Abstract
In the present study, cytotoxic effects of the polycyclic aromatic hydrocarbons benzo[a] pyrene (B[a]P) were investigated in Sparus aurata hepatocytes primary cultures after acute and chronic exposure. Cells were treated with a wide range of B[a]P doses (1 pg/mL to 100 μg/mL) for 24, 48 and 72 h. B[a]P toxicity was quantified in sea bream hepatocytes by MTT assay and immunofluorescence analysis of apoptosis after the various exposure periods, in order to evaluate the hepatic damage and toxicity range. Results showed three cytotoxic responses: B[a]P cell death for primary necrosis after exposure to high concentrations for short times, apoptosis induction with the use of sublethal doses and cell proliferation allied with neoplastic foci formation after exposure to low concentrations for long times. This responses provided an interesting correlation between the damage caused on hepatocytes and the metabolism of this toxic compound, to date mainly studied in vivo. Additionally, the statistical analysis revealed that the effects of time and dose were significant for both parameters and especially the time was extremely significant (P<0.0001), in fact B[a]P induced damage that increased over time. Our findings demonstrated and confirmed that S. aurata is a very sensitive species to B[a]P exposure since adverse effects were found at all tested doses. Furthermore, the new in vitro animal model can be considered a useful tool for studying the cellular effects induced by any contaminant harmful for farmed fish.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are a vast class of organic contaminants formed by two or more benzene rings fused together. This compounds are widely spread in the environment, deriving mainly from the process of incomplete combustion of organic material (coal, wood, oil products, waste) (Neff, Citation2002). Benzo[a]pyrene (B[a]P) is classified as probable human carcinogen (IARC, Citation1989), and belongs to the sixteen PAHs considered as priority pollutants by US Environmental Protection Agency (EPA) and World Health Organization (WHO) (EPA, Citation1987). The greater PAHs contribution to the marine environment originates mostly from human and industrial activities or accidental oil spills, thus they appear principally in urbanized coastal areas (Rodriguez-Ariza et al., Citation1993; Cortazar et al., Citation2008). Due to their hydrophobic nature and the high affinity for organic carbon, PAHs tend to be rapidly adsorbed to particulate, especially with the increase in molecular weight. This characteristic determines their bioavailability to fish and other marine organisms through the food chain, sediments and waterborne exposure (Liang et al., Citation2007; Perugini et al., Citation2007). The Mediterranean basin is by now considered one of the areas at high risk of hydrocarbons pollution being a half-closed basin with limited water exchange subjected to an elevated marine traffic (Galgani et al., Citation2011). In the later years of the 20th century, development of marine aquaculture of sea bream and sea bass, both land-based and in sea floating cages, is raised all over the Mediterranean, in order to meet the soaring global demand for seafood (Basurco, Citation2000; Tacon and Halwart, Citation2007). As a consequence marine farming industry has to face several problems such as environmental concerns and bioaccumulation of various chemicals by fish grown in polluted waters (FAO, Citation2008). Additionally, farmed fish can be exposed to PAHs contaminated feed as raw materials used for the production of fish oil and fish meal can be a possible source of PAHs (Hellou et al., Citation2005). Similarly, the recent replacement of fishmeal by vegetable oils in aquafeed preparation can be a further source of PAHs contamination (Moret and Conte, Citation2000). PAHs exert their toxicity following biotransformation to toxic metabolites, which can be bound covalently to DNA, RNA and proteins causing cell damage, mutagenesis, teratogenesis and carcinogenesis, particularly in the liver (Tuvikene, Citation1995; Xu et al., Citation2011). Although several in vivo studies have been focused on the hepatic metabolism, adduct formation and erythrocytic genotoxic responses to PAHs in sea bass and gilthead sea bream as they are considered very sensitive species (Lamaire et al., Citation1990, Citation1992; Rodriguez-Ariza et al., Citation1999; Gravato et al., Citation2000; Gravato and Santos, Citation2002, Citation2003; Ortiz-Delgado and Sarasquete, Citation2004; Banni et al., Citation2009), no data are available in literature on the toxic effect of benzo[a]pyrene in Sparus aurata hepatocytes. Consequently, this work was carried out to overcome this lack applying an innovative in vitro animal model as S. aurata hepatocytes in primary culture, as required by the latest European directives on replacement of in vivo studies (EC, Citation2006). Primary cultured hepatocytes keep most of their original in vivo properties and therefore facilitate the extrapolation of the results in vivo (Chen and Bunce, Citation2003). Additionally, the liver has the higher level of PAHs concentration because is the receiving organ of the enterohepatic circle. The new in vitro liver model resulted metabolically active and previous studies have successfully used it to determine the toxicity of feed contaminants in gilthead sea bream (Santacroce et al., Citation2011a, Citation2011b, Citation2011c). The main objectives of the present study were to evaluate the cytotoxic effects induced in vitro by benzo[a]pyrene on Sparus aurata hepatocytes in primary culture after short and long exposure times, to screen the sensitivity of these cells and determine the hepatic damage and toxicity range.
Materials and methods
Preparation of cultured S. aurata hepatocytes
For hepatocyte culture, 45 Sparus aurata juveniles of 25-30 g body weight were obtained from a local fish farm (Panittica Pugliese, Brindisi, Italy). They were kept in oversaturated natural seawater (O2 at 10 mg/L, 37.5‰ salinity) holding tanks at a temperature of 18±1°C with a density of 10 kg/m3 and transferred to the laboratory. Fish were anaesthetized by immersion in a tank with seawater plus tricaine methane sulphonate (MS 222, 0.02%; Sigma Aldrich Ltd, Milano, Italy) and then decapitated. The isolation and primary culture of S. aurata hepatocytes were aseptically carried out in accordance with the method of Centoducati et al. (Citation2009). Briefly, livers from all fish were carefully excised from the abdominal cavity, transferred onto a glass petri dish and rinsed with a washing buffer (Hank’s Balanced Salt Solution without Ca2+ and Mg2+, supplemented with 10 mM HEPES, 0.5 mM EDTA, 25 mM NaHCO3, 200 U/mL penicillin, 200 μg/mL streptomycin, 200 μg/mL amphotericin B and 100 μg/mL gentamicin). Livers were then mashed through a stainless-steel sieve 380 μm mesh and digested for 20 min at 20°C with a digestion medium (7 mM CaCl2, 200 U/mL penicillin, 200 μg/mL streptomycin, 200 μg/ml amphotericin B, 100 μg/ml gentamicin, 10 mM HEPES, 25 mM NaHCO3 in Leibovitz’s L-15) supplemented with a cocktail of four enzymes (0.1% collagenase type IV, 0.05% hyaluronidase type IV-S, 0.4% dispase type II, 0.03% DNase type I). The resulting cell suspension was filtered throughout 230 μm and 104 μm stainless-steel filters, transferred to a sterilized tube (50 mL, Falcon, BD Biosciences, Franklin Lakes, NJ, USA) and washed twice by centrifugation in cold 1× phosphate-buffered saline (PBS) at 80 g for 5 min at 4 °C. After the last wash, the cell pellet was re-suspended in Leibovitz’s L-15 culture medium supplemented with 2 mM glutamine, 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL amphotericine, 50 μg/mL gentamycin, 1 mM Na pyruvate, 5 mM d-glucose, 10 mM HEPES, 12 mM NaHCO3, 0.05% ITS plus, 0.01 mM MEM non-essential amino acid (BioWhittaker), 0.01 mM MEM-vitamin mix (BioWhittaker), 0.1 mM ascorbic acid, 0.01 μg/mL epidermal growth factor (EGF) and 0.005 μg/mL hepatocyte growth factor (HGF). Based on previous results, cells were counted using a haemocytometer and then seeded at a density of 3×104 cell/cm2 in 96-well plates precoated with collagen I and cultivated in a refrigerated incubator (3% CO2) at 18°C. Cells were left to adhere for 12 h, then fresh medium was replaced every 48 h. Before treatments, cells were allowed to complete monolayer formation for four days after seeding. Unless specified, all chemicals were purchased from Sigma Aldrich Ltd, Milano, Italy.
Cell culture treatments
On the fifth day after seeding, confluent monolayers were incubated for 24, 48 and 72 h with 100 μL/well of new culture medium (L-15) containing serial dilutions of B[a]P (Sigma). The working solutions ranged from 1 pg/mL to 100 μg/m and eleven concentrations were tested. Each exposure dose included control wells were repeated in triplicates as well as the all experiment. At the end of each incubation period with B[a]P (24, 48, and 72 h), the medium was removed and cells were rapidly observed by inverted contrast-phase microscope for morphological alterations in order to examine survival of S. aurata hepatocytes.
Cytotoxicity assessment
The monotetrazolium (MTT) assay was used to determine the cytotoxicity of B[a]P on S. aurata hepatocytes primary culture. This assay measures the activity of the mitochondrial dehydrogenase enzyme that reduces MTT to formazan dyes, giving a purple color and allowing to assess the viability and the proliferation of cells. The tetrazolium MTT test was performed according to the method of Mosmann (Citation1983). Briefly, following treatments with B[a]P, the media was removed from each culture plate, and cells were washed with sterile PBS. Cell cultures were incubated for 4 h at room temperature with MTT (Sigma) Nutrient Mixture F-12 Ham (Ham’s F-12, Sigma), at a final concentration of 0.5 mg/mL. The medium was then replaced by a buffer solution for formazan solubilization, before reading absorbance at 570 nm using a Spectrafluor microplate reader (Bio-Rad). The ratio between the optical density of treated cultures and the optical density of untreated cultures leads to quantify the percentage of vitality.
Immunofluorescence analysis for the detection of apoptosis and cell proliferation
In order to detect apoptotic cell death after B[a]P treatments, annexin V-Cy3 apoptosis detection kit (Sigma) was used following manufacturer’s instructions. In brief, after 24, 48 and 72 h of B[a]P exposure, cell cultures were washed with 1x Binding Buffer (10 mM HEPES, pH 7.5, containing 140 mM NaCl and 2.5 mM CaCl2) and successively incubated with double label staining solution (1 mg/ml annexin V-Cy3 and 500 mM 6-carboxyfluorescein diacetate (6-CFDA) in 1x Bindin Buffer) for 15 min at room temperature. After staining, cells were washed with 1x Binding Buffer to remove excess label. Microscopy analysis was performed, for brightfield and fluorescence, by a Motic AE31 Epi-Fluorescent Inverted Microscope, equipped with DAPI/TRITC/FITC fluorescence filter cube set and a Moticam 3000C Cooled CCD digital color camera (Motic, Seneco, Milano, Italy). The Cy3 labeled annexin V binds exposed phosphatidylserine (PS) on cells undergoing the early stages of apoptosis in fluorescence microscopy, while the non-fluorescent compound 6-CFDA, enters the cell and is hydrolyzed by the esterases present in living cells to the fluorescent compound 6-carboxyfluorescein, indicating that the cells are viable. This combination allows the differentiation among early apoptotic cells (annexin V positive, 6-CFDA positive), necrotic cells (annexin V positive, 6-CFDA negative), and viable cells (annexin V negative, 6-CFDA positive). Additionally, tumorigenesis was evaluated by the proliferating cell nuclear antigen (PCNA) labeling (Sigma) in indirect immunofluorescence, an intranuclear protein cell cycle dependent considered as marker of DNA synthesis.
Statistical analysis
Statistical analysis was carried out using the two-way analysis of variance (ANOVA) with Bonferroni as post-hoc test. For each exposure time, the IC50 (half maximal inhibitory concentration) values based on the results of MTT assay, reflecting the 50% of inhibition cell viability, were determined by the GraphPad Prism 5.0 using an Hill function non-linear regression analysis. Data represented the arithmetic mean ± standard deviation of at least three independent experiments. Differences were considered statistically significant at P≤0.05.
Results
In order to evaluate the susceptibility of S. aurata hepatocytes in primary culture upon B[a]P treatment, we tested in vitro a wide range of concentrations miming short and long exposure times. At the end of each period of treatment, cytotoxicity assessment, morphological and immunocytochemical analysis were performed. The dose-response curves obtained in the MTT assay after exposure of cells to decreasing B[a]P concentrations for 24, 48 and 72 h, showed the presence of three distinct cytotoxic responses and revealed a dose and time dependent cytotoxic effect (). Particularly, in the range of concentrations between 100 μg/mL and 1 μg/mL, the doseresponse curves showed a cell viability lower than 50% after 24 h of exposure, resulting decreased and reaching its plateau after 48 h. This lethality effect was confirmed by contrastphase and immunocytochemical analysis, where loss of monolayer and cell membrane integrity for primary necrosis were revealed by annexin V positive staining of hepatocytes (,). After 72 h, cell death passed from a value of 50% to 20% in almost all the concentrations taken into account in this range, till reaching values of controls (100% of viable cells) at the dose of 0.1 μg/mL (). As a result, this concentration defined the boundary between B[a]P lethal effects and cell proliferation. In fact, the immunofluorescence analysis registered an increase of cell viability (annexin V- and 6-CFDA+ staining) paralleled with signs of early induction of apoptosis in correspondence of the small nests of actively proliferating hepatocytes (annexin V+, 6-CFDA+; early apoptotic cells yellow-orange coloured) (,). In the range of doses between 1 μg/mL and 1 ng/mL after 24 h of exposure, cultures showed signs of damage as cell shrinkage and presence of vacuolar degeneration. Immunofluorescence analysis revealed a decrease of necrotic cells along with an increase of vitality, presence of delayed apoptosis (cells red-orange coloured) and the appearance of early apoptotic cells at dose of 1 ng/mL (,). After 72 h, a progressive increase of uncontrolled cell proliferation was observed in correspondence of the small nests of actively proliferating hepatocytes till the maximum level registered at the concentration of 1 ng/mL ( and ). At this dosage, the mitotic index calculated by the number of mitotic cell positives using immunofluorescent labeling of DNA (PCNA antigen +) revealed the formation of neoplastic foci (). Additionally, cell cultures demonstrating an high mitotic index, highlighted by contrastphase analysis and known as progenitor oval cells, showed signs of early apoptotic induction (,). In the last range of concentrations (from 1 ng/mL to 1 pg/mL) identified by dose-response curves, although after 24 h of exposure cell viability grown with the decrease of B[a]P concentrations, reaming upper than 70%, immunofluorescence analysis revealed signs of apoptosis and cell damage (). Prolonged exposure times induced uncontrolled cell proliferation and an increase in cell viability (annexin V-,6-CFDA+; living cells green), even though the cell proliferation resulted less sustained than previous concentration range as shown in the dose-response curves ( and ). Cell viability expressed as IC50 was found to be 10 μg/mL in MTT assay (), outlining the threshold dose that allows to distinguish acute lethal effects (massive cell death for primary necrosis) from subcytotoxic effects (apoptosis induction and cell proliferation). Statistical analysis showed that B[a]P exhibited a dose and time dependent cytotoxic effect (). The two parameters were calculated using a two way analysis of variance (ANOVA) with Bonferroni as post-hoc test. Calculations revealed that the effects of time and dose were significant for both parameters and especially the time was extremely significant (P<0.0001) (). Comparing the mean of each treatment time by Bonferroni post-hoc test, all the differences between the three incubation periods resulted significant and particularly the comparison between 24 and 72 h exposure (P=0.05). The interactions among time and dose were significant at concentrations of 0.01 μg/mL and 1 ng/mL, in correspondence of cell proliferation peak.
Discussion
Our findings demonstrated that S. aurata is a very sensitive species to B[a]P exposure since adverse effects were found at all tested doses. Particularly, cells treatment with the highest concentrations of B[a]P led to a significant cell death for primary necrosis within 24 h. This early lethal effect agrees with the B[a]P metabolism studied in vivo in Teleosts by several authors. Banni et al.(Citation2009) found that liver uptake of B[a]P after intraperitoneal injection of S. aurata shows a bifasic behaviour reaching the maximum level after 6 h, followed by a decreasing phase till 24 h and a subsequent new increase.
On the contrary, the data showed a pronounced Phase I biotrasformation, measured as EROD activity, when liver B[a]P uptake was minimal (after 24 h) suggesting a maximum B[a]P liver metabolism after only 24 h. Such results demonstrate a strong liver induction of Phase I enzymes (mediated by cytochrome P450-dependent monooxygenases) involved in the metabolism of B[a]P. Since their activity is evident only few hours after the exposure and reaches the plateau within 24 h (Banni et al., Citation2009). The early activation hepatic enzymes in sea bream after B[a]P treatment is also confirmed in other in vivo studies (Viarengo et al.,Citation1997; Gravato and Santos, Citation2002). Additionally, investigations carried out in vivo on Dicentrarchus labrax by Gravato and Santos (Citation2003) reported a time dependent liver EROD activity decrease after exposures longer than 4 h along with an increase of B[a]P toxic metabolites in the cytosol. Despite authors found liver Phase II conjugation, measured as GST activity, significantly increased till 8 h, the presence of liver cytosolic B[a]P-type metabolites after short term exposure to B[a]P suggested a failure of Phase II system. All these data can explain the possible correlation between B[a]P metabolism and hepatic lesions. In fact, the significant liver damage caused on S. aurata hepatocytes in primary culture within 24 h of exposure to high concentrations can be due either to the high content of B[a]P reactive metabolites in the cytosol that in turn influence the Phase I activity enzymes, or to impaired Phase II biotrasformation allied with Phase II metabolite saturation and/or conjugation exhaustion. Consequently, prevalence of B[a]P expoxide metabolites accumulated in the cytosol leads to the formation of covalent adducts with DNA in fish as described in literature, exhibiting the genotoxic potential of this compound (Mitchelmore and Chipman, Citation1998; Woo et al., Citation2006; Wessel et al., Citation2010). In our findings, the lethal effect caused by B[a]P exposure significantly decreased after 24 h when low concentrations were applied, but hepatocytes showed signs of damage and immunocytochemical analysis revealed induction of apoptosis. Additionally, after 72 h of exposure, cell cultures exhibited an increase in cell proliferation in correspondence of actively proliferating hepatocytes (oval cells) till the maximum level registered at the concentration of 1 ng/mL, revealing the formation of neoplastic foci. Such results can be related to adduct formation that causes DNA strand breaks leading either to cell death for apoptosis activation, or neoplastic transformation due to somatic mutation. Trosko and Upham (Citation2005) have indicated the proliferating progenitor cells in rat liver as the sensitive target of genotoxic compounds like B[a]P. Furthermore, this cell population extensively proliferates in response to diverse chemical carcinogens playing a significant role in hepatocarcinogenesis (Alison and Lowell, Citation2005; Roskams, Citation2006). As confirmed by our analysis, B[a]P damage increased over time in accordance with a study carried out in vitro on cultured primary rainbow trout hepatocytes treated with low B[a]P concentrations for various intervals of time where extensive time-dependent covalent binding to cellular DNA occurred (Tsuji and Walle, Citation2007).
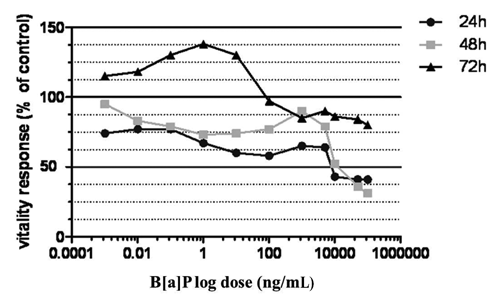
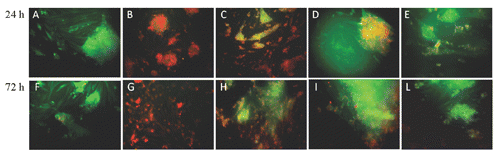
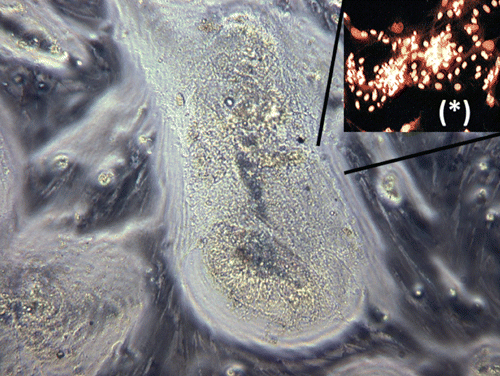
Table 1. Statistical analysis of variance; two way ANOVA, followed by Bonferroni’s multiple comparison post-hoc test.
Conclusions
Benzo[a]pyrene is one of the most genotoxic compound that is readily absorbed by fish during exposure to contaminated food, water and sediments. The cytotoxic responses of S. aurata hepatocytes in primary culture to acute and chronic exposure to a wide range of B[a]P concentrations provided an interesting correlation between the damage caused on hepatocytes and the metabolism of this toxic compound, mainly studied in vivo. The innovative in vitro animal model used simulated the biological response of S. aurata, providing useful information on the susceptibility of this species towards B[a]P. Since adverse effects were found at all tested doses, the possible interaction between this toxic compound and farmed fish should be taken into account by aquaculture producers and any source of contamination should be taken under strict control in order to guarantee a product free of residues and suitable to human consumption.
References
- AlisonM. R.LovellM. J. 2005. Liver cancer: the role of stem cells. Cell. Prolif. 38:407-421.
- BanniM.BouraouiZ.GhediraJ.ClerandeauC.GuerbejH.NarbonneJ. F.BoussettaH. 2009. Acute effects of benzo[a]pyrene on liver phase I and II enzymes, and DNA damage on sea bream Sparus aurata. Fish. Physiol. Biochem. 35:293-299.
- BasurcoB. 2000. Offshore mariculture. CIHEAM-IAMZ Publ. N. 30, Zaragoza, Spain. Available from: http://ressources. ciheam.org/om/pdf/b30/00600643.pdf
- CentoducatiG.SantacroceM.P.ConversanoM.C.CrescenzoG. 2009. Biotechnological process for isolation of hepatocytes from marine organisms. Bulletin 317 2009/39. Espace Publication No. EP2103686 (A1), European Patent Office, Munchen, Germany.
- ChenG.BunceN.J. 2003. Polybrominated diphenyl ethers as ah receptor agonists and antagonists. Toxicol. Sci. 76:310-320.
- CortazarE.BartoloméL.ArrasateS.UsobiagaA.RaposoJ.C.ZuloagaO.EtxebarriaN. 2008. Distribution and bioaccumulation of PAHs in the UNESCO protected natural reserve of Urdaibai, Bay of Biscay. Chemosphere 72:1467-1474.
- Environmental Protection Agency, 1987. Quality criteria for water. EPA no. 440/5-86-001. US Environmental Protection Agency Publ., Washington, DC, USA.
- European Commission, 2006. Regulation No. 1907/2006 of the European parliament and of the council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). In: Official Journal, L 396, 30/12/2006, pp 1-849.
- FAO. 2008. Opportunities for addressing the challenges in meeting the rising global demand for food fish from aquaculture. Committee on Fisheries, Sub-committee on Aquaculture, 4th Session. Puerto Varas, Chile. FAO Publ., Roma, Italy.
- GalganiF.Martínez-GómezbC.GiovanardicF.RomanellicG.CaixachdJ.CentoA.ScarpatocA.BenBrahimfS.MessaoudigS.DeuderohS.BoulahdidiM.BenedictobJ.AndralaB. 2011. Assessment of polycyclic aromatic hydrocarbon concentrations in mussels (Mytilus galloprovincialis) from the Western basin of the Mediterranean Sea. Environ. Monit. Assess. 172:301-317.
- GravatoC.SantosM.A. 2002. Juvenile sea bass liver P450, EROD induction and erythrocytic genotoxic responses to PAH and PAH-like compounds. Ecotox. Environ. Safe. 51:115-127.
- GravatoC.SantosM.A. 2003. Genotoxicity biomarkers’association with B[a]P biotransformation in Dicentrarchus labrax L. Ecotox. Environ. Safe. 55:352-358.
- GravatoC.SantosM.A.MagalhaesI. 2000. Juvenile Dicentrarchus labrax L. biochemical and genotoxic responses after short term exposure to b-naphthoflavone and contaminated harbor waters. Fresen. Environ. Bull. 9:269-274.
- HellouJ.HayaK.StellerS.BurridgeL. 2005. Presence and distribution of PAHs, PCBs and DDE in feed and sediments under salmon aquaculture cages in the Bay of Fundy, New Brunswick, Canada. Aquat. Conserv. 15: 349-365.
- International Agency for Research of Cancer, 1989. Diesel and Gasoline Engine Exhausts and some Nitroarenes. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to humans, N. 46, IARC Publ., Lyon, France.
- LemaireP.BerhautJ.Lemaire-GonyS.LafaurieM. 1992. Ultrastructural changes induced by benzo[a]pyrene in sea bass (Dicentrarchus labrax) liver and intestine: importance of the intoxication route. Environ. Res. 57:59-72.
- LemaireP.MathieuA.CarriereS.DraiP.GiudicelliJ.LafaurieM. 1990. The uptake metabolism and biological halflife of benzo(a)pyrene in different tissues of sea-bass, Dicentrarchus labrax. Ecotox. Environ. Safe. 20:223-233.
- LiangY.TseM.F.YoungL.WongM.H. 2007. Distribution patterns of polycyclic aromatic hydrocarbons (PAHs) in the sediments and fish at Mai Po Marshes Nature Reserve, Hong Kong. Water Res. 41:1303-1311.
- MitchelmoreC.L.ChipmanJ.K. 1998. Detection of DNA strand breaks in brown trout (Salmo trutta) hepatocytes and blood cells using the single cell gel electrophoresis (comet) assay. Aquat.Toxicol. 41:161-182.
- MoretS.ConteL.S. 2000. Polycyclic aromatic hydrocarbons in edible fats and oils: occurrence and analytical methods. J. Chromatogr. A 882:245-253.
- MosmannT. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63.
- NeffJ. M. 2002. Bioaccumulation in Marine Organisms - Effects of Contaminants from Oil Well Produced Water. Elsevier Publ., Amsterdam, The Netherlands.
- Ortiz-DelgadoJ.B.SarasqueteC. 2004. Toxicity, histopathological alterations and immunohistochemical CYP1A induction in the early life stages of the sea bream, Sparus aurata, following waterborne exposure to B(a)P and TCDD. J. Mol. Histol. 35:29-45.
- PeruginiM.ViscianoP.GiammarinoA.ManeraM.Di NardoW.AmorenaM. 2007. Polycyclic aromatic hydrocarbons in marine organisms from the Adriatic Sea, Italy. Chemosphere 66:1904-1910.
- Rodriguez-ArizaA.Dıaz-MendezF.M.NavasJ.I.PueyoC.Lopez-BareaJ. 1999. Metabolic activation of carcinogenic aromatic amines by fish exposed to environmental pollutants. Environ. Mol. Mutagen. 25:50-57.
- Rodriguez-ArizaA.PeinadoJ.PueyoC.Lopez-BereaJ. 1993. Biochemical indicators of oxidative stress in fish from polluted littoral areas. Can. J. Aquat. Sci. 50: 2568-2573.
- RoskamsT. 2006. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma Oncogene 25: 3818-3822.
- SantacroceM.P.NarracciM.AcquavivaM.I.CavalloR.A.ZacchinoV.CentoducatiG. 2011a. New development in Aflatoxin research: from aquafeed to marine cells. Chapter 12. In: Torres-PachecoI. ( ed.) Aflatoxins - Detection, Measurement and Control. InTech On-line Publ., pp 209-234.
- SantacroceM.P.NarracciM.AcquavivaM.I.ZacchinoV.Lo NoceR.CentoducatiG.CavalloR.A. 2011b. Effects induced in vitro by aflatoxin B1 on Vibrio fischeri and on primary cultures of Sparus aurata hepatocytes. Chem. Ecol. 27:67-76.
- SantacroceM.P.ZacchinoV.CasalinoE.MerraE.TateoA.De PaloP.CrescenzoG.CentoducatiG. 2011c. Expression of a highly differentiated phenotype and hepatic functionality markers in gilthead sea bream (Sparus aurata L.) long-cultured hepatocytes: first morphological and functional in vitro characterization. Rev. Fish Biol. Fisher. 21:571-590.
- TaconA.G.J.HalwartM. 2007. Cage aquaculture: a global review. In: HalwartM.D.S.ArthurJ.R. ( eds.) Cage aquaculture: regional views and global review. FAO Fish.Tech. Paper No. 498, FAO Publ., Roma, Italy, pp 1-16.
- TroskoJ.E.UphamB.L. 2005. The emperor wears no clothes in the field of carcinogen risk assessment: ignored concepts in cancer risk assessment. Mutagenesis 20:81-92.
- TsujiP.A.WalleT. 2007. Benzo(a)pyreneinduced cytochrome P450 1A and DNA binding in cultured trout hepatocytes-Inhibition by plant polyphenols. Chem.-Biol. Interact. 169:25-31.
- TuvikeneA. 1995. Responses of fish to polycyclic aromatic hydrocarbons (PAHs). Ann. Zool. Fenn. 32:295-309.
- ViarengoA.BettellaEFabbriR.BurlandoB.LafaurieM. 1997. Heavy metal inhibition of EROD activity in liver microsomes from the bass Dicentrarchus labrax exposed to organic xenobiotics: role of GSH in the reduction of heavy metal effects. Mar. Environ. Res. 43:1-11.
- XuX.W.HuangS.ScholzM.DongY. 2011. Remediation of polycyclic aromatic hydrocarbons: a review. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 6:1-9.
- WesselN.MenardD.Pichavant-RafiniK.OllivierH.Le GoffJ.M.BurgeotT.AkchaF. 2010. The toxicity of benzo [a]pyrene on sole (Solea solea) hepatocytes: assessment of genotoxic and enzymatic effects. Polycycl. Aromat. Comp. 30:346-354.
- WooS.KimS.YumS.YimH.U.LeeT.K. 2006. Comet assay for the detection of genotoxicity in blood cells of flounder (Paralichthys olivaceus) exposed to sediments and polycyclic aromatic hydrocarbons. Mar. Pollut. Bull. 52:1768-1775.