Abstract
The aim of this study was to determine the effect of age of female ide on spawning success under controlled conditions. All ide breeders were collected from one wild population. Data concerning reproductive parameters were collected during induced spawning under hatchery conditions. The age of mature females ranged from 4 (average weight 504 g) to 9 years (average weight 2540 g) and the youngest collected females (3+) (average weight 330 g) were immature. The females from group 4+ produced smaller oocytes (1.28 mm diameter after hydration) than older fish (1.44–1.57). There were no differences in oocyte sizes in females 6+ and older. The highest relative fecundity (mean 44,621 eggs kg−1) was noted for fish aged from 5 to 7. Eggs obtained from the oldest females had the lowest biological quality: the highest percentage of dead embryos during incubation (49.9) and the highest percentage of morphological abnormalities in hatched larvae (5.6%). In addition, in the group of the oldest females, only 67% of fish produced eggs.
Key Words:
Introduction
Spawning effectiveness in fish is affected by many factors. These include the species and the population origin, fish condition, size, age as well as a number of environmental factors including photo-thermal conditions (CitationBrooks et al., 1997; CitationAnguis and Canavate, 2005; CitationBromage et al., 2001). Proper manipulations of thermal regime, photoperiod (CitationHilder and Pankhurst, 2003; CitationKucharczyk et al., 2008a; CitationTargońska et al., 2010) and hormonal agents (CitationBrzuska, 2006; CitationYaron et al., 2009) were the main factors determining successful reproduction in captivity. In artificial reproduction of the cyprinids, the type of hormones and its combination affected the latency time, ovulation rate and gamete quality (CitationYaron 1995; CitationKucharczyk et al., 2005; CitationBrzuska et al., 2006; CitationSzabo 2003; CitationKrejszeff et al., 2008; CitationŻarski et al., 2009; CitationCejko et al., 2010; CitationTargońska et al., 2010). Only domesticated stock of cyprinids was reported to be able to spawn after stimulation of photo-thermal conditions (CitationKrejszeff et al., 2009).
Research into the effect of age on the reproductive parameters of fish has been limited (CitationBrooks et al., 1997). There are scarce reports, mainly comparing the females which spawned for the first time with those a year older (CitationBromage and Cumaranatunga, 1988). It has been shown in recent studies of aquarium fish – Buenos Aires tetra, Hyphessobrycon anisitsi (Eigenmann, 1907) and neon tetra, Paracheirodon innesi (Myers, 1936), that viable offspring cannot be obtained from females which have spawned 6–7 times after they reached maturity (CitationKucharczyk et al., 2008b; 2010). This indicates that there is a relationship between the number of spawns and the biological quality of eggs and possibility of fertilizing. However, there have been no comprehensive reports about the effect of female age on spawning.
Ide, Leuciscus idus (L.), is one of the most popular sport fish in eastern and central Europe (CitationKrejszeff et al., 2009). The production of ide fry for restocking purposes is an important source of income for fish farms and open water managers (CitationWojda, 2004). One of the bottlenecks in rheophilic cyprinid fry production is controlled reproduction (CitationKucharczyk et al., 2008b) where hormonal preparations and a thermal regime were reported to be very important factors affecting successful spawning (CitationŻarski et al., 2009; CitationCejko et al., 2010). Among the many preparations tested in ide controlled reproduction, the best results were achieved after applying preparations containing GnRH analogues and the dopamine inhibitors: Ovopel and Ovaprim (CitationKrejszeff et al., 2009; CitationŻarskiarski et al., 2009; CitationCejko et al., 2010). In addition, an elevated water temperature was found to be a limiting factor for embryo viability, which was also reported for asp Aspius aspius (L.) (CitationTargońska et al., 2010). There is no other data concerning other factors affecting ide reproduction effectiveness.
The aim of this study was to examine how ide female age affects spawning effectiveness, defined as the percentage of ovulations, as well as the number and quality of gametes obtained.
Materials and methods
The ide spawners were caught in February 2009 in the lake Mosaag near Olsztyn. After being caught, they were transported in bags with oxygen to the hatchery at the Department of Lake and River Fisheries of the University of Warmia and Mazury, Olsztyn, and placed in 1000 dm3 tanks with controlled photo-thermal conditions (CitationKujawa et al., 1999). The fish were separated by gender, weighed and labeled with floy-tags with individual numbers. Fish age was determined on the basis of scale analysis, where growth rings (year marks) were recognized under a stereoscopic microscope (Leica MZ 12.5, Wetzlar, Germany). Scales were taken from the females from the dorsal part, at the level of the first fin ray of the dorsal fin, above the lateral line. Next, fish were classed into seven age categories (from 3+ to 9+). Before stimulation, oocyte samples were taken with a catheter from all females and their maturity was determined after immersion in the Serra liquid (ethyl alcohol 70%, formaldehyde 96% and 95% acetic acid at the proportion of 6:3:1). Following the clarification of the cytoplasm, the position of the oocyte germinal vesicle was determined under a stereoscopic microscope. Next, oocytes were categorized according to a four-degree scale:
Stage 1: germinal vesicle in the central position.
Stage 2: early migration of germinal vesicle (less than half of the radius).
Stage 3: late migration of germinal vesicle (more than half of the radius).
Stage 4: periphery germinal vesicle or germinal vesicle breakdown (GVBD).
Next, the fish were acclimatized for three days at 10°C (±0.1). The photoperiod was constant (12L:12 D). After the first injection, the temperature was raised to 11°C, after the second one to 12°C, and 24 h later to 13.5°C (CitationKucharczyk et al., 2008c). he control group (n=10) were pooled from different age groups (from 4+ to 8+) and injected with physiological saline (0.9% NaCl). The fish in the treated groups (all age groups from 3+ to 9+) were obtained Ovopel (Unic-trade, Hungary) and diluted in physiological saline. The preparation contains mammalian GnRH analogue [(D-Ala6, Pro9-Net)-mGnRH] (CitationHorvath et al., 1997) and the dopamine antagonist, metoclopramide. One pellet contains 18–20 µg of the analogue and 8–10 mg of the inhibitor. The applied doses given to the female fish were 0.2 and 1.0 pellet kg−1 during 1st and 2nd injection, respectively. All the injections were made intraperitoneally under the ventral fin at 0.5 cm3 kg−1, with a 24-hour interval. Before the manipulations, the spawners were anaesthetized with 2-fenoxyethanol solution (Sigma-Aldrich, Seelze, Germany) (0.5 cm3 dm−3). All the manipulations, including egg collection, were done by the method described by CitationKrejszeff et al., (2009). The ovulation was checked at 30 h after the second (resolving) injection and then in 2-hour intervals. During the study, the ovulation rate, latency time and working fecundity were recorded. Egg quality was determined in the eyed-egg stage when the survival rate of embryos was recognized. For this purpose, eggs from each female (about 150 each in three replications) were incubated on Petri dishes in a closed water system at 14°C (CitationKucharczyk et al., 2008c). Before fertilization, 0.05 cm3 of sperm from the pooled sample was added to each egg sample; subsequently, water was added to activate gametes. The sperm mixture was made by pooling sperm from a minimum of 5 males, using a sperm motility of at least 70%. Sperm motility was evaluated subjectively under a microscope (400×). Before hatching, Petri dishes were transferred to 1 dm3 chambers, where the larvae were hatched and the deformation frequency was determined. One hour after egg hydration, the eggs were photographed and measured (ProgRes® Capture Pro 2.5, Jenoptik, Jena, Germany) under a stereoscopic microscope (Leica MZ 12.5). The females in which no ovulation was observed had oocyte samples taken from them in order to determine their maturity stage. The procedure of maturity determination was the same as described above. Data regarding embryo survival, deformation of larvae and relative working fecundity were analyzed by ANOVA. If that found significant differences, a post-hoc Tukey’s test was performed at a level of significance of α=0.05. Data expressed in percent were subjected to arcsine transformation before the statistical analysis.
Results
Before the hormonal stimulation was started, it was found (based on recognition of the oocyte samples) that female fish aged 3+ were sexually immature (oocytes at stage 1). Oocytes taken from these females were much smaller (0.34 mm) than in older fish (0.76 mm). In the latter, oocytes were at maturity stage 2. It is noteworthy, that it was possible to extract the oocytes from immature females. Oocytes from two individuals from the oldest (9+) group could not be extracted by a catheter. Thus, it could be suggested that these females did not produce oocytes. After reproductive procedures (hormonal and photo-thermal stimulation), the oocytes were not stripped and it was also not possible to catheter from these females at that time. No eggs were stripped from the fish in the lowest age group (3+), although it was possible to extract oocytes with a catheter. When the oocyte maturity stage was checked after the experiment was completed, they had the same size (0.34 mm in diameter) and maturity stage 1. Ovulation took place 36 hours after the resolving injection (). All the fish in age classes 4+ to 8+ ovulated. A slight progression of the oocyte maturation was observed in the control group. The oocytes were at stage 2 – 2/3. No statistical differences were found in embryo survival to the eyed-egg stage in eggs obtained from females aged between 5+ and 8+ (average about 70%) (). Lower egg quality was obtained from fish aged 4+ and 9+. Eggs obtained from the oldest fish were of the lowest quality, where a 50.1% embryo survival to the eyed-egg stage was recorded. Survival of the eggs obtained from the youngest spawning females was significantly better (59.6%). Similar relationships were found after analyzing the percentage of deformed larvae. The highest deformation rate was found in the group with 9-year-old females (5.6%). Significantly better results (P<0.05) were recorded in the 3+ group (2.3% deformed larvae). However, in the remaining groups, the deformation rate did not exceed 0.01% (P<0.05) ().
Table 1 Spawning effects (mean ±SD) of female ide in various age groups. There were 10 females in each group, except the group with age 9+ females, where there were 6 fish.
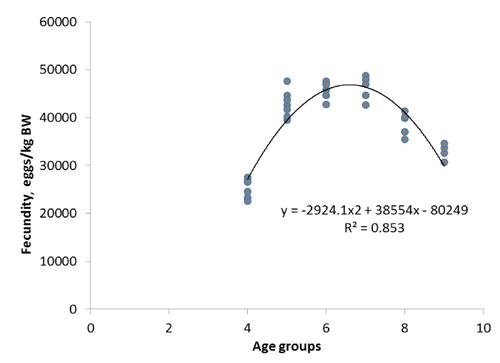
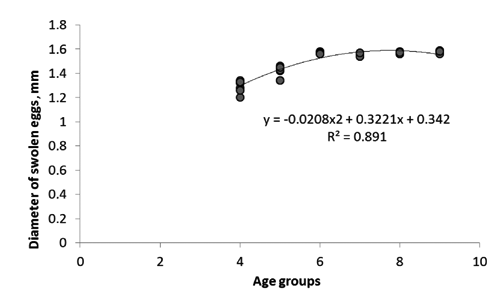
Relative fecundity, expressed as the number of eggs per kg of female body weight before ovulation, showed an increasing tendency between the fourth and the seventh year of life (from 23,584 to 48,789 eggs kg−1) (). Subsequently, the fecundity was seen to decrease to 30,698 eggs kg−1 in the older females. Moreover, a positive correlation was found between the female size and the diameter of swollen oocytes after hydration. The value ranged from 1.2 mm in the fourth to 1.6 mm in the ninth year of life ().
Discussion
A significant amount of recent research has been devoted to various aspects of fish ageing. However, there have been no reports on the direct effect of fish age on gamete quality and spawning effects. In order to determine this, studies were started on species with short life cycles, such as turquoise killfish, Nothobranchius furzeri, Buenos Aires tetra and neon tetra (CitationGerhard, 2007; CitationTerzibasi et al., 2007; CitationKucharczyk et al., 2008b, Citation2010), where the effect of age or the number of spawns on the spawning effectiveness and gamete quality is possible to observe in a relatively short period of time (within a few months). It has been shown in the latter two species that viable offspring were obtained only until 6–7 spawn. Females may lay eggs many times afterwards (over 20 times), but they were of very poor quality. In the present study, the parameters of reproduction effectiveness were also found to be lower in the oldest fish.
The results obtained indicate that using too young fish for reproduction is not a guarantee of breeding success either. As the examples of Buenos Aires tetra (CitationKucharczyk et al., 2008b) and neon tetra (CitationKucharczyk et al., 2010) have shown, fish which spawn for the first time produce fewer oocytes of slightly lower quality than during the next few spawns. Similar results, associated with a distinct decrease in the reproductive capacity of fish with a short life span, have been reported by CitationPatnaik et al., (1994) and CitationGerhard (2007). However, published data on the effect of age on the reproductive outcome in fish with a longer life-span were only fragmentary reports. It has been shown in the European sea bass (Dicentrachus labrax) and rainbow trout (CitationBromage et al., 1992; CitationBrooks et al., 1997) that the quality and amount of eggs obtained from fish which spawn for the first time was lower than in fish which spawn for the second time. However, no differences have been found in the outcome of the first two reproductions in 1-year old tench (CitationRodriguez et al., 2004). It was found in the present study that the quality of oocytes and fecundity of ide which spawn for the first time is also lower than in older fish (aged 5–8 years).
The results obtained indicate that ide females from the lake Mosag, where fish for this experiment were caught, reached sexual maturity at year 4 of their lives. The high ovulation rate in groups aged from 4+ to 8+ was close to the data reported for the species by e.g., CitationKucharczyk et al., (2008a), CitationKrejszeff et al., (2009) and CitationŻarski et al., (2009). Only in the oldest fish in this study (age group 9+) was the ovulation rate found to have decreased. It resulted from the fact that 33% of females did not produce oocytes. These fish may have been too old to reproduce or an annual break in reproduction might have taken place, which has been observed in some species. For example, it has been found that not all burbot, Lota lota (L.), females were ready for reproduction during their spawning season and it was supposed that some of them reproduce every two or more years (CitationPulliainen and Korhonen 1993). However, such observation for ide has never been reported and, in the present study, it was strictly related with the age. It should be pointed out that it is the first report on a reproduction failure of the ide during the spawning season where eggs were not sampled with a catheter (CitationKucharczyk et al., 2008c; CitationKrejszeff et al., 2009; CitationŻarski et al., 2009). This was probably related to the fact that such old fish (9+) with such high weights had not previously been caught for spawning (CitationKucharczyk et al., 2008c). However, it has to be stated that this phenomenon should be studied more precisely.
Latency time after hormonal stimulation was related to inter-populational differences (CitationKujawa et al., 2011), degree of domestication (CitationKrejszeff et al., 2009) and type or dose of hormonal treatment (CitationŻarski et al., 2009; CitationTargońska et al., 2010). Differences in latency resulting from the size and age of females were reported for Eurasian perch, Perca fluviatilis L., where ovulation in older (bigger) fish took place later than in younger ones (CitationKucharczyk et al., 2001). It created the necessity for separation of the spawners into different sizes or age classes to avoid handling stress in females which ovulated later. The age of the ide females has not been found to affect latency time, which was the same in all the age groups. Thus, during the reproduction of ide, spawners of different ages could be kept together.
According to CitationBrooks et al. (1997), oocyte size might be positively correlated with oocyte quality and offspring survival. Higher egg size usually increased the quality. In the halibut, Hipoglossus hipoglossus, larvae quality was strictly related to oocyte size (CitationEvans et al., 1996; CitationMazorra et al., 2003). A similar relationship with respect to the oocyte diameter has been found in the carp (CitationKucharczyk et al., 2008a). In the present study, no clear relationship between egg size and quality was found. Low quality was recorded for both the smallest and largest eggs, obtained from the youngest and oldest females. Thus, it might suggest that the age of females is a more important factor than egg diameter, which directly affects egg and larvae viability.
Conclusions
The current study indicates that the age of fish is a very important factor affecting the effectiveness of reproduction, but only where too young or too old fish were used. Females fish aged from 4+ to 8+ were comparably useful spawners for commercial purposes. It provides farmers with a great opportunity to use such widely-aged broodstock without negative effects on production effectiveness. The culture program should include the highest quality gene pool as possible, especially for restocking purposes and managing the wild populations. Thus, eliminating the oldest fish from a spawning schedule because of the slightly lower biological quality of eggs is not justified as it could reduce the genetic pool of future generations (CitationWildt et al., 1993), excluding specimens in which eggs are not possible to sample with a catheter.
References
- AnguisV. CanavateJ.P. 2005 Spawning of captive Senegal sole (Solea senegalensis) under a naturally fluctuating temperature regime Aquaculture 243 133 145
- BromageN. CumaranatungaR. 1988 Egg production in the rainbow trout MuirJ.F. RobertsR.J. Recent Advances in Aquaculture 3 Croom Helm Publ. London, UK 63 138
- BromageN. JonesJ. RandallC. ThrushM. DaviesB. SpringateJ. DustonJ. BarkerG. 1992 Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss) Aquaculture 100 141 166
- BromageN. PorterM. RandallC. 2001 The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin Aquaculture 197 63 98
- BrooksS. TylerC.R. SumptemJ.P. 1997 Egg quality in fish: what makes a good egg? Rev. Fish Biol. Fish 7 387 416
- BrzuskaE. 2006 Artificial spawning of female Lithuanian strain B carp (Cyprinus carpio L.) after treatment with carp pituitary homogenate, Ovopel or [D–Tle6, ProNHEt9] LH-RH–a (Lecirelin) Aquacult. Res 37 264 271
- CejkoB.I. KowalskiR.K. KucharczykD. TargońskaK. KrejszeffS. ŻarskiD. GlogowskiJ. 2010 Influence of the length of time after hormonal stimulation on selected parameters of milt of ide Leuciscus idus L Aquacult. Res 41 804 813
- EvansR.P. ParrishC.C. BrownJ.A. DavisP.J. 1996 Biochemical composition of eggs repeat and first-time spawning captive Atlantic halibut (Hippoglossus hippoglossus) Aquaculture 139 139 149
- GerhardG.S. 2007 Small laboratory fish as models for ageing research Ageing Res. Rev 6 64 72
- HilderM.L. PankhurstW. 2003 Evidence that temperature change cues reproductive development in the spiny damselfish, Acanthochromis polycanthus Env. Biol. Fish 66 187 196
- HorvathL. SzaboT. BurkeJ. 1997 Hatchery testing of GnRH analogue-containing pellets on ovulation in four cyprinid species Pol. Arch. Hydrobiol 44 221 226
- KrejszeffS. KucharczykD. KuprenK. TargońskaK. MamcarzA. KujawaR. KaczkowskiZ. RatajskiS. 2008 Reproduction of chub, Leuciscus cephalus L., under controlled conditions Aquacult. Res 39 907 912
- KrejszeffS. TargońskaK. ŻarskiD. KucharczykD. 2009 Domestication affects spawning of the ide (Leuciscus idus) – preliminary study Aquaculture 295 145 147
- KucharczykD. KujawaR. MamcarzA. Targońska-DietrichK. WyszomirskaE. GlogowskiJ. BabiakI. SzaboT. 2005 Induced spawning in bream (Abramis brama L.) using pellets containing LH-RH Czech J. Anim. Sci 50 89 95
- KucharczykD. SzczerbowskiA. ŁuczyńskiM.J. KujawaR. MamcarzA. WyszomirskaE. SzaboT. RatajskiS. 2001 Artificial spawning of Eurasian perch, Perca fluviatilis L. using Ovopel Arch. Pol. Fish 9 39 49
- KucharczykD. TargońskaK. HliwaP. GomułkaP. KwiatkowskiM. KrejszeffS. PerkowskiJ. 2008a Reproductive parameters of common carp (Cyprinus carpio L.) spawners during natural season and out-of-season spawning Reprod. Biol 8 285 289
- KucharczykD. TargońskaK. PrusińskaM. KrejszeffS. KuprenK. KujawaR. MamcarzA. 2008b Reproduction of Buenos Aires tetra (Hemigrammus caudovittatus) under controlled conditions Pol. J. Nat. Sci 23 858 865
- KucharczykD. TargońskaK. ŻarskiD. KrejszeffS. KuprenK. ŁuczyńskiM. J. SzczerbowskiA. 2010 The reproduction of neon tetra, Paracheirodon innesi (Myers, 1936), under controlled conditions Pol. J. Nat. Sci 25 81 92
- KucharczykD. TargońskaK. ŻarskiD. KujawaR. MamcarzA. 2008c A review of the reproduction biotechnology for fish from the genus Leuciscus Arch. Pol. Fish 16 319 340
- KujawaR. KucharczykD. MamcarzA. 1999 A model system for keeping spawners of wild and domestic fish before artificial spawning Aquacult. Eng 20 85 89
- KujawaR. KucharczykD. MamcarzA. ŻarskiD. TargońskaK. 2011 Artificial spawning of common tench Tinca tinca (Linnaeus, 1758), obtained from wild and domestic stocks Aquacult. Int 19 513 521
- MazorraC. BruceM. BellJ.G. DavieA. AlorendE. JordanN. ReesJ. PapanikosN. PorterM. BromageN. 2003 Dietary lipid enhancement of brood-stock reproductive performance and egg and larval quality in Atlantic halibut (Hippoglossus hippoglossus) Aquaculture 227 21 33
- PatnaikB.K. MahapatroN. JenaB.S. 1994 Ageing in fishes Gerontology 40 113 132
- PulliainenE. KorhonenK. 1993 Does the burbot, Lota lota, have rest years between normal spawning seasons? J. Fish Biol 43 355 362
- RodriguezR. CeladaJ.D. Saez-RoyuelaM. CarralJ.M. AguileraA. MelendreP.M. 2004 Artificial reproduction in 1-year-old tench (Tinca tinca L.) J. Appl. Ichthyol 20 542 544
- SzaboT. 2003 Ovulation induction in northern pike Esox lucius L. using different LH-RH analogues, Ovaprim, Dagin and carp pituitary Aquacult. Res 34 479 486
- TargońskaK. KucharczykD. KujawaR. MamcarzA. ŻarskiD. 2010 Controlled reproduction of asp, Aspius aspius (L.) using luteinizing hormone releasing hormone (LHRH) analogues with dopamine inhibitors Aquaculture 306 407 410
- TerzibasiE. ValenzanoD.R. CellerinoA. 2007 The short-lived fish Nothobranchius furzeri as a new model system for aging studies Exp. Gerontol 42 81 89
- WildtD.E. SealU.S. RallW.F. 1993 Genetic resource banks and reproductive technology for wildlife CloudJ.G. ThorgaardG.H. Genetic conservation of salmonid fishes. Plenum Press New York, NY, USA 159 173
- WojdaR. 2004 Production of stocking material of rheophilous fishes in Poland in 1995–2002: possibilities and necessities of further increases Arch. Pol. Fish 12 (Suppl.2) 359 369
- YaronZ. 1995 Endocrine control of gameto-genesis and spawning induction in the carp Aquaculture 129 49 73
- YaronZ. BogomolnayaA. DroriS. BitonI. AizenJ. KulikovskyZ. Levavi-SivanB. 2009 Spawning induction in the carp, past experience and future prospects. Isr J. Aquacult-Bamid 61 5 26
- ŻarskiD. KucharczykD. TargońskaK. JamrózM. KrejszeffS. MamcarzA. 2009 Application of Ovopel, Ovaprim and their combination in artificial reproduction of two rheophilic cyprinid fishes Pol. J. Nat. Sci 24 235 244