Abstract
The first aim of this study was to examine the effects of strain and age on phytate phosphorus (PP) utilization and intestinal phytase activity (IPA) in two strains of laying hens, Hy-line Brown (HB) and Hy-line White W-36 (HW) at 32, 52 and 72 weeks of age. A digestion trial was conducted using the indicator method and birds were sampled to measure IPA. The second aim was to examine the effect of feed grade exogenous phytase enzyme on total (TP), water soluble (WSP) and phytate phosphorus (PP) excretion in the HW strain fed varying levels of phosphorus. Hens were fed three concentrations of available P (AP): 0.2, 0.3 and 0.4%. Each level of P was supplemented with three levels of commercial feed grade exogenous phytase enzyme (0, 300, and 600 FTU/kg) and the amount of TP, WSP and PP in excreta per 100 g of feed consumed was calculated. The HB retained more PP as compared to HW. Intestinal phytase activity showed a significant (P<0.01) age effect with the highest activity occurring at 32 weeks. There were significant differences in the amount of TP and SP excreted between birds receiving the 3 levels of phosphorus with 300 units phytase (P<0.01). The results of this study showed that layers are capable of utilizing PP, and that utilization is regulated by IPA and varies with age. Exogenous phytase improved PP utilization but it increased the amount of TP and WSP in excreta.
Introduction
Most ingredients used in poultry feeds are of plant origin. Phytate P in plants occurs as a mixed calcium-magnesium-potassium salt of phytic acid (CitationAnderson, 1912). The chickens’ ability to utilize PP is a subject of debate. Phytate P utilization from corn-soybean type diets is influenced by dietary factors (CitationBallam et al., 1984; CitationMohammed et al., 1991; CitationMarounek et al., 2008). Phytate P utilization in laying hens is increased when dietary Ca is lowered (CitationScheideler and Sell, 1987; CitationApplegate et al., 2000).
The effect of age on the ability of chickens to utilize PP has not been clearly defined. CitationMaddaiah et al. (1964) showed that mature hens utilized phytate more efficiently than chicks as measured by phytate retention. In laying hens fed corn-soybean diet, phytate P retention was 24% in 20-week and 53% in 47-week old hens (CitationMarounek et al., 2008).
A possible explanation for the increased utilization of PP with age is the suggestion that more endogenous phytase is present in the gastrointestinal tract of older birds (CitationRavindran et al., 1995; CitationMarounek et al., 2010).
Despite the contradictory observations and uncertainties concerning PP utilization with age, when birds are fed corn-soybean meal diets, the recommended levels of Ca and P were reduced when compared to earlier recommendations (CitationNRC, 1994). An intake of 300 mg AP per hen/day is recommended to reduce P excretion by 20% (CitationSummer, 1995). However, the results of a recent study with wheat-based diets without feed grade phytase indicated that dietary P concentration of 0.16-0.39% non-phytate P for 12 weeks had little effect on egg and shell production (CitationBoorman and Gunaratne, 2001). Continued feeding of the 0.16% non-phytate P diet for 61 weeks did not influence performance, but plasma phosphorus and bone measurements indicated marginal P depletion (CitationBoorman and Gunaratne, 2001). These results clearly showed that earlier, as well as the current (CitationNRC, 1994) recommendations overestimate P requirements of layers. The equivocal reports of PP utilization by layers fed corn-soybean meal diets, the role of intestinal phytase, and the need to take into account the contribution of phytate, all provided the rationale to examine the effect of age on PP utilization.
Phytase supplementation of layer diets has generally been limited to 250-300 FTU/kg (CitationVan der Kils et al., 1994; CitationGordan and Roland, 1997). Supplementation of 250 FTU of phytase/kg diet in laying hens was equivalent to 0.8 g of P from mono-calcium phosphate. Higher levels of phytase resulted in a lower equivalence per unit of phytase due to the significant quadratic response in P absorption (CitationVan der Klis et al., 1994). CitationUm and Paik (1999) reported that there were no significant differences between 0.2 to 0.5% NPP diets and supplementation of phytase gave no further improvement in performance.
Two experiments were conducted with layers to (a) examine the effects of age and strain on PP retention and IPA in laying hens, and (b) to evaluate the response of laying hens to graded levels of exogenous phytase supplementation.
Materials and methods
Experiment #1
A total of 80 30-week old commercial laying hens, 40 Hy-line Brown (HB) and 40 Hy-line White W-36 (HW), were used. Hens of each strain were selected from individually caged hens according to egg production and were caged individually in an open-sided poultry house. Birds received a corn-soybean meal basal diet formulated to contain 3.7% Ca and 0.58% TP from 30 to 72 weeks of age ().
Table 1 Dietary ingredients and chemical composition of the experimental layer diets.
To determine PP and TP retention, 0.3% chromic oxide was added to the basal diet of 12 birds per strain and fed for a 10-day adaptation period before collecting the excreta for three days at 32, 52 and 72 weeks of age. The same birds were used in all three collection periods. On Day 10 of each collection period, dropping trays were cleaned and lined with aluminum foil for excreta collection. Each day’s collection was dried at 80°C to inhibit phytate hydrolysis by microorganisms. Fecal samples from the same bird were pooled and ground to pass a 0.5 mm screen, and stored in sealed plastic containers until they could be assayed. The diet was sampled and treated in the same manner. Extreme care was taken in collecting the fecal samples to ensure they were not contaminated with feed or feathers. All values from chemical analysis performed on the diet and excreta samples were made on a dry matter basis. Sub-samples of dried excreta and diet were analyzed for percentage of TP, PP (using a method modified from that of CitationLatta and Eskin, 1980) and chromium (using a method modified from that of CitationBolin et al., 1952). The data were used to calculate PP and TP retentions (CitationEdwards and Gillis, 1959).
An additional 12 birds per strain per period were sampled for intestinal phytase activity (CitationIqbal et al., 1994; CitationBiehl and Baker, 1997). The duodenal loop mucosa from three birds per strain was pooled. Four pooled samples per strain were used for the assay.
Experiment #2
A total of 108 84-week old Hy-Line white (W-36) hens were selected and housed individually according to previous egg production data. Diets were formulated to meet or exceed CitationNRC (1994) nutrient requirement except for P (). The hens were fed nine dietary combinations; diets consisted of 3 concentrations of AP (0.2, 0.3 and 0.4%) and each level of AP was supplemented with 3 levels of commercial feed grade phytase enzyme (Ronozyme P CT, Lot # HB950031, 2500 FYT/g) at 0, 300 and 600 FYT/kg.
Hens were fed these diets for a 2-week acclimation period, then dropping trays were lined with aluminum foil for total excreta collection for two consecutive days. Excreta collected and feed samples were subjected to the same preparation as described previously in Experiment 1. Subsamples of the excreta and feed samples were analyzed for percentage of TP, PP and WSP.
Statistical analysis
Analysis of Experiment 1 was based on repeated measures design. The pen was used as the experimental unit. All calculations were performed using CitationSAS (1996). A model was defined for the experiment that included the following sources of variation: hen strain; age; age × strain. An analysis of variance (ANOVA) was performed to test for significance for each model term. Analysis of Experiment 2 was based on a 3×3 factorial arrangement of AP and phytase treatments in a randomized complete block design. The pen was used as the experimental unit. A model was defined that included the main effects of AP, phytase level and an interaction of AP × phytase. Significant model term differences in the analysis of variance were further tested using pair wise comparisons. A probability of P<0.05 or less was considered significant, and when appropriate the means±SEM are presented.
Results
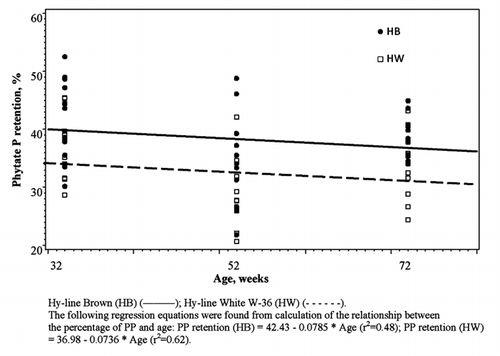
Values of PP retention measured at 32, 52 and 72 weeks of age for both strains are presented in . Phytate P utilization was influenced by strain (P<0.02) and age (P<0.001) but a strain × age interaction was not observed (). Hy-Line Brown strain retained 15.4% more PP (P<0.01) compared to strain HW (38.3 vs 33.2%±1.07, respectively). At 32 weeks of age birds retained a significantly (P<0.01) higher amount of PP compared to 52 weeks of age and a numerically (P>0.05) higher amount of PP compared to 72 weeks of age (39.5, 31.3 and 36.5%±1.18, respectively). The Hy-Line Brown birds retained more PP than HW at 32 weeks of age (42.1 vs 36.9%±1.67, respectively) and it declined 0.079% and 0.074% per week of age in strains HB and HW, respectively.
Table 2 Effects of age and strain on phytate phosphorus and phosphorus retentions and phytase activity in laying hens.
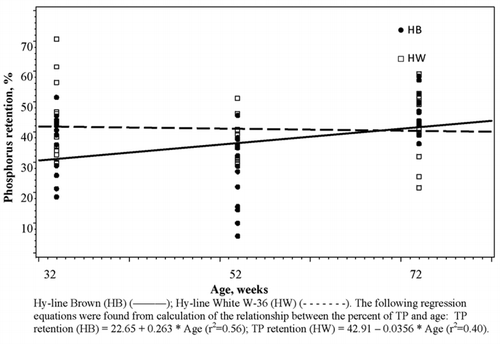
Total phosphorus retention measured at 32, 52 and 72 weeks of age for the 2 strains are presented in . A significant strain × age interaction was detected for TP retention (P<0.05) indicating differences in the slopes of the two strains (). At 32 and 52 weeks of age, HW retained higher TP compared to HB (43.7, 37.2 vs 36.3, 25.9±3.1, respectively). At 72 weeks of age, HW retained lower TP (42.3 vs 46.8±3.1, respectively) resulting in a significant strain × age interaction. Retention of TP increased by 0.263% per week for HB while it decreased by 0.036% for HW birds. At 32 weeks of age, birds retained significantly higher amounts (P<0.001) of TP compared to 52 weeks of age and numerically lower amounts (P>0.05) of TP compared to 72 weeks of age (40.0, 31.6 and 44.5%±2.2, respectively). Strain showed no significant effect on TP retention values (P>0.05).
No significant strain × age interaction was observed for IPA but it was influenced by age of the birds (P<0.001). Higher activity was observed at 32 weeks of age compared to 52 and 72 weeks of age (36.3, 25.9 and 30.0±1.4, respectively) ().
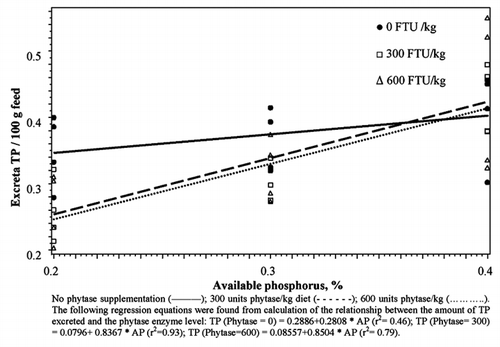
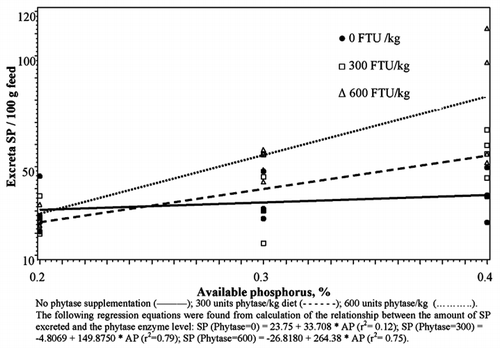
The addition of exogenous phytase to laying hen diets in Experiment 2 improved the degradation of PP. Phytase addition resulted in lower amounts of PP recovered in excreta (). The effect of the addition of the first 300 units of phytase was greater than that of the second 300 units. The first 300 units of phytase lowered PP in excreta per 100 g feed from 0.27 to 0.20 g, and the further addition of 300 units resulted in no further drop in PP (0.19 g). A comparison between the level of phosphorus in the diets indicates that the degradation of PP by phytase and measured by PP content in excreta is not dependent on the P content in the feed (P>0.05). Mean PP excreted were 0.23, 0.22 and 0.21 per 100 g feed intake for birds that received 0.2, 0.3 and 0.4% AP, respectively (). The phytase × phosphorus level term was not significant indicating that the slopes of the three lines do not differ from each other. A significant P × phytase interaction for the TP excreted per 100 g of feed intake indicated a significant difference in the slopes of the lines (). The addition of 300 or 600 units of phytase to the diet that contained 0.2% available phosphorus lowered the amount of TP in excreta (). No further improvement in TP excretion was seen from the addition of 600 units of phytase. Birds that consumed the diet containing 0.4% AP without phytase excreted lower amounts of TP compared to the other two groups, resulting in a significant phosphorus × phytase interaction.
Table 3 Effects of available phosphorus and exogenous phytase on phytate, total and soluble phosphorus excretion in laying hens.
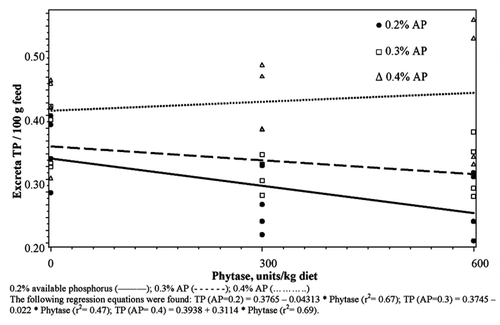
There was no significant difference in TP excreted between birds that received 0.2, 0.3 or 0.4% AP without phytase enzyme (P>0.05), while there were significant differences in the amount of TP excreted between birds that received the 3 levels of AP with 300 units phytase (P<0.01). Birds that received the diet with 0.2% AP excreted the lowest amount and those that received 0.4% excreted the highest amount of TP per 100 g feed (). The difference between the 3 levels increased with the addition of 600 units of phytase. Birds that received 0.2 and 0.3% AP had negative slopes while those that received 0.4% had a positive slope (P<0.01) ().
Significant differences in the slopes of the 3-phytase levels indicated a significant phosphorus × phytase interaction on the WSP in excreta (). At 0.2% AP, there were no significant differences in the amounts of WSP in excreta from birds that received 0, 300 and 600 units of phytase/kg diet (P>0.05). While at 0.3 and 0.4% AP levels the amount of WSP in excreta was higher for those receiving 300 or 600 units of phytase (P<0.01) than those that received no supplement. Increasing the amount of enzyme added had no influence on the amount of SP in excreta from birds receiving 0.2% AP. Increasing the enzyme from 0 to 600 units resulted in a significant increase in the WSP content of excreta (P<0.01).
Discussion
It has been reported that the ability of laying hens to utilize PP may decline with age, averaging 46.7, 9.1 and 16.5% at 34, 50 and 72 weeks of age, respectively (CitationScheideler and Sell, 1987). Other investigators reported that PP utilization increases with age in chicks and laying hens (CitationMaddaiah et al., 1964; CitationEdwards et al., 1989; CitationMarounek et al., 2008). In this study, the highest PP retention occurred at 32 weeks of age. This agrees with the fact that there is higher demand for P during the early stages of the laying cycle when egg production, egg weight and body weight are increasing. The high PP retention at 32 weeks was also associated with high IPA and, as a result, TP retention was significantly higher compared to the other two periods.
Feeding diets high in phosphorus have a tendency to lower the utilization of PP. Phytate P retention increased when low levels of NPP were fed to 3-week old broiler chicks (CitationBallam et al., 1984). In general, the data reported in this experiment did not show a consistent effect of dietary P in the range of 0.2 to 0.4% AP on PP retention.
It was reported that the addition of 250 units of phytase/kg feed of 24-week old laying pullets resulted in an improvement in PP degradation and a further addition of phytase resulted in slight improvement (CitationVan der Klis and Versteegh, 1991). The results of this experiment show that phytase is effective in improving the availability of PP in corn-soybean meal diets and the effect of the first 300 units of phytase was greater than that of the second 300 units. Phytase supplementation also increased the amount of TP and SP even at the low level (300 units), which could have negative effects on the environment. An addition of 300 units of phytase to the diet that contained 0.2% AP resulted in a reduction of TP excreted but no further improvement was seen after increasing the amount of phytase in the diets to 600 units. Addition of phytase at either level to the diet with 0.4% AP resulted in more TP being excreted. These results indicated that environmental contamination by P can be reduced by lowering dietary P levels. Phosphorus in excreta consists of an undigested portion of bound phytate and NPP from ingredients, and undigested portions of P from mineral supplements and surplus amounts of bioavailable P in excess of the bird’s need. Phosphorus losses from soil are generally associated with SP, which is dissolved in run off. Organic P associated with manure has greater bioavailability and solubility, and most dissolved P is immediately available for biological uptake (CitationSharpley, 1999). The CitationNRC (1994) requirement for available P in laying hens is 250 mg/day. Several authors (CitationTemperton et al., 1965a,Citationb; CitationBoorman and Gunaratne, 2001) have shown that the P requirements of laying hens can be met entirely from plant ingredients to sustain egg production and egg shell quality. However, examination of the diets used by these researchers showed that they included phytase rich ingredients such as wheat. CitationOwings et al. (1977) showed that 0.19% NPP (0.09% must be in inorganic form) will sustain a high rate of egg production but to maintain livability, the requirement was at least 0.28%. CitationGarlich et al. (1975) reported that 0.227% NPP in the diet did not maintain normal hen weight gains. CitationGordan and Roland (1997) reported that hens consuming 0.1% NPP without supplemental phytase had a decrease in egg production, compared to 0.2 to 0.5 NPP. But a 0.1% NPP diet supplemented with 300 units phytase corrected the adverse effect. There were no significant differences between 0.2 to 0.5% NPP diets, and supplementation of phytase to 0.2 to 0.5% NPP diets gave no further improvement in performance. CitationBoling et al. (2000a,Citationb) obtained similar results in a study involving 0.15% NPP with phytase. Supplementation of 500 units of phytase/kg diet of 21-week old ISA Brown hens reduced the NPP level to 0.12% in layer diet without affecting laying performance (CitationUm and Paik, 1999). The use of an NPP regimen of 0.25, 0.2 and 0.15% plus 300 units phytase/kg diet for the periods 20 to 35, 36 to 51, and 52 to 63 weeks of age, reduced the daily TP excretion by 55.6% over the control 0.45% NPP for the period of 20 to 63 weeks of age (CitationKeshavarz, 2003). Equivalency of phytase to P is very important in diet formulation to determine how much inorganic P can be saved by supplementation with phytase.
Conclusions
Birds are able to utilize PP and this is regulated by intestinal phytase. Exogenous phytase improved the utilization of PP but at the same time, the amounts of TP and SP in excreta were increased by phytase and inorganic supplementation. One portion of P in excreta consists of surplus amounts of bioavailable P in excess of the bird’s need. The most significant factor in reducing P in excreta is dietary P level. These results clearly indicate that levels of P pollution can be reduced by lowering dietary inorganic P level and by the judicious use of exogenous phytase.
Acknowledgments:
this project was supported by the King Saud University, Deanship of Scientific Research, College of Food & Agriculture Sciences, Research Center.
References
- AndersonR.J. 1912 Phytin and phosphoric acid esters of inosite J. Biol. Chem 17 141
- ApplegateT.J. AngelC.R. ClassenH.L. NewkirkR.W. MaenzD.D. 2000 Effect of dietary calcium concentration and 25-hydroxycholecalciferol on phytate hydrolysis and intestinal phytase activity in broilers Poultry Sci 79 (Suppl.1) 21
- BallamG.C. NelsonT.S. KirbyL.K. 1984 Effect of fiber and phytate source and of calcium and phosphorus for chicks Poultry Sci 63 333 338
- BiehlR.R. BakersD.H. 1997 1αa-hydroxycholecalciferol does not increase specific activity of intestinal phytase but does improve phosphorus utilization in both cecectomized and sham-operated chicks fed cholecalciferol-adequate diets J. Nutr 127 2054 2059
- BolinD.W. RichardP.K. KlostermanE.W. 1952 A simplified method for the determination of chromic oxide when used as an index substance Science 116 634 635
- BolingS.D. DouglasM.W. JohnsonM.L. WangX. ParsonsC.W. KoelkebeckK.W. ZimmermanR.A. 2000a The effects of dietary available phosphorus levels and phytase on performance of young and older laying hens Poultry Sci 79 224 225
- BolingS.D. DouglasM.W. ShirleyR.B. ParsonsC.W. KoelkebeckK.W. 2000b The effects of various dietary levels of phytase and available phosphorus on performance of laying hens Poultry Sci 79 535 538
- BoormanK.V. GunaratneS.P. 2001 Dietary phosphorus supply, egg-shell deposition and plasma inorganic phosphorus in laying hens Brit. Poultry Sci 42 81 91
- EdwardsH.M.Jr. GillisM.B. 1959 A chromic oxide balance method for determining phosphorus availability Poultry Sci 38 569 574
- EdwardsH.M.Jr. PaloP. SoonchaerernyingS. ElliotM.A. 1989 Factors influencing the bioavailability of phytate phosphorus to chickens SouthgateD. JohnsonI. FenwickG.R. Nutrient availability: chemical and biological aspects The Royal Society of Chemistry Publ. Cambridge, UK
- GarlichJ.D. 1975 Effects of short-term phosphorus deprivation on laying hens Poultry Sci 54 1193 1199
- GordanR.W. RolandD.A.Sr. 1997 Performance of commercial laying hens fed various phosphorus levels, with and without supplemental phytase Poultry Sci 76 1172 1177
- IqbalT.H. LewisK.O. CooperB.T. 1994 Phytase activity in the human and rat small intestine Gut 35 1233 1236
- KeshavarzK. 2003 The effect of different levels of non phytate phosphorus with and without phytase on the performance of four strains of laying hens Poultry Sci 82 71 91
- LattaM. EskinM. 1980 A simple and rapid colorimetric method for phytate determination J Agr. Food Chem 28 1313 1315
- MaddaiahV.T. KurnickA.A. ReidB.L. 1964 Phytic acid studies P. Soc. Exp. Biol. Med 115 391 (abstr.)
- MarounekM. SkřivanM. DlouháG. BřeňováN. 2008 Availability of phytate phosphorus and endogenous phytase activity in the digestive tract of laying hens 20 and 47 weeks old Anim. Feed Sci. Tech 146 353 359
- MarounekM. SkřivanM. RoseroO. RopO. 2010 Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat-maize-soybean diet J. Anim. Feed Sci 19 430 439
- MohammedA.A. GibneyM.J. TaylorT.G. 1991 The effects of dietary levels ofinorganic phosphorus, calcium and cholecalciferol on the digestibility of phytate-P by the chick Brit. J. Nutr 66 251 259
- National Research Council 1994 Nutrient requirements of poultry 9th rev. ed. Academy Press Washington, DC, USA
- OwingW.J. SellJ.L. BallounS.L. 1977 Dietary phosphorus needs of laying hens Poultry Sci 56 2056 2060
- RavindranV. BrydenW.L. KornegayE.T. 1995 Phytates: Occurrence, bioavailability and implications in poultry research Poult. Avian Biol. Rev 6 125 143
- SAS 1996 Users Guide: Statistics. Version 7.0 SAS Inst. Inc. Cary, NC, USA
- ScheidelerS.E. SellJ.L. 1987 Utilization of phytate phosphorus in laying hens influenced by dietary phosphorus and calcium Nutr. Rep. Int 35 1073 1081
- SharpleyA. 1999 Agricultural phosphorus, water quality, and poultry production: Are they compatible? Poultry Sci 78 660 673
- SummerJ.D. 1995 Reduced dietary phosphorus levels for layers Poultry Sci 74 1977 1983
- TempertonH. DudleyF.J. PickeringG.J. 1965a Phosphorus requirements of poultry. IV. The effects on growing pullets of feeding diets containing no animal fat protein or supplementary phosphorus Brit. Poultry Sci 6 125 133
- TempertonH. DudleyF.J. PickeringG.J. 1965b Phosphorus requirements of poultry. V. The effects during the subsequent laying year of feeding growing diets containing no animal fat protein or supplementary phosphorus Brit. Poultry Sci 6 135 141
- UmJ.S. PaikI.K. 1999 Effects of microbial phytase supplementation on egg production, eggshell quality, and mineral retention of laying hens fed different levels of phosphorus Poultry Sci 78 75 79
- Van der KlisJ.D. VersteeghH.A.J. 1991 Ideal absorption of phosphorus in light-weight white laying hens using microbial phytase and various calcium in laying hen feed IPR publication No. 563, Institute for Poultry Research, Spelderholt The Netherlands
- Van der KlisJ.D. VersteeghH.A.J. ScheeleC.W. 1994 Practical enzyme use in poutry diets: phytase and NSP enzymes 113 128 in 21st Proc. Carolina Poultry Nutrition Conf Charlotte, NC, USA