Abstract
The aim of this study was to evaluate the bioavailability of ingested selenium (Se) yeast in laying hens and its effects on performance, eggshell quality, and tissue Se distribution. Forty-eight ISA brown laying hens were divided into 3 treatment groups: Group C, fed a basal diet containing 0.11 mg Se/kg of feed; Group SS, fed a basal diet plus 0.4 mg/kg of feed of Se from sodium selenite; and Group SY, fed a basal diet plus 0.4 mg/kg of feed of Se from selenium yeast. Feed intake, egg mass ratio, and production performance were not affected by Se supplementation, regardless of the Se source. Egg weight (+3.61% and +2.95%), eggshell weight (+4.26% and +5.38%), and eggshell surface (+2.43% and +1.96%) were higher (P<0.05) in SS and SY than C, whereas breaking strength was increased in SY (P<0.01). Breast muscle, liver and skin Se levels were higher in SY than in C, while kidney Se content was higher in SS hens. Eggs from SY had higher Se levels than SS. Blood metabolites were not affected in SS or SY groups than C. A higher Se level was detected in eggs and breast muscle of SY hens (P<0.05). Seleniumenriched eggs and edible tissues from organic Se sources in poultry diet could improve antioxidant status in humans and reduce possible Se deficiency-related diseases.
Introduction
Selenium, as an essential trace element for animals and humans, is required for growth, health, and many physiological functions such as antioxidant defence (Rotruck et al., Citation1973), immune function (McKenzie et al., Citation1998), thyroid dysfunction and reproduction (Rayman, Citation2000). The antioxidant effect of selenium (Se) seems to be relative to the reduction via oxidation of selenocysteine (SeCys) (Surai, Citation2006) as part of the active centre of glutathione peroxidase (GSH-Px) (Navarro-Alarcón and López-Martínez, Citation2000). This causes the enzyme to catalyse the reduction of hydrogen and lipid peroxides into less harmful hydroxides (Arthur, Citation2000; Juniper et al., Citation2011). GSH-Px levels in plasma are indicative of the supply level of Se in the diet (Zhou and Wang, Citation2011). Deficiencies in Se, as can still be found is some EU countries or regions (Čobanová et al., Citation2011), can contribute to more than 40 human diseases or health problems (Reilly, Citation1998) including cardiovascular disease (Yoshizawa, et al., Citation2003), cancer (Rayman, Citation2005), asthma (Hoffmann, Citation2008), diabetes (Faure, Citation2003), and hypothyroidism (Reid and Territ, Citation1997). Therefore, higher Se content in both eggs and edible tissues could increase health and general antioxidant status in humans (Čobanová et al., Citation2011).
The uptake and assimilation of Se are dependent upon its inorganic or organic form; among them, inorganic Se is the most used in poultry diets as sodium selenite (Na2SeO3). Over past years, organic sources of Se, such as selenium yeast, have been explored as an alternative to inorganic supplementation (Payne et al., Citation2005; Utterback et al., Citation2005; Schrauzer, Citation2006). Selenium yeast from Saccharomyces cerevisiae may assimilate up to 3000 μg of Se/g (Schrauzer, Citation2003) where the major product is Se-Met. This is incorporated into yeast proteins or is physically associated with cell-wall constituents (Polatajko et al., Citation2004) with the result that there is more Se deposition than inorganic form into body tissues and eggs (Utterback et al., Citation2005; Juniper et al., Citation2011). The differences in tissue deposition among Se dietary sources are relative to the absorbtion mechanisms. Inorganic forms such as sodium selenite are absorbed by passive diffusion and incorporated into selenoenzymes or excreted; organic forms can be either utilised for incorporation into selenoenzymes or incorporated non-specifically in place of methionine into general body proteins via methionine transporter mechanisms (Suzuki and Ogra, Citation2002; Weiss, Citation2003). Furthermore, several studies on supplementation indicated that Se from selenium yeast exhibits greater bioavailability than that from inorganic Se sources, and that increased Se levels are maintained for a longer period after supplementation has ceased (Dumont et al., Citation2006; Schrauzer, Citation2006; Yoon et al., Citation2007). While many studies have suggested positive results on egg (Davis et al., Citation1996; Cantor et al., Citation2000), blood, liver and kidney (Jiakui and Xiaolong, Citation2004) Se content, feeding organic rather than inorganic forms of Se in poultry, very few papers have considered Se accumulation and distribution in tissues using organic or inorganic sources.
The aim of this study was to evaluate the bioavailability of ingested selenium yeast in laying hens and its effects on performance, eggshell quality, and tissue Se distribution from 22 to 30 weeks of age.
Materials and methods
Animals and experimental design
Animal care and treatment were in accordance with the European Community Citation1986 guidelines n. 609 (EEC, Citation1986) approved by the Italian Ministry of Health.
Forty-eight ISA Brown Warren laying hens from the same stock with homogeneous genetics and initial live weight (1590±187 g) were randomly distributed into 3 experimental groups of 16 hens each and housed 2 birds per cage in 34 x 40 x 45 cm cages. Each experimental group consisted of 8 cages in order to obtain 8 replicates per group, and 3 dietary treatments were assigned: C (control, initial live weight 1638±149 g), fed the basal diet containing 0.11 mg of Se/kg of feed; SS (inorganic Se, 1519±261 g), fed the control diet plus Se from sodium selenite at 0.4 mg Se/kg of feed; SY (organic Se, 1613±152 g) fed the control diet plus Se from selenium yeast at 0.4 mg Se/kg of feed. The selenium yeast (Alkosel R397, EU n. 3b8.11, Lallemand SAS, Blagnac, France) was a commercial product containing 2000 ppm of total Se with 98% organic Se; 65-75% of the organic Se was composed of selenomethionine. Birds were housed in the same shed and environmental conditions were set according to the ISA Warren layer management guide. Animals were fed ad libitum, and the feed was formulated to meet nutritional requirements according to the ISA Warren management manual (ISA SAS, Citation2005).
From 17 to 22 weeks of age, all hens were fed the C diet without any Se supplementation of additional Se sources as adaptation period; the experimental period started at 22 weeks of age and lasted until 30 weeks of age. At the beginning of the trial and for the whole experiment, control and treated feeds from the same batch were sampled and analysed weekly for dry matter, crude protein, ether extract, neutral detergent fibre, metabolisable energy, ash, calcium, phosphorus, lysine, methionine, methionine+ cysteine, non-phytate P, and Se content following AOAC methods (AOAC, Citation1995). Feed composition and the analysed chemical composition of the experimental diets are reported in and .
Feed intake per cage was evaluated daily from 22 weeks of age, and the feed conversion ratio was calculated during the laying period as weekly feed intake per cage over weekly produced egg mass per cage. Daily egg production per cage as well as egg weight were recorded at the same time of day (6.00 pm), and daily laying rate per cage was calculated as the ratio between the number of eggs produced and an ideal number of eggs (2 eggs per cage per day). The selenium content in the eggs was analysed at 0, 18, 36 and 56 days from the beginning of the trial. Two eggs per replicate were collected and refrigerated. Subsequently collected samples were individually cracked, shells were discarded, and the fluid content homogenised and frozen until Se and dry matter content determination.
Two days before each egg-sampling procedure for Se and dry matter content, 4 eggs per replicate were collected to assess eggshell quality. At the end of the trial, 8 animals per group were sacrificed by anesthesia and subsequent decapitation. Breast muscle, liver, kidneys, and skin samples were collected and analysed for Se content (Tam and Lacroix, Citation1982); results are expressed on a dry matter basis. A necropsy was performed on the slaughtered animals via macroscopic observation. Two sets of blood samples were collected before slaughtering from 8 animals per group from the jugular vein, put into two 10-mL vacuum tubes (Venojet VT050SP Terumo, Japan) and subsequently centrifuged (1400 ×g ×10 min). Two serum aliquots from each tube were than collected and frozen (-20°C) until analysis for Se (Machát et al., Citation2002), glucose (Centers for Disease Control, Citation1976), total protein (Hiller et al., Citation1948), albumin (Doumas et al., Citation1971), cholesterol (Allain et al., Citation1974), alanine transaminase (ALAT) and aspartate transaminase (ASAT) (Henry et al., Citation1960), bilirubin (Mori, Citation1978), alkaline phosphatase (ALP) content (Browers and Comb, Citation1966), and serum glutathione peroxidase activity (GSH-Px) (Paglia and Valentine, Citation1967).
ALAT, ASAT, albumin, total protein, glucose, ALP, and cholesterol content were analysed using a Synchron CX5® Delta chemistry analyser (Beckman Coulter, Brea, CA, USA), while GSH-Px activity was measured using a commercial assay kit from Cayman Chemical Company (Ann Arbor, MI, USA) adapted for spectrophotometer. The activity was assayed in a 190 μL reaction mixture containing 100 μL assay buffer (50 mM Tris-HCl, pH 7.6, containing 5 mM EDTA), 50 μL of co-substrate mixture containing NADPH, glutathione, and glutathione reductase, and 20 μL of cumene hydroperoxide as the starter reactive. The decrease in absorbance caused by the reduction of hydroperoxide by GSH-Px was monitored at 340 nm. GSH-Px activity was calculated using an extinction coefficient for NADPH at 340 nm of 0.00622 μM/cm.
Eggshell quality
The length and breadth (mm) of each egg were measured, and a shape index (SI) was calculated (SI=egg length:breadth). Shell weight (g) was measured after washing the shells and drying them overnight at 80°C. Eggshell percentage, eggshell index, and egg surface area were calculated as described by Mabe et al. (Citation2003). Eggshell thickness (without shell membranes) was measured at 3 positions (top, middle and bottom) using a micrometer (Digimatic 0-25 mm 0.001 mm, Mitutoyo Corp., Kanagawa, Japan). Eggshell mechanical stiffness (N/mm) and breaking strength (N) were measured by quasi-static compression using a testing machine (model 5542, Instron, Norwood, MA, USA) fitted with a 500-N load cell and equipped with a food texture fixture compression anvil (catalogue n. 2830-009, Instron, Norwood, MA, USA). Breaking strength was measured as the maximum force required to fracture each egg at a compression speed of 5 mm/min. Static stiffness was calculated as a linear slope of the force deformation curve resulting from the load applied up to 10 N at a compression speed of 5 mm/min on the equator of each egg. The elastic modulus (N/mm2) and fracture toughness (N/mm3/2) of each egg were estimated using formulae developed by Bain (Citation1990) and described by Mabe et al. (Citation2003).
Selenium
To determine selenium levels in the blood by inductively coupled plasma atomic emission spectrometry (ICP-AES), serum sample solutions were prepared by acid digestion in an open system in order to eliminate spectral interference caused by carbon, as described by Machát et al. (Citation2002). Briefly, 2 mL of each serum sample were heated in 8 mL of HNO3 (65%) (Baker Instra-Analyzed) at 145°C for 6 hours. After cooling, 2 mL of H2O2 (30%) was added to the solution until a light yellow colour developed, and samples were heated again until evaporation. The serum reference material (SeronormTM Trace Element Serum, Sero AS, Norway) with a certified Se content of 0.136 mg/L was used to test the accuracy and precision of the analytical procedure. For liver and kidney samples, a closed-vessel microwave (MARS 5, CEM Corp., Matthews, NC, USA) mineralization procedure was performed. Liver aliquots were weighed (0.8-0.9 g) in Teflon vessels, and then 10 mL of HNO3 (65%) was added. The vessels were sealed tightly and kept in the microwave for 25 minutes under 600 W of microwave power at 210°C and 170 psi. After digestion and cooling down to room temperature, the samples were carefully transferred to glass tubes; 2 mL of HNO3 (65%) was added to each sample which was then heated at 105°C until complete evaporation. Serum, liver, and kidney samples were finally resuspended in 2 mL of HNO3 (5%) prior to determination of Se by ICP-AES. An ICP emission spectrometer (OPTIMA 3300 XL, Perkin-Elmer Corp., Waltham, MA, USA) with a standard axial torch was used. The instrument was optimised to obtain the maximum signal-to-background ratio and minimum relative standard deviation (RSD) of signal and background. The most sensitive line Se 196.026 nm was used. In all digestates, the Se concentration (SeronormTM included) was determined using a calibration curve constituted by standard solutions (0-0.5 mg/L) of 100 ppm inorganic Se (AccuTrace™, AccuStandard Inc., New Haven, CT, USA).
Skin, muscle, eggs, and feed samples were submitted to a closed-vessel acid digestion protocol prior to analysis by an atomic absorption spectrophotometer (Tam and Lacroix, Citation1982). Samples were treated with a hydrated mixture of MgO:Mg(NO3)2 at a 1:10 ratio and digested at low temperatures on a hotplate until completely dry. Afterwards, the samples were placed in a muffle furnace at 500 °C for 1 hour in order to remove the entire organic matrix. The residues were mixed with an acid solution and treated to reduce Se to the IV form. A 4100 ZL atomic absorption spectrophotometer with a FIAS 100 hydride generator was used (Perkin-Elmer Corp., Waltham, MA, USA). The instrument was optimised to obtain the maximum signal-tobackground ratio and the minimum RSD of signal and background. The most sensitive line Se 192 nm was used. Selenium amount was determined using a calibration curve obtained from standard solutions at 3 levels.
Statistical analysis
Data relative to feed consumptions, egg Se content, egg weight, feed:egg mass ratio, egg production, and eggshell quality were analyzed by an ANOVA using a MIXED procedure of SAS for repeated measures (SAS, Citation2006). The model considered the effects of Se source, treatment day, and their interaction, the random effect of animals nested within treatment, and the residual error. The applied model was:
where
Yij is dependent variable feed consumptions, egg Se content, egg weight, feed:egg mass ratio, egg production, and eggshell quality parameters;
µ is general mean;
Ti is effect of Se source;
Dj is effect of day of sampling;
(T×D)ij is effect of the interaction between treatment and time;
eij is casual effect of each observation.
Data obtained for blood parameters and selenium tissue deposition were analysed by ANOVA using an SAS General Linear Model (SAS, Citation2006). The cage was considered as the experimental unit for statistical evaluation. P≤0.05 was considered significant.
Results
Performance of laying hens during the whole trial period are summarised in . The administration of organic or inorganic sources of Se in the feed for 8 consecutive weeks did not significantly affect either average daily feed intake (ADFI), feed:egg mass ratio, egg production or egg mass.
On the contrary, egg quality was generally influenced by the administration of both organic or inorganic forms of Se with increased weight of egg (P<0.05) and eggshell (P<0.05), and higher egg surface area (P<0.05) (). In particular, egg weight in Se-enriched diets groups was increased by 3.61% and 2.95%, respectively, for SS and SY hens than C, while eggshell weight was 4.26% and 5.38% higher in SS and SY. Such increased egg and eggshell weights, did not lead to the same amount of increment in eggshell surface area that was less influenced by the administration of organic or inorganic Se sources (+2.43% in SS, +1.96% in SY compared with C, respectively). No difference was observed for these parameters between SS and SY groups, as for eggshell percentage, index, stiffness, elastic modulus, fracture, and thickness. SY hens had higher eggshell index (P<0.05) than control animals, while the breaking strength was considerably higher than SS and C hens (P<0.01). There was no difference in total egg Se content between the experimental groups when hens were fed a non-supplemented diet either during the adaptation period or at the beginning of the trial ().
Eggs from SS and SY had greater Se content than those from hens fed the basal diet (0.94 ppm and 1.38 ppm vs 0.54 ppm, respectively; P<0.01). SY eggs had 46.81% higher Se content (P<0.01) than those from SS group (), while higher serum Se content at the end of the trial was found in SY group than C (P<0.01) and SS (P<0.05) (). Tissue samples revealed increased Se content in breast muscle, liver, and skin in SY hens than C, while no differences were found between SS and C animals except for a higher Se content in kidney (). The source of dietary Se did not affect the selenium content in sampled tissues except for lower breast muscle level in sodium selenite fed animals.
Inorganic or organic supplementation of Se did not affect plasma total protein, albumin, cholesterol, total bilirubin, ALAT, ASAT, ALP, or glucose (), but significantly increased serumglutathione peroxidase concentration (P< 0.05)with respect to C.
Discussion
Laying hens performance
Increasing Se content in the diet of poultry and, generally, in livestock (Petrera et al., Citation2009; Calamari et al., Citation2010; Cozzi et al., Citation2011; Tufarelli and Laudadio, Citation2011) is strongly dosedependent: higher dosages lead to enhanced performance (Scheideler et al., Citation2011). In particular, Se-enriched yeast should be more effective than inorganic sources due to the higher bioavailability relative to the presence of huge amounts of selenomethionine (Wu et al., Citation2011). Furthermore, organic Se sources could have positive effects on the environment, decreasing pollution with a less toxic activity than the inorganic form of the element (Kim and Mahan, Citation2001). In the present trial, there was no difference in performance of laying hens between the 3 experimental groups. There was no difference in organic or inorganic dietary Se supplementation in poultry versus the control group, and a similar performance was found when comparing SY and SS hens. Similar feed intake and feed efficiency between organic or inorganic supplemented hens are in accordance with data from Payne et al. (Citation2005) who, however, observed an increase in feed intake in hens fed higher levels of Se both from organic or inorganic sources than animals fed the basal diet.
The lack of any significant difference in egg production during the present trial agrees with some previous reports by Cantor et al. (Citation2000) and Payne et al. (Citation2005) who found no effects when selenium selenite or organic Se were added to poultry diet. Furthermore, Pavlovi et al. (Citation2009) did not observe any differences in egg production during the first 8 weeks of dietary Se administration in laying hens, whereas in the last 8 weeks selenium yeast increased egg production compared to control and sodium selenite.
In this trial, the lack of higher performance when Se was added to poultry diet can be related to the relatively high amount of Se in the basal diet (0.11 ppm) in contrast to reports by other authors, such as Cantor and Scott (Citation1974), using 0.02 ppm.
Egg weight and eggshell quality
In our trial, the supplementation with different sources of Se led to heavier eggs than those reported in other studies (Utterback et al., Citation2005; Chantiratikul et al., Citation2008). However, our findings are in agreement with data obtained by Rutz et al. (Citation2003) and Sk ivan et al. (Citation2006) who found heavier eggs in organic-selenium supplemented hens than control or hens receiving sodium selenite-supplemented diets. Similarly, eggshell weight and egg surface area in SS and SY were higher than in C. The shape index was higher in SY than in C whereas eggshell percentage was equal between treatment groups, indicating that egg and shell weight increased proportionally. In agreement with Renema (Citation2004), Se supplementation, particularly SY, resulted in the greatest positive changes in eggshell quality, although Pavlovi et al. (Citation2010) revealed that neither the source nor the level of Se affected eggshell quality.
Results on increased breaking strength are confirmed by previous studies (Paton et al., Citation2000; Siske et al., Citation2000; Golubkina and Papazyan, Citation2006): this corresponds with a higher Se concentration in the shell and shell membrane. These two last factors are particularly increased when organic dietary Se supplementation is adopted, suggesting that the high Se concentration could be the reason for increased shell strength. There was no difference in eggshell thickness between treatment groups; this is consistent with results of Arnold et al. (Citation1973), who added 2 and 8 ppm of sodium selenite to the hens’ diet, and Chantiratikul et al. (Citation2008), who added 0.3, 1 and 3 ppm of both sodium selenite and a chelated type of Se source, such as zinc-L selenomethionine.
Selenium in eggs
When hens received diet without any Se supplementation, the Se content of eggs was similar in the 3 experimental groups, but when Se was added as selenium yeast or in an inorganic form, the Se egg content doubled and increased by 74%, respectively. SY increased Se egg content by 47% more than the increase observed in SS. Over the years, many studies have been conducted on whole egg Se concentration when diets were supplemented with Se (Cantor and Scott, Citation1974; Latshaw and Biggert, Citation1981; Cantor et al., Citation2000). Several authors, using basal diets within 0.02 and 0.11 ppm Se, reported that organic Se supplementation of the diet with SY is more effective than a SS diet for increasing the Se content of egg (Payne et al., Citation2005; Pan et al., Citation2007), although positive results were also found with the supplementation of inorganic Se (Rizzi et al., Citation2009).
Latshaw and Biggert (Citation1981) reported that whole egg, egg white, and egg yolk Se levels were 44%, 79%, and 15% greater, respectively, in hens fed organic Se compared with those fed SS; this was confirmed by results obtained by Cantor et al. (Citation2000) who used the same range of supplementation. The main reason for the increased Se deposition in eggs by SY is that the majority of Se in SY is selenomethionine, a Se analogue of methionine (Kelly and Power, Citation1995). The other organic Se components have not yet been clearly identified, but act as Se-Met precursors, although there is some recent evidence (Polatajko et al., Citation2005) that selenomethionine represents approximately 80% of the organic selenium in Se yeast, in some cases reaching levels of over 90% (Schrauzer, Citation2006). Se-Met is deposited in the egg to a greater extent than selenium selenite, and is actively absorbed and incorporated into eggs as effectively as methionine (Combs and Combs, Citation1986).
Blood chemistry and selenium content
Supplementation of Se increased the serum Se concentration, but the difference was statistically significant only when Se yeast was included in the diet. Jiakui and Xiaolong (Citation2004) and Petrovič et al. (Citation2006) observed a higher blood Se content when inorganic or organic Se was added to a hen’s diet, while Scott and Thompson (Citation1971) reported an increase in blood Se concentrations when Se was provided in an organic form compared with sodium selenite.
Serum GSH-Px was significantly higher when Se was added to the basal diet, but no differences were observed in hens fed Se-supplemented diet in previous studies by Kuricovà et al. (Citation2003) and Petrovič et al. (Citation2006). These results can be explained by the fact that GSH-Px mRNA is regulated by absorbed Se during a post-transcriptional step (Toyoda et al., Citation1990; Petrovič et al., Citation2006) and all Se sources have to be split into H2Se before selenocysteine is synthesised de novo for incorporation into an active centre of selenoenzymes (Schrauzer, Citation2003).
Selenium in tissues
SY has a higher Se bioavailability than inorganic Se sources (Yoshida et al., Citation2002); this means that when SY is administered to hens, an increase in the Se content of tissues is expected. Indeed, we observed that breast muscle Se content was higher in SY-supplemented hens compared with SS-supplemented animals. Petrovič et al. (Citation2006), Pan et al. (Citation2007), and Leeson et al. (Citation2008) observed an increased Se concentration in the breast muscle of hens fed selenium yeast when compared to hens fed sodium selenite. According to Petrovič et al. (Citation2006), the muscle tissue of birds fed selenised yeast becomes the most significant site of Se deposition, since in poultry striated muscle mass represents approximately 52-56% of body weight.
It has been suggested that selenomethionine deposited in the muscle tissue of an animal fed with Se yeast may account for more than 50% of the total Se in the body (Daniels, Citation1996). The possible benefit of selenomethionine being deposited in body tissues is that it may serve as quantitatively important storage (Oster et al., Citation1988), capable of releasing Se during episodes of an insufficient dietary Se supply (Zuberbuehler et al., Citation2006). The portion of selenomethionine absorbed from the digestive tract that is not immediately used for synthesis of specialised selenoproteins is incorporated non-specifically into the structural proteins of muscle, gizzard, heart, and other organ tissues. Selenomethionine substitutes for the common amino acid methionine that contains sulfur instead of Se (Schrauzer, Citation2003). In this way, muscle tissue becomes the most significant site of Se deposition in the form of selenomethionine when using organic Se dietary supplementation in animals. The intensive uptake of selenomethionine by muscle proteins is also very important for the increased transport of Se from hens to eggs and embryos, for the subsequent development of chicken immunocompetence, and for the overall health of the birds (Surai, Citation2000).
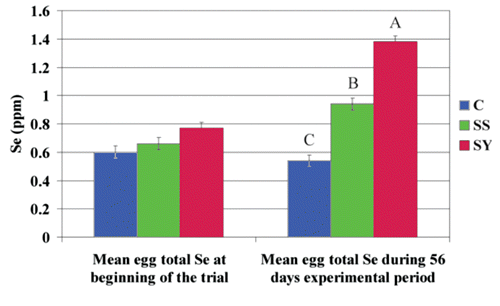
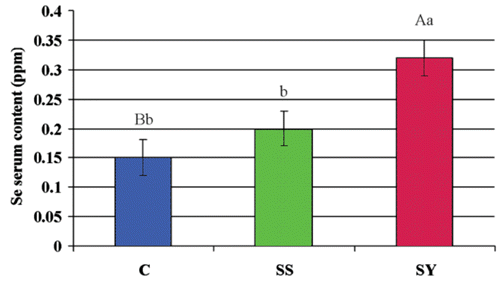
Table 1. Composition of the basal diet.
Table 2. Analysed content of selenium in the experimental diets.
Table 3. Performance of laying hens fed different sources of selenium.
Table 4. Eggshell quality of laying hens fed different sources of selenium.
Table 5. Selenium content of breast muscle, liver, skin, and kidneys in laying hens fed different sources of selenium.
Table 6. Blood metabolites of laying hens fed different sources of selenium.
Pan et al. (Citation2007) and Leeson et al. (Citation2008) reported that either SS or SY supplementation increased the Se concentration in hens’ liver, but this effect was higher when organic Se was used compared with inorganic Se. In agreement with these authors, we observed an increase in liver Se concentration when Se was supplemented, but the difference compared with non-supplemented hens was statistically significant only in SY-supplemented hens. The selenium concentration in skin follows the same pattern shown by the liver Se concentration. No recent data are available on the effect of the source of Se on Se content in skin: Scott and Thompson (Citation1971) reported an increase in Se content in the skin of chicks and poults when SS was added to a basal diet. Kidney selenium content was higher in hens fed a supplemented diet than in non-supplemented hens, but the difference achieved statistical significance only for SS-supplemented animals. Even if the difference between the Se concentration in kidney between SS- and SY-supplemented hens was not statistically significant, we can suppose that when the diet is supplemented with SS a greater excretion of Se occurs via the kidney, as argued by Pan et al. (Citation2007) who found a decrease in the Se concentration of kidneys when hens were fed SY compared with hens fed SS. This relationship may be explained by the fact that the kidneys contain abundant capillary vessels, and these capillaries are filled with blood. When selenium absorption from an inorganic Se source exceeds the nutritional or production need, excessive inorganic Se is excreted via the urinary route. On the other hand, reabsorbed Se-Met is captured in kidney capillaries and reenters whole-body metabolism via the bloodstream, and no urinary losses of Se in the form of selenomethionine occur.
Furthermore, kidney Se content reflects the amount of Se deposited in the kidneys, as well as the Se eliminated from the body via urine (Aspila, Citation1991; Mahan and Parrett, Citation1996). Within a few minutes, selenite absorbed from the gut is metabolised into selenide (H2Se) that forms non-specific bonds with plasma albumin (Suzuki and Itoh, Citation1997). After multiple recycling of Se via the selenide-to-selenite transformation pathway and its methylation, the surplus of inorganic Se is rapidly excreted via the urine. However, glomerular filtration of H2Se seems to be limited due to its albumin bond, and the rapid urinary elimination of Se of inorganic origin is another significant disadvantage in comparison to selenoamino acids (Boldizárová et al., Citation2001).
Conclusions
The present trial represents one of the few available reports of Se content in both egg and tissues studied at the same time in poultry. Results of our study indicate that supplementation with 0.4 ppm of Se from SS or SY does not affect hen performance or blood metabolites except for the increased weight of the egg obtained from hens supplemented with both sources of Se. On the other hand, organic Se, particularly in its organic form, improves eggshell quality. Specific selenium sources influence selenium distribution in hen tissues. Indeed, egg and breast muscle Se concentrations were higher when hens were fed selenium yeast because of the greater bioavailability of organic Se sources when compared with inorganic sources. Eggs and edible tissues enriched with selenium from organic Se sources in poultry diet could improve antioxidant status in humans and reduce possible Se deficiencyrelated diseases.
Acknowledgments
The authors wish to thank Dr. Eric Chevaux (Lallemand SAS, France) for his cooperation and providing yeast.
References
- AllainC.C.PoonL.S.ChanC.S.G.RichmondW.FuP.C. 1974. Enzymatic determination of total serum cholesterol. Clin. Chem. 20:470-475.
- AOAC,1995. Official Methods of Analysis. 16th ed. Association of official Analytical Chemists. Washington, DC, USA.
- ArnoldR.L.OlsonO.E.CarlsonC.W. 1973. Dietary selenium and arsenic additions and their effects on tissue and egg selenium. Poultry Sci. 52:847-854.
- ArthurJ.R. 2000. The glutathione peroxidases. Cell. Mol. Life Sci. 57:1825-1835.
- AspilaP. 1991. Metabolism of selenite, selenomethionine and feed-incorporated selenium in lactating goats and dairy-cows. J. Agr. Sci. Finland 63:9-73.
- BainM.M. 1990. Eggshell strength: a mechanical/ultrastructural evaluation. Degree Diss., Faculty of Veterinary Medicine, University of Glasgow, UK.
- BoldizárováK.FaixŠ.LengL. 2001. The effects of intravenous infusion of sodium selenite on the renal excretion of selenium in sheep. Page 64 in Proc. 19th Days on Animal Physiology, Košice, Slovakia.
- BowersG.N.Jr.McCombR.B. 1966. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin. Chem. 12:70-89.
- CalamariL.PetreraF.BertinG. 2010. Effects of either sodium selenite or Se yeast (Sc CNCM I-3060) supplementation on selenium status and milk characteristics in dairy cows. Livest. Sci. 128:154-165.
- CantorA.H.ScottM.L. 1974. The effect of selenium in the hen’s diet on egg production, hatchability, performance of progeny and selenium concentration in eggs. Poultry Sci. 53:1870-1880.
- CantorA.H.StrawM.L.FordM.J.PescatoreA.J.DunlapL.K. 2000. Effect of feeding organic selenium in diets of laying hens on egg selenium content. In: SimJ.S.NakaiS.GuenterW. ( eds.) Egg nutrition and biotechnology. CABI Publ., New York, NY, USA, pp 473-476.
- Centers for Disease Control, 1976. A proposed method for determining blood glucose using Hexokinase and glucose-6-Phosphate dehydrogenase. CDC Publ., Atlanta, GA, USA.
- ChantiratikulA.ChinrasriO.ChantiratikulP. 2008. Effect of sodium selenite and zincl-selenomethionine on performance and selenium concentrations in eggs of laying hens. Asian Aust. J. Anim. Sci. 21:1048-1052.
- čobanováK.PetrovičV.MellenM.ArpášovaH.GrešákováL.FaixS. 2011. Effects of dietary form of selenium on its distribution in eggs. Biol. Trace Elem. Res. 144:736-746.
- CombsG.F.CombsS.B. 1986. The role of selenium in nutrition. Academic Press Inc., Orlando, FL, USA.
- CozziG.PrevedelloP.StefaniA.L.PironA.ContieroB.LanteA.GottardoF.ChevauxE. 2011. Effect of dietary supplementation with different sources of selenium on growth response, selenium blood levels and meat quality of intensively finished Charolais young bulls. Animal 5:1531-1538.
- DanielsL.A. 1996. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 54:185-199.
- DavisR.H.FearJ.WintonA.C. 1996. Interactions between dietary selenium, copper and sodium nitroprusside, a source of cyanide in growing chicks and laying hens. Brit. Poultry Sci. 37:87-94.
- DoumasB.T.WatsonW.A.BiggsH.G. 1971. Albumin standards and measurements of serum albumin with bromocreasol green. Clin. Chim. Acta 31:87-96.
- DumontE.VanhaeckeF.CornelisR. 2006. Selenium speciation from food source to metabolites: a critical review. Anal. Bioanal. Chem. 385:1304-1323.
- European Communities Council, 1986. Council Directive of 24 november 1986 on the approximation of laws, regulations, and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes, 86/609/EEC. In: Official Journal, L 358, 18/12/1986, pp 1-28.
- FaureP. 2003. Protective effects of antioxidant micronutrients (vitamin E, zinc and selenium) in type 2 diabetes mellitus. Clin. Chem. Lab. Med. 41:995-998.
- GolubkinaN.A.PapazyanT.T. 2006. Selenium distribution in eggs of avian species. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 145:384-388.
- HenryR.J.ChiamoriN.Golub0.J.BerkmanS. 1960. Revised spectrophotometric methods for the determination of glutamic oxalacetic transaminase, glutamic pyruvic transaminase and lactic acid dehydrogenase. Am. J. Clin. Pathol. 34:381 -398.
- HillerA.PlazinJ.Van SlykeD.D. 1948. A study of conditions for Kjeldahl determination of nitrogen in protein: decription of methods with Mercury as catalyst, and titrimetric and gasometric measurements of the ammonia formed. J. Biol. Chem. 176:1401-1420.
- HoffmannP.R. 2008. Selenium and asthma: a complex relationship. Allergy 63:854-856.
- ISA SAS, 2005. Layer Management Guide ISA Warren by ISA. ISA SAS Publ., Saint-Brieuc, France.
- JiakuiL.XiaolongW. 2004. Effect of dietary organic versus inorganic selenium in laying hens on the productivity, selenium distribution in egg and selenium content in blood, liver and kidney. J. Trace Elem. Med. Biol. 18:65-68.
- JuniperD.T.PhippsR.H.BertinG. 2011. Effect of dietary supplementation with selenium-enriched yeast or sodium selenite on selenium tissue distribution and meat quality in commercial-line turkeys. Animal 5:1751-1760.
- KellyM.P.PowerR.F. 1995. Fractionation and identification of the major selenium containing compounds in selenized yeast. J. Dairy Sci. 78:237-242.
- KimY.Y.MahanD.C. 2001. Comparative effects of high dietary levels of organic and inorganic selenium on selenium toxicity of growing-finishing pigs. J. Anim. Sci. 79:942-948.
- KuricovaS.BoldizarovaK.GresakovaL.BobcekR.LevkutM.LengL. 2003. Chicken selenium status when fed a diet supplemented with Se-yeast. Acta Vet. Brno 72:339-346.
- LatshawJ.D.BiggertM.D. 1981. Incorporation of selenium into egg proteins after feeding selenomethionine or sodium selenite. Poultry Sci. 60:1309-1313.
- LeesonS.NamkungH.CastonL.DurosoyS.SchlegelP. 2008. Comparison of selenium levels and sources and dietary fat quality in diets for broiler breeders and layer hens. Poultry Sci. 87:2605-2612.
- MabeI.RappC.BainM.M.NysY. 2003. Supplementation of a corn-soybean meal diet with manganese, copper, and zinc from organic or inorganic sources improves eggshell quality in aged laying hens. Poultry Sci. 82:1903-1913.
- MachátJ.KanickyV.OtrubaV. 2002. Determination of selenium in blood serum by inductively coupled plasma atomic emission spectrometry with pneumatic nebulization. Anal. Bioanal. Chem. 372: 576-581.
- MahanD.C.ParrettN.A. 1996. Evaluating the efficacy of selenium-enriched yeast and sodium selenite on tissue selenium retention and serum glutathione peroxidase activity in grower and finisher swine. J. Anim Sci. 74:2967-2974.
- McKenzieR.C.RaffertyT.S.BeckettG.J. 1998. Selenium: an essential element for immune function. Immunol. Today 19:342-345.
- MoriL. 1978. Modified Jendrassik-Grof method for bilirubins adapted to the Abbott Bichromatic Analyzer. Clin. Chem. 1841-1845.
- Navarro-AlarcónM.López-MartínezM.C. 2000. Essentiality of selenium in the human body: Relationship with difference diseases. Sci. Total Environ. 249:347-371.
- OsterO.SchmiedelG.PrellwitzW. 1988. The organ distribution of selenium in German adults. Biol. Trace Elem. Res. 15:23-45.
- PagliaD.E.ValentineW.N. 1967. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 70:158-168.
- PanC.HuangK.ZhaoY.QinS.ChenF.HuQ. 2007. Effect of selenium source and level in hen’s diet on tissue selenium deposition and egg selenium concentrations. J. Agric. Food Chem. 55:1027-1032.
- PatonN.D.CantorA.H.PescatoreA.J.FordM.J. 2000. Effect of dietary selenium source and storage on internal quality and shell strength of eggs. Poultry Sci. 79(Suppl.1):116 (abstr.).
- PavloviZ.MiletiI.JokiŽ.PavlovskiZ.ŠkrbiZ.ŠobajiS. 2010. The effect of level and source of dietary selenium supplementation on eggshell quality. Biol. Trace Elem. Res. 133:197-202.
- PavloviZ.MiletiI.JokiŽ.ŠobajiS. 2009. The effect of dietary selenium source and level on hen production and egg selenium concentration. Biol. Trace Elem. Res. 131:263-270.
- PayneR.L.LavergneT.K.SouthernL.L. 2005. Effect of inorganic versus organic selenium on hen production and egg selenium concentration. Poultry Sci. 84:232-237.
- PetreraF.CalamariL.BertinG. 2009. Effect of either sodium selenite or Se-yeast supplementation on selenium status and milk characteristics in dairy goats. Small Ruminant Res. 82:130-138.
- PetrovičV.BoldizarovaK.FaixS.MellenM.ArpasovaH.LengL. 2006. Antioxidant and selenium status of laying hens fed with diets supplemented with selenite or Seyeast. J. Anim. Feed Sci. 15:435-444.
- PolatajkoA.BanasB.EncinarJ.R.SzpunarJ. 2005. Investigation of the recovery of selenomethionine from selenized yeast by two-dimensional LC-ICP MS. Anal. Bioanal. Chem. 381:844-849.
- PolatajkoA.Sliwka-KaszynskaM.DernovicsM.RuzikR.EncinarJ.R.SzpunarJ. 2004. A systematic approach to selenium speciation in selenized yeast. J. Anal. Atom. Spectrom. 19:114-120.
- RaymanM.P. 2000. The importance of selenium to human health. Lancet 365:233-241.
- RaymanM.P. 2005. Selenium in cancer prevention: a review of the evidence and mechanism of action. P. Nutr. Soc. 64:527-554.
- ReidG.M.TerritH. 1997. Sudden infant death syndrome and placental disorders: the thyroid-selenium link. Med. Hypotheses 48:317-324.
- ReillyC. 1998. Selenium: a new entrant into the functional food arena. Trends Food Sci. Tech. 9:114-118.
- RenemaR. 2004. Reproductive responses to Sel-Plex® organic selenium in male and female broiler breeders: impact on production traits and hatchability. pp 81-91 in Proc. 20th Alltech’s Annual Symp., Lexington, KY, USA.
- RizziL.BochicchioD.BargelliniA.ParazzaP.SimioliM. 2009. Effects of dietary microalgae, other lipid sources, inorganic selenium and iodine on yolk n-3 fatty acid composition, selenium content and quality of eggs in laying hens. J. Sci. Food Agr. 89:1775-1781.
- RotruckJ.T.PopeA.L.GantherH.E.SwansonA.B.HafemanD.G.HoekstraW.G. 1973. Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588-590.
- RutzF.PanE.A.XavierG.B.AnciutiM.A. 2003. Meeting selenium demands of modern poultry: Responses to Sel-Plex® organic selenium in broiler and breeder diets. pp 147-161 in Proc. 19th Alltech’s Annual Symp., Nottingham, UK.
- SAS, 2006. Statistics User’s Guide, ver.9.1. SAS Inst. Inc., Cary, NC, USA.
- ScheidelerS.E.WeberP.MonsalveD. 2011. Supplemental vitamin E and selenium effects on egg production, egg quality, and egg deposition of α-tocopherol and selenium. J. Appl. Poultry Res. 19:354-360.
- SchrauzerG.N. 2003. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 47:73-112.
- SchrauzerG.N. 2006. Selenium yeast: composition, quality, analysis, and safety. Pure Appl. Chem. 78:105-109.
- ScottM.L.ThompsomJ.N. 1971. Selenium content of feedstuffs and effects of dietary selenium levels upon tissue selenium in chicks and poults. Poultry Sci. 50:1742-1748.
- SiskeV.ZemanL.KleckerD. 2000. The egg shell: a case study in improving quality by altering mineral metabolism-naturally. Page 327 in Proc. 16th Alltech’s Annual Symp., Nottingham, UK.
- SkrivanM.SimaneJ.DlouhaG.DouchaJ. 2006. Effect of dietary sodium selenite, Seenriched yeast and Se-enriched Chlorella on egg Se concentration, physical parameters of eggs and laying hen production. Czech J. Anim. Sci. 51:163-167.
- SuraiP.F. 2000. Effect of selenium and vitamin E content of the maternal diet on the antioxidant system of the yolk and the developing chick. Brit. Poultry Sci. 41:235-243.
- SuraiP.F. 2006. Selenium absorption and metabolism. In: SuraiP.F. ( ed.) Selenium nutrition and health, Nottingham University Press, Nottingham, UK, pp 161-171.
- SuzukiK.T.ItohM. 1997. Metabolism of selenite labelled with enriched stable isotope in the bloodstream. J. Chromatogr. Biomed. 692:15-22.
- SuzukiK.T.OgraY. 2002. Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit. Contam. 19:974-983.
- TamG.K.LacroixG. 1982. Dry ashing, hydride generation atomic absorption spectrometric determination of arsenic and selenium in foods. J. Assoc. Off. Ana. Chem. 65:647-650.
- ToyodaH.HimenoS.ImuraN. 1990. Regulation of glutathione peroxidase mRNA level by dietary selenium manipulation. Biochim. Biophys. Acta 1049:213-215.
- TufarelliV.LaudadioV. 2011. Dietary supplementation with selenium and vitamin E improves milk yield, composition and rheological properties of dairy Jonica goats. J. Dairy Res. 78:144-148.
- UtterbackP.L.ParsonsC.M.YoonI.ButlerJ. 2005. Effect of supplementing selenium yeast in diets of laying hens on egg selenium content. Poultry Sci. 84:1900-1901.
- WeissW.P. 2003. Selenium nutrition of dairy cows: comparing responses to organic and inorganic selenium forms. pp 333-343 in Proc. 19th Alltech Annual Symp., Nottingham, UK.
- WuR.ZhanX.WangY.ZhangX.WangM.YuanD. 2011. Effect of different selenomethionine forms and levels on performance of breeder hens and Se distribution of tissue and egg inclusion. Biol. Trace Elem. Res. 143:923-931.
- YoonI.WernerT.M.ButlerJ.M. 2007. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poultry Sci. 86:727-730.
- YoshidaM.SugiharaS.SuenagaT.NaitoC.FukunagaK.TsuchitaH. 2002. Digestibility and chemical species of selenium contained in high-selenium yeast. J. Nutr. Sci. Vitaminol. 48:401-404.
- YoshizawaK.AscherioA.MorrisJ.S.StampferM.J.GiovannucciE.BaskettC.K. 2003. Prospective study of selenium levels in toenails and risk of coronary heart disease in men. Am. J. Epidemiol. 158:852-860.
- ZhouX.WangY. 2011. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poultry Sci. 90:680-686.
- ZuberbuehlerC.A.MessikommerR.E.ArnoldM.M.ForrerR.S.WenkC. 2006. Effects of selenium depletion and selenium repletion by choice feeding on selenium status of young and old laying hens. Physiol. Behav. 87:430-440.