Abstract
The effect of replacement of dietary sunflower oil (SO) with linseed oil (LO) on Stearoyl CoA desaturase (SCD) gene expression was investigated. Thirty-six lambs were randomly assigned to four groups and fed with one of the experimental diets, consisting of lucerne pellets with oil (60 g/Kg). The diets varied in the percentage of the oil supplemented and were: 100% SO; 66.6% SO plus 33.3% LO; 33.3% SO plus 66.6% LO and 100% LO. The trial period was of 7 weeks. Longissimus dorsi was removed from each carcass and stored at −80°C until the analysis. Total fatty acids composition was determined by gas-chromatograph, while SCD gene expression was assessed by Real-Time Reverse-Transcription PCR. Replacement of SO with LO decreases significantly the SCD mRNA content with a concomitant increment of polyunsatured fatty acids (PUFA) n-3. These results are related to the higher level of PUFA n-3 present in linseed than sunflower. Although, there were differences on mRNA level, there was not a simultaneously changes on SCD activity. In conclusion, PUFA n-3 act on the regulation of mRNA SCD level without affecting the activity of the relative enzyme.
Key Words:
Introduction
Stearoyl-CoA Desaturase enzyme (SCD) (also known as Δ9-desaturase) plays a key role in the lipid metabolism, because it introduces a double bond at the Δ9-position in a large spectrum of fatty acids (FA) (CitationNtambi, 1995; CitationNtambi and Miyazaki, 2004). The most important substrates of SCD are acyl-CoA of myristic, palmitic, stearic acid and C18:1 t11 (known as vaccenic acid) which are converted into C14:1 c9, C16:1 c9, C18:1 c9, and C18:2 c9,t11, respectively (CitationCorl et al., 2001), the latter being a conjugated linoleic acid (CLA) isomer of potential interest in human health. In fact, different in vivo and in vitro studies reported anti-diabetic (CitationBelury and Kempa-Steczko, 1997), anti-carcinogenic and anti-atherogenic effects (CitationScollan et al., 2006) of CLA. Milk and meat from ruminants are the major sources of CLA in the human diet (CitationRitzenthaler et al., 2001). Ovine SCD protein is encoded by a gene located on chromosome 22, in a region where CitationCarta et al. (2008) have detected a positional quantitative trait locus (QTL) for the CLA:vaccenic acid ratio in milk.
SCD gene expression in liver and adipose tissue is regulated by different factors (CitationDobrzyn and Dobrzyn, 2006): dietary fat (e.g. polyunsatured FA, cholesterol, vitamin A), hormonal signals (e.g. insulin, glucagon), environmental factors (temperature, metals, alcohol, thiazolinediones), peroxisomal proliferators and development processes (CitationNtambi and Miyazaki, 2004). These factors may act on translational or post-translational regulation, affecting the enzyme expression or activity, respectively. Polyunsaturated FA (PUFA) may inhibit transcription of key genes involved in lipid synthesis, including Acetyl-CoA Carboxylase (ACACA), fatty acids synthase (FASN), SCD, Malic Enzyme, L-pyruvate kinase, adipocyte lipid-binding protein (aP2) resulting in a decrease of de novo lipogenesis (CitationJump and Clarke, 1999; CitationAl-Hasani and Joost, 2005). PUFA reduce the SCD expression, as well as the other lipogenic genes, by a repression of sterol regulator element binding protein (SREBP-1) activity (CitationMiyazaki and Ntambi, 2003). Moreover, PUFA activate peroxisome proliferator-activated receptor proteins (PPARs) to modulate the gene expression, in response to environmental stimuli (CitationMiyazaki and Ntambi, 2003). In bovine, some studies reported the effect of SREBP-1 and PPAR modulation on SCD gene expression (CitationGraugnard et al., 2009; CitationWaters et al., 2009). In the literature, the effect of dietary PUFA on SCD gene expression or activity in ruminants are related to the use of linseed oil (CitationBas et al., 2007; CitationBernard et al., 2005, Citation2009a, Citation2009b; CitationHerdman et al., 2010) and/or CLA (CitationBaumgard et al., 2002) on beef, lamb and goat. Studies on rodents reported that alpha-linolenic acid inhibited the SCD gene expression with a greater extent than linoleic acid (CitationSessler et al., 1996), but no data are available about the comparison of dietary PUFA n-3 and n-6 on SCD expression in lamb muscles. In sheep, feeding trials shown that the SCD protein activity in muscle from concentrate fed lambs did not differ to the SCD activity in muscle from forage fed animal (CitationVasta et al., 2009). On the contrary, CitationBas et al. (2007) revealed a reduction of SCD activity (expressed as desaturation ratio) only in subcutaneous adipose tissue as a consequence of linseed increment in the diet. The supplementation of ruminant diets with PUFA rich lipids is the most effective approach to decrease saturated FA and promote the enrichment in unsaturated FA of meat and milk (CitationJeronimo et al., 2009; CitationMele, 2009; CitationLanza et al., 2011). However, more knowledge are needed in order to evaluate how different sources of PUFA in the diet may affect the expression of lipogenic genes in animal tissues.
In the present study the effect of dietary substitution of sunflower oil (SO) with linseed oil (LO) was evaluated, in order to investigate the role of dietary PUFA in regulating the SCD expression in skeletal muscle of lambs. The two types of plant oil were chosen as source of 18:2 n-6 (SO) and 18:3 n-3 (LO) to evaluate the effect of PUFA n-6 and PUFA n-3 series on lipid metabolism.
Materials and methods
Experimental design
Animals, diets and experimental design are fully described by CitationJerónimo et al. (2009). Briefly, animal handling followed the EU directive 86/609/EEC, concerning animal care. The trial tacked place in the Centro de Experimentação do Centro Alentejo (Reguen gos de Monsaraz, Portugal), using thirty-six Merino Branco ram lambs. The lambs were reared on pasture with their dams until wea ning, at about 90 days of age. The average initial weight of lambs was 22.9±2.78 kg (mean ± SD). Animals were randomly assigned to four groups of nine lambs each and were fed one of the four experimental diets: pelletted dehydrated lucerne with 6% of SO (diet 6S); pelletted dehydrated lucerne with 4% of SO and 2% of LO (diet 4S2L); pelletted dehydrated lucerne with 2% of SO and 4% of LO (diet 2S4L); pelletted dehydrated lucerne with 6% of LO (6L). The target for oil inclusion was 60 g/kg on a dry matter basis, resulting in pellets with ether extract range between 70 and 76 g/kg of dry matter. The chemical composition of the diets is reported in .
Table 1 Chemical composition of the experimental diets.
After 7 weeks of trial the lambs were slaughtered on an experimental abattoir. Samples of Longissimus dorsi muscle were removed immediately after slaughter and divided in two portions: one portion was frozen quickly in liquid nitrogen and stored at −80°C, in order to quantify the SCD mRNA, while the second portion was minced after removing the epimysium, vacuum packed, freeze-dried and stored at −80°C until the fatty acid composition analysis.
Lipid analysis
Intramuscular lipids were extracted by the method of CitationFolch et al. (1957), using a dichloromethane and methanol (2:1, vol/vol) solution instead of the chloroform and methanol (2:1, vol/vol) solution (CitationCarlson, 1985). The FA were transesterificated with sodium methoxide followed by hydrochloric acid in methanol (1:1, vol/vol) as described by CitationRaes et al. (2001). Quantification of FA methyl ester was done using nonadecanoic acid (C19:0) as internal standard. The FA methyl esters were analysed using a HP6890A chromatograph (Hewlett-Packard, Avondale, PA, USA), equipped with a flame-ionization detector (GC-FID) and fused silica capillary column (CP-Sil 88; 100 m x 0.25 mm id 0.20 µm of film thickness; Chrompack, Varian Inc., Walnut Creek, CA, USA). The identification of FA methyl esters and chromatographic conditions are described in CitationJerónimo et al. (2009).
RNA extraction
Longissimus muscle total RNA was extracted using the RNAqueous Kit (Qiagen, Melbourne, Australia) following the manufacturer’s instructions. Integrity of RNA was verified by visualization of the 18S and 28S ribosomal bands of individual samples subjected to ethi dium bromide staining agarose gel electrophoresis (). Concentration and purity of RNA were determined by spectrophotometry analysis (Lambda 25 UV/ visible spectrometer, Perkin-Elmer Instruments, Les Ulis, France) at 260, 280, and 320 nm (A260-A320/A280-A320, where A is absorbance). All the RNA samples shown a ratios ranged from 1.807 to 2.072. Genomic DNA contamination was eliminated by DNase treatment, using TURBO DNase kit (Applied Biosystem, Carlsbad, CA, USA), following the manufacturer’s instructions. The absence of genomic DNA was controlled by PCR using a pair of primers (named SCD1genF and SCD1genR, ) from ovine SCD gene, including the 4th intron (1667 bp), that would yield PCR products of either 1913 or 347 bp when starting from genomic DNA or cDNA, respectively. The PCR was performed by GeneAmp PCR 9600 Thermo cycler (Perkin Elmer Life Sciences) according the follow program: the DNA was preheated at 94°C for 5 min and then amplified with 30 cycles of 94°C for 45 s, 56°C for 45 s, and 72°C for 1 min. The final cycle had an extension time of 7 min at 72°C. The PCR mix was constituted by 12.5 µL of 2× PCR Master Mix (Fermentas Internatio nal Inc., Burlington, Canada; Taq DNA polymerase 0.05 units/µL, MgCl2, 4 mM, and dNTP, 0.4 mM of each), 0.5 µL of 10 pmol/µL primer forward, 0.5 µL of 10 pmol/µL reverse primer, 250 ng of DNA and ddH2O up to 25 µL. Primers used for PCR were designed with Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) with the ovine mammary gland SCD mRNA sequence (NM_001009254; CitationWard et al., 1998).
Table 2 Primer sequences used and relative gene bank accession number.
Analysis of mRNA using Real-Time Reverse-Transcription PCR
Level of SCD mRNA in Longissimus dorsi muscle was quantified by real-time reverse-transcription PCR. Single-strand cDNA was obtained from 4 µg of purified total RNA, using High Quality cDNA Archive kit (Applied Biosystem) containing the MultiScribe™ Reverse Transcriptase, 50 U/ L, following the manufacture’s instructions. Quantitative PCR was performed in 100-µL tubes using a Rotorgene 2000k (Corbett Research, Sydney, Australia) thermal cycler with real-time detection of fluorescence. PCR was conducted in a volume of 50 µL using 1x MESA GREEN qPCR MasterMix Plus for SYBR® (including dNTPs, Meteor Taq DNA polymerase, 4mM MgCl2, SYBR® Green I and stabilizers) (Eurogentec, San Diego, CA, USA) and 0.3 µM of each primer. PCR program consisted of a Meteor Taq DNA polymerase activation at 95 °C for 5 min, followed by 3-steps cycles, repeated 40 times: denaturation for 15 s at 95°C, annealing for 20 s at 60°C and extension for 40 s at 72°C. The fluorescence intensity of SYBR Green I was read and acquired at 72°C after completion of the extension step of each cycle. PCR conditions for individual primer sets were optimized by varying template cDNA and magnesium ion concentration, in order to obtain amplifications yielding a single product and melt curves with a single uniform peak. Quantification of individual transcripts was performed using the Comparative Quantitation option of the software supplied by Corbett Research for the Rotorgene. This option allows to assess the relative expression of the SCD gene compared to the housekeeping gene. The gene expression intensity was measured as cycle thresholds (Ct). The Ct of SCD gene (Ct1) was normalized according to the transcription level of the housekeeping gene (Ct2), and the relative fold-change ( Ct) was obtained. Ct1 and Ct2 were calculated for each sample. The mean efficiency of a group of cycling curves is calculated at the point that the cycling curves take off and used to calculate a fold-change according to the formula (CitationWarton et al., 2004):
where A is the efficiency of amplification reaction (for an efficiency of 100%, A = 2; this data is calculated directly by the software); Ct1 (Ct of SCD-1 gene) and Ct2 (Ct of housekeeping gene) are the take off values of the cycling curves being compared.
Cyclophilin A (PPIA) was used as a housekeeping gene for comparison of RNA extraction efficiency. Although, relying on treatment effects as a guide for the selection of reference genes for normalization is not the most optimal, the lack of response of cyclophilin A (PPIA) mRNA to nutritional factors in previous studies (CitationBernard et al., 2006, Citation2009; CitationOllier et al., 2009) allowed to use this gene as an internal standard reference gene. Results were expressed as the mRNA copy number of SCD gene relative to cyclophilin. Reactions were repeated three times for each sample. Primers for SCD and cyclophilin A for quantitative RT-PCR were designed with Primer3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) with the ovine mammary gland SCD (NM_001009254; CitationWard et al., 1998) and cyclophilin A (AY251270; Etschmann et al., 2004, unpublished) mRNA sequence and are listed in .
Desaturase activity
The extent of FA desaturation was determined by calculating the ratio c9-unsaturated to c9-unsaturated + saturated for the different FA that SCD catalyzes (CitationPalmquist et al., 2004). The following ratio were calculated:
c9-14:1 to c9-14:1 + 14:0: desaturation index for C14:0 (DI14);
c9-16:1 to c9-16:1 + 16:0: desaturation index for C16:0 (DI16);
c9-18:1 to c9-18:1 + 18:0: desaturation index for C18:0 (DI18);
c9,t11-18:2 to c9,t11-18:2 + t11-18:1: desaturation index for Vaccenic acid (RA/VA).
Statistical analysis
The effect of dietary replacement of SO with LO was analyzed using the GLM procedure of SAS (1999), using a linear model where dietary oil replacement was included as linear and quadratic effect.
Results and discussion
In this work, the effect of dietary PUFA on SCD gene expression and activity was investigated. In particular, diets with different n-6/n-3 ratio were used, in order to modulate the content of SO (rich in PUFA n-6) and LO (rich in PUFA n-3).
The effect of experimental diets on FA composition of polar, neutral and total lipid of muscle samples has been previously reported and discussed in CitationJeronimo et al. (2009). Descriptive statistic for the investigated traits is reported in . In the present paper, only FA ratios, which are a proxy of SCD activity, and total PUFA n-3 content were reported in and , respectively.
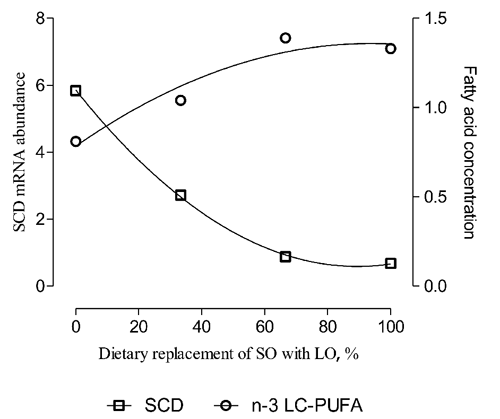
Table 3 Descriptive statistics of desaturase index and expression level (expressed as mRNA amount). mRNA amount was expressed as mRNA copy number of SCD gene relative to cyclophilin gene.
Table 4 Diet effect on SCD activity (expressed as product to substrate ratio) and expression level (expressed as mRNA amount). mRNA amount was expressed as mRNA copy number of SCD gene relative to cyclophilin.
SCD gene showed a higher expression level in the diet 6S and 4S2L (6 and 3 times respectively), while it was slightly lower in the diets 2S4L and 6L (0.88 and 0.68 times, respectively) (). Data reported in showed a significant quadratic decrease of mRNA content when SO was replaced with LO in the diet. The SCD mRNA amount significantly decreased until the 2S4L diet, which represent the 66.6% of replacement of SO with LO, and then stabilized (). On the other hand, the content of total PUFA n-3 in the intramuscular fat showed an increasing trend with the increment of LO in the diet (). Muscle samples from lambs fed 6S diet contained a higher amount of SCD mRNA (8.6 times) and a lower amount of PUFA n-3 than muscle samples from lambs fed 6L diet (). This result agreed with CitationWaters et al. (2009) and CitationDervishi et al. (2010), which found a negative relationship between SCD mRNA in muscles and the amount of dietary PUFA n-3, in beef and lamb, respectively. In particular, CitationDervishi et al. (2010) shown a higher SCD gene expression in the lambs fed a diet rich in PUFA n-6, similarly to what found in the present study. The modulation of the rate of n-6 and n-3 in the diet plays an important role to define the SCD gene expression through the SREBP-1c in muscle, as reported by CitationWaters et al. (2009). On the contrary CitationBernard et al. (2009b) revealed no effect on SCD mRNA abundance in mammary, liver or perirenal adipose tissues of goat after LO treatment.
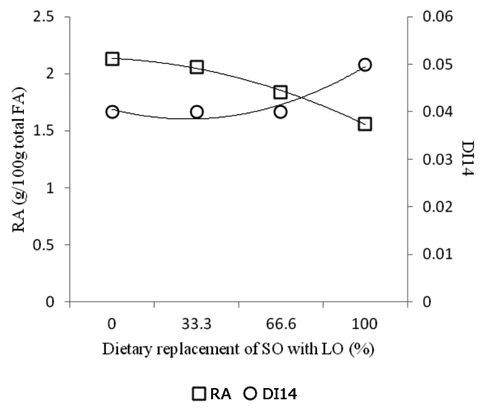
The product/substrate ratios reported in are considered a proxy of SCD activity (CitationMalau-Aduli et al., 1997). CitationPalmquist et al. (2004) proposed that the DI14 can be used to quantify endogenous RA synthesis in lamb tissues. Results from the present study, indicated that DI14 was not related to RA concentration in intramuscular fat (). Keeping into consideration also the other product:substrate ratios, the pattern of SCD activity did not seem related to the pattern of SCD mRNA expression (). The only FA ratio significantly, but marginally, affected by the replacement of SO with LO was the RA/VA ratio (). In particular, intramuscular fat from lambs fed 6L diet shown significant lower RA/VA ratio (−11.4%) than that from lamb fed the other diets (). In a previous study, CitationBas et al. (2007) reported that feeding lamb diet supplemented with linseed oil did not affect Δ9-desaturase indexes in the intramuscular fat.
The lack of association between desaturation indexes and SCD mRNA expression was reported also in previous studies on beef cattle (CitationArchibeque et al., 2005), on dairy cow (CitationBionaz and Loor, 2008) and goat (CitationBernard et al., 2009a). On the contrary, CitationHerdman et al. (2010) revealed that the SCD protein expression was significantly affected by dietary PUFA n-3 in bovine adipocytes. According to CitationArchibeque et al. (2005), these contrasting results may be due to individual variation of enzyme activity not related with indices of desaturation, probably because ingestion of SCD substrates and products may vary between diets (CitationRenaville et al., 2006).
Therefore, data of the present study seemed to suggest that in the intramuscular fat of lambs, dietary PUFA n-3 affected mainly SCD gene transcription rather than the activity of the SCD enzyme, as reported also in mouse and cow by CitationSessler et al. (1996) and CitationHiller et al. (2011) respectively. CitationHerdman and co-workers (2010) observed a reduction of SCD enzyme content in adipose tissue of bulls fed with linseed, without significant decreasing of MUFA. The authors concluded that the lack of significant differences might be related to large between-individual variations within the groups and relatively small number of animals per group. However, further investigation based on direct measurements of SCD enzyme activity in vivo are needed, in order to obtain additional insights about the regulation of SCD enzyme by PUFA n-3 in ruminant tissues.
Some hypothesis have been proposed in order to explain the regulation of SCD gene transcription by PUFA n-3. Some authors suggested that PUFA may regulate the expression of the adipocyte SCD gene by acting on the stability of mRNA transcripts (CitationSessler et al., 1996; CitationBernard et al., 2009a). Other authors proposed that PUFA n-3 may affect SCD gene transcription by regulating the nuclear abundance of the mature sterol response element binding protein-1 (i.e. nSREBP-1) (CitationNtambi, 1999; CitationHiller et al., 2011). However, at present it is still not completely clear the mechanism of the SCD gene regulation by PUFA n-3 in ruminants. Despite the relatively small number of animals per group, results of this work suggested an active role of these FA in lipid metabolism and gave more information about their dietary effect on quality of ruminant product. Since PUFA n-3 are implicated in the prevention of various diseases, including obesity and diabetes, by several different molecular mechanisms, including signal transduction and regulation of gene expression in liver, adipose tissue and brain (CitationAl-Hasani and Joost, 2005), further researches with a larger number of animals are required to completely understand the role of PUFA n-3 in the gene expression.
Conclusions
Replacing SO with LO in the diet of lambs resulted in a reduction of SCD mRNA level, and a marginally decrease of the SCD enzyme activity (expressed as RA/VA ratio). This results may be due either to a lack of effect of PUFA n-3 on the enzyme activity or to a lack of correlation between SCD product:substrate ratios and actual enzyme activity. Finally, results seemed to indicate that PUFA n-3 are more effective in affecting SCD expression in lamb muscle than PUFA n-6.
References
- Al-HasaniH. JoostH.G. 2005 Nutrition/diet-induced changes in gene expression in white adipose tissue Best Pract. Res. Cl. En 19 589 603
- ArchibequeS.L. LuntD.K. GilbertC.D. TumeR.K. SmithS.B. 2005 Fatty acid indices of stearoyl-CoA desaturase do not reflect actual stearoyl-CoA desaturase enzyme activities in adipose tissues of beef steers finished with corn-, flaxseed-, or sorghum-based diets J. Anim. Sci 83 1153 1166
- BasP. BerthelotV. PottierE. NormandJ. 2007 Effect of level of linseed on fatty acid composition of muscles and adipose tissues of lambs with emphasis on trans fatty acids Meat Sci 77 678 688
- BaumgardL. H. MatitashviliE. CorlB. A. DwyerD. A. BaumanD. E. 2002 Trans-10, cis-12 conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows J. Dairy Sci 85 2155 2163
- BeluryM.A. KempasteczkoA. 1997 Conjugated linoleic acid modulates hepatic lipid composition in mice Lipids 32 199 204
- BernardL. BonnetM. LerouxC. ShingfieldK.J. ChilliardY. 2009a Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in alpine goats fed maize silage-based diets J. Dairy Sci 92 6083 6094
- BernardL. LerouxC. ChilliardY. 2006 Characterization and nutritional regulation of the main genes in the lactating mammary gland SejrsenK. HvelplundT. NielsenM.O. Ruminant Physiology Wageningen Academic Publ. Wageningen, The Netherlands 295 326
- BernardL. LerouxC. FaulconnierY. DurandD. ShingfieldK. J. ChilliardY. 2009b Effect of sunflower-seed oil or linseed oil on milk fatty acid secretion and lipogenic gene expression in goats fed hay-based diets J. Dairy Res 76 241 248
- BernardL. RouelJ. LerouxC. FerlayA. FaulconnierY. LegrandP. ChilliardY. 2005 Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed vegetable lipids J. Dairy Sci 88 1478 1489
- BionazM. LoorJ.J. 2008 Gene network driving bovine milk fat synthesis during the lactation cycle BMC Genomics 9 366
- CarlsonL.A. 1985 Extration of lipids from human whole serum and lipoproteins and from rat liver tissue with methylene chloride-methanol: a comparison with extration chloroform-methanol Clin. Chim. Acta 149 89 93
- CartaA. CasuS. UsaiM. G. AddisM. FioriM. FraghìA. MiariS. MuraL. PireddaG. SchiblerL. SechiT. ElsenJ.M. BarilletF. 2008 Investigating the genetic component of fatty acid content in sheep milk Small Ruminant Res 79 22 28
- CorlB.A. BaumgardL.H. DwyerD.A. GriinariJ.M. PhillipsB.S. BaumanD.E. 2001 The role of Δ9-desaturase in the production of cis-9, trans-11 CLA J. Nutr. Biochem 12 622 630
- DervishiE. SerranoC. MargalidaJ. SerranoM. RodellarC. CalvoJ.H. 2010 Effect of the feeding system on the fatty acid composition, expression of the Δ9-desaturase, peroxisome proliferator-activated receptor alpha, gamma, and sterol regulatory element binding protein 1 genes in the semitendinous muscle of light lambs of the Rasa Aragonesa breed BMC vet. Res 6 40 50
- DobrzynA. DobrzynP. 2006 Stearoyl-CoA desaturase - a new player in skeletal muscle metabolism regulation J. Physiol. Pharmacol 57 (Suppl.10) 31 42
- FolchJ. LeesM. StanleyG.H.S. 1957 A simple method for the isolation and purification of total lipides from animal tissues J. Biol. Chem 226 497 509
- GraugnardD.E. PiantoniP. BionazM. BergerL.L. FaulknerD.B. LoorJ.J. 2009 Adipogenic and energy metabolism gene networks in longissimus lumborum during rapid post-weaning growth in Angus and Angus × Simmental cattle fed high-starch or low-starch diets BMC Genomics 10 142
- HerdmanA. NuernbergK. MartinJ. NuerbergG. DoranO. 2010 Effect of dietary fatty acids on expression of lipogenic enzymes and fatty acid profile in tissues of bulls Animal 4 755 762
- HillerB. HocquetteJ. F. Cassar-MalekI. NuernbergG. NuernbergK. 2011 Dietary n-3 PUFA affect lipid metabolism and tissue function-related genes in bovine muscle Brit. J. Nutr 108 858 863
- JerónimoE. AlvesS.P. PratesJ.A.M. Santos-SilvaJ. BessaR.J.B. 2009 Effect of dietary replacement of sunflower oil with linseed oil on intramuscular polyunsaturated fatty acids of lamb meat Meat Sci 83 499 505
- JumpD.B. ClarkeS.D. 1999 Regulation of gene expression by dietary fat Annu. Rev. Nutr 19 63 90
- LanzaM. FabroC. ScerraM. BellaM. PaganoR. BrognaD.M.R. PennisiP. 2011 Lamb meat quality and intramuscular fatty acid composition as affected by concentrates including different legume seeds Ital. J. Anim. Sci 10 e18
- Malau-AduliA.E.O. SiebertB.D. BottemaC.D.K. PitchfordW.S. 1997 A comparison of the fatty acids composition of triacylglycerols in adipose tissue from Limousin and Jersey cattle Aust. J. Agric. Res 48 715 722
- MeleM. 2009 Designing milk fat to improve healthfulness and functional properties of dairy products: from feeding strategies to a genetic approach Ital. J. Anim. Sci 8 Suppl.2 365 373
- MiyazakiM. NtambiJ.M. 2003 Role of stearoyl-coenzyme a desaturase in lipid metabolism Prostag. Leukotr. Ess 68 113 121
- NtambiJ.M. 1995 The regulation of stearoyl-CoA desaturase (SCD) Prog. Lipid Res 34 139 150
- NtambiJ.M. 1999 Regulation of stearoyl-CoA desaturase by polyunsatured fatty acids and cholesterol J. Lipid Res 40 1549 1558
- NtambiJ.M. MiyazakiM. 2004 Regulation of Stearoyl CoA desaturases and role in metabolism Prog. Lipid Res 43 91 104
- OllierS. LerouxC. de la FoyeA. BernardL. RouelJ. ChilliardY. 2009 Whole intact rapeseeds or sunflower oil in high-forage or high-concentrate diets affects milk yield, milk composition, and mammary gene expression profile in goats J. Dairy Sci 92 5544 5560
- PalmquistD.L. St-PierreN. McClureK.E. 2004 Tissue fatty acid profiles can be used to quantify endogenous rumenic acid synthesis in lambs J. Nutr 134 2407 2414
- RaesK. SmetS.D. DemeyerD. 2001 Effect of double-muscling in Belgian Blue young bulls on the intramuscular fatty acid composition with emphasis on conjugated linoleic acid and polyunsaturated fatty acids Anim. Sci 73 253 260
- RenavilleB. MullenA. MoloneyF. LarondelleY. SchneiderY.J. RocheH.M. 2006 Eicosapentaenoic acid and 3,10 dithia stearic acid inhibit the desaturation of trans-vaccenic acid into cis-9, trans-11-conjugated linoleic acid through different pathways in Caco-2 and T84 cells Brit. J. Nutr 95 688 695
- RitzenthalerK.L. McGuireM.K. FalenR. ShultzT.D. DasguptaN. McGuireM.A. 2001 Estimation of conjugated linoleic acid intake by written dietary assessment methodologies underestimates actual intake evaluated by food duplicate methodology J. Nutr 131 1548 1554
- ScollanN. HocquetteJ. F. NuernbergK. DannenbergerD. RichardsonI. MoloneyA. 2006 Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality Meat Sci 74 17 33
- SesslerA. M. KaurN. PaltaJ. P. NtambiJ. M. 1996 Regulation of stearoyl-CoA desaturase 1 mRNA stability by polyunsaturated fatty acids in 3T3-L1 adipocytes J. Biol. Chem 271 29854 29858
- VastaV. PrioloA. ScerraM. HallettK.G. WoodJ.D. DoranO. 2009 Δ9 desaturase protein expression and fatty acid composition of longissimus dorsi muscle in lambs fed green herbage or concentrate with or without added tannins Meat Sci 82 357 364
- WardR.J. TraversM.T. RichardsS.E. VernonR.G. SalterA.M. ButteryP.J. BarberM.C. 1998 Stearoyl-CoA desaturase mRNA is transcribed from a single gene in the ovine genome. J. Biochim. Biophys Acta 1391 145 156
- WartonK. FosterN.C. GoldW.A. StanleyK.K. 2004 A novel gene family induced by acute inflammation in endothelial cells Gene 342 85 95
- WatersS.M. KellyJ.P. O'BoyleP. MoloneyA.P. KennyD.A. 2009 Effect of level and duration of dietary n-3 polyunsaturated fatty acid supplementation on the transcriptional regulation of delta 9- desaturase in muscle of beef cattle J. Anim. Sci 87 244 252