienic practices on farm level.
Conclusions
Based on our results potential antimicrobial activity and good shelf life of raw donkey milk might be attributed to its natural compounds acting in synergism each other and to the hygienic milking procedures applied at farm level. Thus it might be considered as healthy base ingredient for feeding in case of low immune defense system, elderly, convalescent, children with cow milk allergy and when breast-feeding is not possible. As limitation of our study the experimental farming conditions should be considered and further studies are required to investigate milk quality in intensive farming conditions in order to facilitate its production and distribution on a large scale.
Acknowledgments:
the authors wish to thank all the staff involved in the experiment.
References
- BeghelliD RosciniA ValianiA VincenzettiS CavallucciC PolidoriP 2009 Somatic (CSS) and differential cell count (DCC) during a lactation period in ass' milk Ital. J. Anim. Sci 8 (Suppl.2) 691 693
- BusincoL GianpietroP.G LucentiP LucaroniF PiniC Di FeliceG lacovacciP CuradiC OrlandiM 2000 Allergenicity of mare's milk in children with cow's milk allergy J. Allergy Clin. Immunol 105 1031 1034
- ChiavariC ColorettiF NanniM SorrentinoE GraziaL 2005 Use of donkey's milk for a fermented beverage with lactobacilli Lait 85 481 490
- ConteF PassantinoA 2007 Isolation of Enterobacter sakazakii from ass' milk in Sicily: case report, safety and legal issues Travel Med. Infect. Dis 6 413 413
- CoppolaR SalimeiE SucciM SorrentinoE NanniM RanieriP Belli BlanesR GraziaL 2002 Behaviour of Lactobacillus rhamnosus strains in ass's milk Ann. Microbiol 52 55 60
- FantuzF VincenzettiS PolidoriP VitaA PolidoriF SalimeiE 2001 Study on protein fractions of donkey milk 635 637 Proc. 14th Nat. ASPA Congr. Firenze, Italy
- MontiG BertinoE MuratoreM.C CosciaA CresiF SilvestroL 2007 Efficacy of donkey's milk in treating highly problematic cow's milk allergic children: an in vivo and in vitro study Pediatr. Allergy Immunol 18 258 264
- PillaR DapràV ZecconiA PiccininiR 2010 Hygienic and health characteristics of donkey milk during a follow-up study J. Dairy Res 77 392 397
- PolidoriP BeghelliD MarianiP VincenzettiS 2009 Donkey milk production: state of the art Ital. J. Anim. Sci 8 (Suppl.2) 677 683
- SalimeiE FantuzF CoppolaR ChiofaloB PolidoriP VariscoG 2004 Composition and characteristics of ass's milk Anim. Res 53 67 78
- ŠarićL.Ć ŠarićB.M MandićA.I TorbicaA.M TomićJ.M CvetkovićD.D OkanovićÐ.G 2012 Antibacterial properties of domestic Balkan donkeys' milk Int. Dairy J 25 142 146
- VincenzettiS PolidoriP MarianiP CammertoniN FantuzF VitaA 2008 Donkey's milk protein fractions characterization Food Chem 106 640 649
- ZhangX.Y ZhaoL JiangL DongM.L RenF.Z 2008 The antimicrobial activity of donkey milk and its microflora changes during storage Food Control 19 1191 1195