Abstract
The objective of this experiment was to determine the degradation of L-arginine (ARG) and N-carbamoyl glutamate (NCG) and to examine their effect on rumen fermentation. Rumen fluids were collected from 3 rumen-fistulated cows and then incubated with ARG or NCG at 1 mmol/L in a glass syringe system at 39°C for 24 h. The control treatment was given neither ARG nor NCG. Gas production (GP) was recorded, and pH at 2, 4, 6, 12, and 24 h was also determined. At 12 and 24 h, the measurements were also made for ammonia-nitrogen (N), volatile fatty acids (VFA) and microbial crude protein (MCP) yield on purine quantification basis. At 24 h, the proportion of ARG and NCG degradation in rumen fluid was 100.0 and 17.8%, respectively. Gas production and the acetate to propionate ratio increased in groups treated with ARG and NCG, compared with the control (P<0.01). Ammonia nitrogen concentration was higher (P<0.01) in the ARG group than in the NCG and control groups. Microbial crude protein concentration diminished in ARG and NCG groups, in comparison with the control (P<0.01). In conclusion, the effects of ARG and NCG on rumen fermentation were numerically relatively similar. Rapid degradation of ARG in rumen is a nutritionally wasteful process. Thus, ARG should be spared from rumen degradation, while NCG could be fed to ruminant without need for coating.
Introduction
L-arginine (ARG) is a functional amino acid which plays a KEY role in urea cycle regulation, hepatic detoxification and protein synthesis. As a precursor of polyamines and nitric oxide (NO), ARG could improve nitrogen metabolism, angiogenesis and lactogensis (CitationMorris, 2006; CitationKim and Wu, 2009). L-arginine supplementation increases milk production in cattle and growth hormone in sheep (CitationChew et al.,1984; CitationDavenport et al., 1990a, Citation1990b), plasma flow in the portal and hepatic veins in steers (CitationMaltby et al., 2005), and stimulates the production of luteinizing hormone in prepubertal ewes (CitationRecabarren et al., 1996). Recently, injected Arg-HCl has decreased embryonic loss in ewes (CitationLuther et al., 2008), increased lamb birth weight in gestationally nutrient restricted ewes (CitationLassala et al., 2010), and improved fetal lamb survival to term in prolific ewes (CitationLassala et al., 2011). Deletion of ARG from a mixture of essential amino acids proved to be limited for milk protein synthesis in dairy cows (CitationDoepel and Lapierret, 2011). As a consequence, supplementation of ARG is needed to optimize production of ruminants. However, ARG is rapidly degraded by ruminal microbes, and its high cost as a feed additive limits its use in ruminant feeding.
The ARG is a direct allosteric activator of N-acetyl glutamate (NAG) synthase, a mitochondrial enzyme converting glutamate and acetyl coenzyme A (acetyl-CoA) into NAG, while N-carbamoyl glutamate (NCG) is a structural analogue of NAG that is co-factor of carbamoyl phosphate synthase 1 (CPS1) (CitationWu and Morris, 1998). The CPS1 is a first rate-limiting enzyme of the urea cycle (CitationWaterlow, 1999), which is inactive in the absence of NAG. The ARG can be synthesized endogenously from glutamate via pyrroline-5-carboxylate (P5C), ornithine, citrulline, and argininosuccinate. Therefore, P5C synthase and NAG synthase are the two key regulatory enzymes in the intestinal citrulline synthesis (CitationWu and Morris, 1998). That is the reason why NCG is also called the ARG raiser. However, information on rumen degradation of NCG and its effect on rumen fermentation is not available. The purpose of the present study was to determine the rumen degradation of NCG in comparison with ARG, and to investigate their effect on in vitro rumen fermentation.
Materials and methods
Experimental design
L-arginine with 98.5% purity and NCG with 97% purity were used. Calibrated glass syringes (Model Fortuna, Häberle Laborte - chnik, Lonsee-Ettlenschieß, Germany) were used as incubators. The ARG or NCG was added at 0 (control) or 1 mmol/L in triplicate. The amount of ARG used was based on CitationSultana et al. (2003). The same amount of NCG was used to compare the degradation in rumen fluid as well as its effects on ruminal fermentation parameters.
At 2, 4, 6, 12, and 24 h, gas production (GP) was recorded and rumen fluids were sampled for pH determination and ARG and NCG degradation. For each time point the sampled syringes were discarded after sampling, and the remaining ones were still used for incubation. At 12 and 24 h, measurements were also made for ammonia-nitrogen (N), volatile fatty acids (VFA) and microbial crude protein (MCP) yield.
In vitro incubation
The GP was determined according to CitationMenke and Steingass (1988). About 200 mg substrates (Chinese wild rye grass and corn at equal ratio) were weighed into 100-mL glass syringes fitted with plungers. Syringes in triplicate were filled with 30 mL of medium consisting of 10 mL rumen fluid and 20 mL buffer solution. This was done anaerobically as described by CitationMenke and Steingass (1988). Syringes were incubated in water bath at 39°C for 24 h. Blanks containing 30 mL of medium were included only for calibrating GP from the rumen fluids. Rumen fluids were collected from 3 rumen-fistulated dairy cows fed a diet with the forage to concentrate mixture ratio at 55:45. The diet contained 15.1, 34.4 and 22.1% dry matter (DM) of crude protein, neutral detergent fiber and acid detergent fiber, respectively. Content of net energy for lactation (NEL) was 1.56 Mcal/kgDM.
Measurement of in vitro fermentation variables
The pH value of rumen fluid was determined immediately after incubation stopped with a pH meter (Model PB-20, Sartorius AG, Göttingen, Germany). The ammonia N concentration in rumen fluids was determined by using the colorimetry (Spectra Max M5, Molecular Devices LLC, Sunnyvale, CA, USA) method (CitationFeng and Gao, 1993). To determine VFA concentration, 1 mL sample was mixed with 0.2 mL of 8% meta-phosphoric acid and centrifuged at 20,000 g for 10 min at 4°C. Then, 0.2 µL supernant was obtained and injected into gas chromatography (GC-2010, Shimadzu, Kyoto, Japan) with N gas as a carrier as already applied by CitationHu et al. (2006). The MCP concentration was determined on the basis of purine with the method described by CitationMakkar and Becker (1999).
Analysis of L-arginine and N-carbamoyl glutamate in rumen fluid
Incubated rumen fluid samples were centrifuged at 3000 rpm for 10 min and the supernatant was stored at −4°C until analysis. L-argi-nine concentration was determined with the method described by CitationWang et al. (2008) and calculated against the value in the standard solution. Two hundred µL of each sample were put into 96 well plates and the absorbance at 500 nm was measured through a spectrophometer (Spectra Max M5, Molecular Devices LLC, Sunnyvale, CA, USA). The analysis of NCG in rumen fluid was carried out according to the method described by CitationZhang and Zhu (2007) using the Dionex ICS-2000 RFIC ion chromatography system (Dionex, Sunnyvale, CA, USA). The supernatant containing NCG was diluted with Milli-Q water 500 times (10 mL water plus 20 µL rumen sample), and then injected into the Dionex ICS-2000 RFIC ion chromatography system (Dionex, Sunnyvale, CA, USA) for the chromatographic determination.
Data calculation and statistical analysis
The proportion of degradation of ARG and NCG was calculated from their initial and residual amount in rumen fluid. This data was then fitted to the model of CitationØrskov and McDonald (1979):
where
p is proportion of degradation at time t (h); a is the rapidly degradable fraction in the rumen;
b is the fraction slowly degraded at rate c (c>0). Effective degradability (dg) was calculated assuming a passage rate of 5 and 10%/h (CitationMadsen and Hvelplund, 1985) and using the formula of CitationØrskov and McDonald (1979):
where
a, b and c are the constants as indicated above, and k is the passage rate.
Fermentable variables were analyzed with one way analysis of variance (ANOVA) using the GLM procedure of the statical analysis system SAS (CitationSAS, 1999). Results were subjected to multiple range tests at P<0.05 and standard error of means (SEM).
Results and discussion
Degradation of L-arginine and N-carbamoyl glutamate in rumen fluid
The ARG was degraded to almost 100% within 12 h, while NCG was degraded 17.8% within 24 h (). The proportions of degradation in rumen fluid at 12 and 24 h incubation were 99.3 and 100% for ARG, and 14.2 and 17.8% for NCG. The rumen degradation parameters are given in . The rapidly degradable fraction (a) was low in both ARG and NCG, but the fraction slowly degraded (b) was 100 for ARG and 16.1 for NCG. The dg value was 80.0 and 63.8 for ARG, and 14.1 and 12.5 for NCG, by assuming a passage rate (kp) of 5 or 10%/h (CitationMadsen and Hvelplund, 1985). Our data indicated that the rumen microbes hardly degrade NCG, as also reported by CitationMartin et al. (1983), who identified NCG as a slowly-releasing source of non-protein nitrogen for ruminant feed additives. CitationChalupa (1976) reported that ARG was rapidly degraded by rumen microbials up to 100% within 6 h, while CitationSultana et al. (2003) found that ARG degradation rates after 6 and 12 h incubation were 52.8 and 85.2%, respectively. These findings imply that NCG might be a potential post-ruminal feed additive with no need of coating.
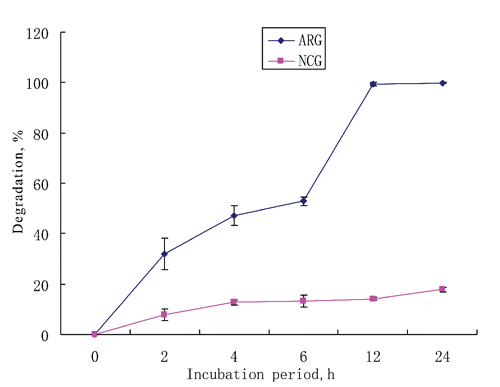
Table 1 Rumen degradation parameters and effective degradability of L-arginine and N-carbamoyl glutamate.
Effects on gas production and fermentation variables
Findings about GP and fermentation variables are shown in . The GP values were higher in ARG and NCG groups at both 12 and 24 h, compared to the control (P<0.01). These values are related to both the amount and rate of substrates degradation (CitationBlümmel and Becker, 1997). Increased GP in ARG and NCG groups indicated that in these two groups DM digestibility might be improved. Total VFA concentration increased at 12 h in ARG and NCG groups (P<0.05), but was not statistically different (P>0.05) at 24 h in the three treatments. Acetate content decreased in the ARG group and propionate increased in ARG and NCG groups at 24 h (P<0.01), in comparison with the control. The increase in propionate content might be due to the glucogenic nature of the substance. Thus, as amino acids are the other main source of glucose precursors, propionate could play a protein-sparing role (CitationLeng, 1970). The acetate to propionate ratio at 24 h was lower in ARG and NCG groups than in the control (P<0.01). Ruminal ammonia N concentration was higher in the ARG group than in the control (P<0.01), thus showing a rapid degradation of ARG by rumen microbes. The microbial crude protein content decreased (P<0.05) in ARG and NCG groups, compared with that of the control at 12 and 24 h. Negative correlations were detected between MCP and ammonia N in ARG (−0.471) and NCG (−0.845) groups, as already demonstrated in previous studies (CitationPilgrim et al., 1970; CitationNolan et al., 1976). Our findings let us infer that the addition of ARG and NCG had similar effect on MCP synthesis. However, we still cannot explain the reasons for a decrease in MCP yield in both ARG and NCG groups. In order to clarify this point, additional studies need to be performed.
Table 2 Effect of L-arginine and N-carbamoyl glutamate on rumen fermentation at 12 and 24 h incubation period.
Conclusions
L-arginine was rapidly degraded in rumen fluid in vitro, while NCG underwent a lower degradation. Adding ARG or NCG improved the rumen fermentation, but reduced microbial protein concentration. The effects of NCG on rumen fermentation variables were similar to those of ARG. To conclude, our study showed how ARG may be well replaced by NCG as a potential feed additive as NCG is a stable analogue of NAG which helps the endogenous synthesis of ARG.
Acknowledgments:
this study was partly supported by the China Agriculture Research System (CARS-37) and by a grant from the Key Project of Science and Technology Department of Zhejiang Province (No. 2008C12050).
The authors are thankful to Dr. Qiao Shiyan (MOA Feed Industry Centre, China) for his technical support to determine NCG in rumen fluid.
References
- BlümmelB. BeckerK. 1997 The degradability characteristics of fifty-four roughages and roughages neutral detergent fiber as described in vitro gas production and their relationship to voluntary feed intake Brit.J. Nutr 77 757 768
- ChalupaW. 1976 Degradation of amino acids by the mixed rumen microbial population J. Anim. Sci 43 828 834
- ChewB.P. EisenmanJ.R. TanakaT.S. 1984 Arginine infusion stimulates prolactin, growth hormone, insulin, and subsequent lactation in pregnant dairy cows J. Dairy Sci 67 2507 2518
- DavenportG.M. BolingJ.A. SchilloK.K. 1990a Nitrogen metabolism and somatotropin secretion in beef heifers receiving abomasal arginine infusions J. Anim. Sci 68 1683 1692
- DavenportG.M. BolingJ.A. SchilloK.K. AaronD.K. 1990b Nitrogen metabolism and somatotropin secretion in lambs receiving arginine and ornithine via abomasal infusion J. Anim. Sci 68 222 232
- DoepelL. LapierretH. 2011 Deletion of arginine from an abomasal infusion of amino acids does not decrease milk protein yield in Holstein cows J. Dairy Sci 94 864 873
- FengZ.C. GaoM. 1993 The improved method in determining the ammonia-N in rumen fluid by colorimetry. Inner Mongolia Anim. Sci 14 40 41
- HuW.L. LiuJ.X. WuY.M. GuoY.Q. YeJ.A. 2006 Effects of tea saponins on in vitro ruminal fermentation and growth performance in growing Boer goat Arch. Anim. Nutr 1 89 97
- KimS.W. WuG. 2009 Regulatory role of amino acid in mammary gland growth and milk synthesis Amino acids 37 89 95
- LassalaA. BazerF.W. CuddT.A. DattaS. KeislerD.H. SatterfieldM.C. SpencerT.E. WuG. 2010 Parenteral administration of L-arginine prevents fetal growth restriction in undernourished ewes J. Nutr 140 1242 1248
- LassalaA. BazerF.W. CuddT.A. DattaS. KeislerD.H. SatterfieldM.C. SpencerT.E. WuG. 2011 Parenteral administration of L-arginine enhances fetal survival and growth in sheep carrying multiple fetuses J. Nutr 141 849 855
- LengR.A. 1970 Glucose synthesis in ruminant Adv. Vet. Sci. Comp. Med 14 209 260
- LutherJ.S. WindorskiE.J. SchauerC.S. KirschiJ.D. VonnahmeK.A. ReynoldsL.P. CatonJ.S. WuG. 2008 Impacts of L-arginine on ovarian function and reproductive performance in ewes J. Anim. Sci 86 (E Suppl. 2) LB5 (abstr.)
- MadsenJ. HvelplundT. 1985 Protein degradation in the rumen. A comparison between in vivo, nylon bag, in vitro and buffer measurements Acta Agric. Scand 25 103 124
- MakkarH.P.S. BeckerK. 1999 Purine quantification in digesta from ruminants by spectrophotometer and HPLC methods Brit. J. Nutr 81 107 113
- MaltbyS.A. ReynoldsC.K. LomaxM.A. BeeverD.E. 2005 Splanchnic metabolism of nutrients and hormones in steers fed alfalfa under conditions of increased absorption of ammonia and L-arginine supply across the portal-drained viscera J. Anim. Sci 83 1088 1096
- MartinP.J. MathisonG.W. MilliganL.P. 1983 Evaluation of two glucopyranosylamine as non-protein nitrogen sources for cattle: digestibility and effect on growth and feed conversion Can. J. Anim. Sci 63 105 116
- MenkeK.H. SteingassH. 1988 Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid Anim. Res. Dev 28 7 55
- MorrisS.M.Jr. 2006 Arginine beyond the protein Am. J. Clin. Nutr 83 S508 S512
- NolanJ.V. NortonB.W. LengR.A. 1976 Further studies on dynamic nitrogen metabolism in sheep Brit. J. Nutr 35 127 147
- ØrskovE.R. McDonaldI. 1979 The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage J. Agr. Sci 92 499 503
- PilgrimA.F GrayF.V. WellerR.A. BellingC.B. 1970 Synthesis of microbial protein from the rumen of sheep, and the proportion of dietary nitrogen converted into microbial nitrogen Brit. J. Nutr 2 589 598
- RecabarrenS.E. JofreA. LobosA. OrellanaP. PariloJ. 1996 Effect of arginine and ornithine infusions on Luteinizing hormone secretion in prepubertal ewes J. Anim. Sci 74 162 166
- SAS 1999 SAS/STAT package SAS Inst. Inc. Cary, NC, USA
- SultanaH. Hussain-YusufH. TakashiT. MoritaT. OnoderaR. 2003 A quantitative study on arginine catabolism by mixed ruminal bacteria, protozoa and their mixture in vitro Anim. Sci. J 74 11 16
- WangH. LiangX.H. ZhaoR.X. FengL.D. LiH. 2008 Spectrophotometer determination of arginine in grape juice using 8-hydroquinoline Agric. Sci. China 7 1210 1215
- WaterlowJ.C. 1999 The mysteries of nitrogen balance Nutr. Res. Rev 12 25 54
- WuG. MorrisS.M.Jr. 1998 Arginine metabolism: nitric oxide and beyond Biochem. J 336 1 7
- ZhangJ. ZhuY. 2007 Determination of betaine, choline and trimethylamine in feed additive by ion-exchange liquid chromatography/non-suppressed conductivity detection J. Chromatogr. A 1170 114 117