Abstract
The objective of this study was to quantify lactoferrin (Lfe) in buffalo milk and to examine the factors affecting milk Lfe, such as the lactation stage, daily milk yield, parity, and milk somatic cells count (SCC). Milk Lfe concentration was detected by the SDS-polyacrilamide gel electrophoresis (SDS-PAGE). The overall mean of Lfe concentration was 0.332±0.165 g/kg and ranged from 0.030 to 0.813 g/kg. Milk Lfe concentrations increased (P<0.01) with the increase of days in milk, but it was not affected by parity. Milk Lfe concentration was significantly affected by SCC. The differences became significant when the levels of SCC increased up to 200,000/mL. This is the first investigation on the levels of Lfe in buffalo milk in reference to daily milk production, lactation stage, parity and SCC. Further studies are needed to clarify the relationship between Lfe and SCC in buffalo milk.
Keywords:
Introduction
Lactoferrin (Lfe), an iron-binding glycoprotein, is present in milk and synthesized by specific granules of polymorphonuclear leukocytes (Lonnerdal et al., Citation1995) and by glandular epithelial cells (Baynes and Bezwoda, Citation1994). The concentration of the protein during colostrogenesis, lactation and mammary gland involution are well documented in dairy cows (Smith et al., Citation1977; Newman et al., Citation2009). Lactoferrin plays a key role in the defense mechanisms of the mammary gland, contributing to the prevention of microbiological infection diseases (Smith et al., Citation1977; Kutila et al., Citation2003; Lee et al., Citation2004). Lactoferrin also may limit the oxidative degeneration of cellular components that can occur during periods of tissue disruption such as during inflammation and involution of mammary gland. In bovine, Lfe concentration in milk was significantly associated to somatic cells count (SCC), levels of bovine serum albumin (BSA), stage of lactation, and milk yield (Harmon et al., Citation1975, Liu et al., Citation2010).
A quite wide range of Lfe concentration has been determined in bovine milk. The value varies from 1.15 μg/mL to 485.63 μg/mL in milk from healthy animals (Hagiwara et al., Citation2003). Lactoferrin concentration is high in colostrum, varying between 1 and 5 mg/mL, and dry secretions, varying between 20 and 30 mg/mL (Welty et al., Citation1976; Stelwagen et al., Citation2009).
Most of the existing studies on the Lfe have been carried out on bovine milk, and available information is very scarce for buffalo milk. Therefore, the main objective of this study was to quantify Lfe concentrations in buffalo milk and to examine the factors affecting milk Lfe, such as the lactation stage, daily milk production, parity, and milk SCC.
Materials and methods
Dataset
The study was carried out in fifteen dairy buffalo farms in the south part of Lazio region. Dairy farms were homogeneous in terms of the production system adopted (intensive) and the barn design and management (total confinement free barn housing with no time at pasture, TMR feeding practices and animals were milked twice a day with pipeline milking machines).
A total of 225 lactating buffaloes were monitored during the entire lactation. Individual milk yield was registered, and individual milk samples were collected at four weeks intervals, starting from the first week to the end of lactation. Duplicate milk samples from each buffalo were collected on the sampling day. Milk samples were immediately put into a refrigerate box and transported to the laboratory. One set of milk samples (50 mL preserved with bronopol-B2) was analysed within 6 h from the sampling-time for fat, proteins and lactose content (Milkoscan FT 6000, Foss Electric, HillerØd, Denmark), somatic cells count (Fossomatic 5000, Foss Electric), titratable acidity (°SH/100mL) by NaOH N/4, and pH. Another set of milk samples (10 mL) was centrifuged at 1500 rpm for 15 min at 4°C to remove fat. The skimmed milk was collected, stored at -20°C and then analysed for Lfe quantification within 3-4 weeks from the samplingday. A total of 2236 milk samples were collected and analysed. Dataset was edited so that only animals having at least 6 records per lactation were maintained. After editing, the final dataset consisted of 196 subjects and 1412-1440 records ().
Quantitative determination of lactoferrin in milk
Milk Lfe concentration was detected by the SDS-polyacrilamide gel electrophoresis (SDSPAGE) performed according to Laemmli (Citation1970) on 12.5% slab gel (). The fractions of milk soluble proteins containing Lfe were diluted by adding an equal volume of doubleconcentrated sample buffer (125 mM Tris-HCl, pH 6.8, 4% SDS, 0.8 M dithiothreitol, 0.4% bromophenol blue and 20% glycerol) and heated at 100°C for 3 min, to thermally denature proteins and to promote interactions of the proteins with SDS. Low molecular weight standards (Bio-Rad, Watford, UK) were prepared in sample buffer. The electrophoresis was performed in a Mini-Protean III dual slab cell (Bio-Rad). Electrophoresis was performed at room temperature using a voltage stepped procedure: voltage was kept constant (30 V) until the samples completely left the stacking gel and then the voltage was increased 15 V per min for 4 times. Voltage was maintained constant (90-100 V) until the tracking dye reached the bottom of the gel. Immediately after ending electrophoresis, gels were removed from the plates and placed in a staining solution containing 40% methanol, 10% acetic acid and 0.1% Coomassie Brilliant Blue R-250. Gels were left for 30 min in the staining solution and then destained in the methanol/acetic acid solution (5% methanol, 7% acetic acid).
Quantitative analysis of electrophoretically separated Lfe was done by densitometry using BSA (Sigma, Milano, Italy) as external standard, with Kodak EDAS-290 densitometer and analyzed with ID Image Analysis software (Kodak Company, Rochester, NY, USA). Detected polypeptides were identified using the standards of BSA, Lfe, α-La, β-Lg, κcasein, α-casein and β-casein (Sigma).
Statistical analysis
Data were analysed by repeated measures using the MIXED procedure of the Statistica-7 Software package (Stat Soft, Inc., Tulsa, OK, USA) with day of sampling as the repeated effect. Data were tested for normality using Kolmogorov-Smirnov test for Gaussian distribution. Except for SCC all data were normally distributed. For this reason somatic cells score (SCS) was calculated [log base 2 (SCC/100,000) + 3] and used in the statistical analysis. The milk Lfe variation was evaluated with a model that included parity, daily milk yield, and somatic cells count classes as fixed effects, and buffalo was the uncorrelated random effect. To describe changes of Lfe and SCS during lactation a model that included days in milk class as fixed effect and buffalo as uncorrelated random effect was used. Random variable was assumed to have a normal distribution. Parity was classified as follow: 1 = 1stlactation; 2 = 2ndand 3rdlactation; 3 = 4thand 5thlactation; 4 = 6thand 7thlactation; 5 = lactation >7th. Daily milk yield was classified as follow: Y1<6 kg; 6≤Y2<8 kg; 8≤Y3<10 kg; 10≤Y4<12 kg; Y5>12. SCC was classified by the number of somatic cells in milk as follow: 0≤SCC1<50.000:50.000≤ SCC2<100.000; 100.000≤ SCC3<200.000; 200.000≤ SCC4<300.000; 300.000≤ SCC5<400.000; SCC6>400.000.
To investigate the relationship between Lfe and milk characteristics, partial correlation coefficients corrected for repeated measures (namely days in milk) were calculated.
The differences were analysed by t- test and the significances were set at a value of P<0.05.
Results
Buffalo milk characteristics and milk lactoferrin concentration
The basic statistics (mean ±SD) of the milk yield and characteristics of all samples are given in . The milk Lfe concentration in lactating buffaloes was 0.332±0.165 g/kg and ranged from 0.030 to 0.813 g/kg (). The change of Lfe content in buffalo milk throughout the lactation period is showed in . Milk Lfe concentrations increased (P<0.01) with the increase of days in milk showing the minimum levels in the first 50-60 days (0.207±0.173 g/kg) and the maximum levels at the end of lactation (0.503±0.135 g/kg). Somatic cells score increased after 90 days in milk and then remained constant until the end of the lactation period ().
The Lfe concentration of buffalo milk was not affected by parity (). The Lfe concentration decreased as the daily milk yield increased (). Milk Lfe concentration was significantly affected by SCC class (). In particular, the differences become significant when the levels of SCC increase up to 100,000 and the highest levels were observed with SCC greater than 200,000/mL ().
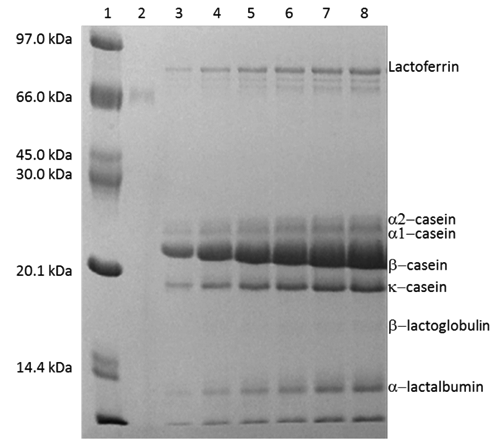
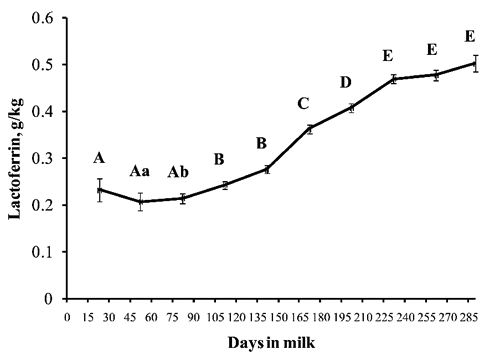
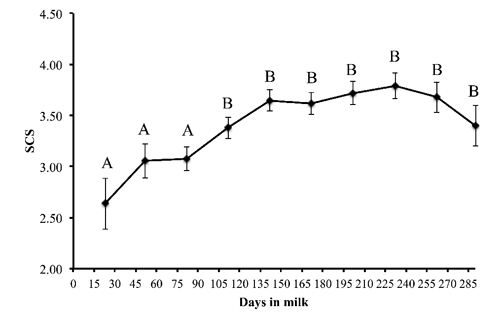
Table 1. Overall means of buffalo milk yield and milk characteristics.
Table 2. Ls means ±SD of lactoferrin concentration in the milk from lactating buffaloes.
Partial correlations between lactoferrin and milk characteristics
Lactoferrin was positively correlated with total proteins (r=0.41; P<0.0001) and SCS (r=0.21; P<0.001) and negatively correlated with lactose (r=-0.31; P<0.001) and titratable acidity (r=-0.11; P<0.001).
Discussion
In this study, the productive performance data and milk analysis on buffalo’s milk samples were in agreement with the data reported in other studies on buffalo’s milk (Ceron-Munoz et al. Citation2002; Rosati and Van Vleck, Citation2002; Zicarelli, Citation2004; Potena et al., Citation2007).
Several different methods have been used to quantify Lfe in bovine and human milk such as capillary electrophoresis (Riechel et al., Citation1998) reversed-phase HPLC (Palman and Elgar, Citation2002) and several immunoassays. In this study, Lfe was quantified by SDS-PAGE method and it average level was of 0.332 g/kg. This level was similar to those of Campanella et al.(Citation2009). Using immunosensor method, those authors found Lfe concentrations of 232.0 mg/L in raw buffalo milk. The difference between our and Campanella and co-workers data might be due to the different number of samples (more that 1400 in our trial 9 in Campanella and co-workers trial) and to the different source: individual samples in our study, bulk milk samples in Campanella and coworkers study. A quite wide range of Lfe concentration has been determined in healthy bovine milk. The values varied from 1.15 μg/mL to 485.63 μg/mL (Hagiwara et al., Citation2003). Lactoferrin concentrations in human milk were reported to be in the range of 1.0-3.2 mg/mL depending on the method used. Hiss et al.(Citation2008) observed that the weekly mean concentrations of Lfe in goat milk ranged between 10 to 28 μg/mL. Lactoferrin levels in mare colostrums and milk have been also measured. The obtained results were 21.7 μg/mL, and 0.82 g/kg, respectively (Malacarne et al., Citation2002; Barton et al., Citation2006). The mean milk Lfe concentration was reported to be 0.229±0.135 mg/mL in the camel (Konuspayeva et al., Citation2007). A comparative study on Lfe content in camel, cow, sheep, goat, donkey, mare and human normal milk was done (El-Agamy and Nawar, Citation2000). The study showed that Lfe concentration varied considerably. The highest level was found in human milk (1.7 mg/mL), while donkey milk had the lowest content (0.07 mg/mL). In our study the concentration of the Lfe in buffalo’s milk appeared to be lower than that reported in human milk, but higher than that in bovine milk. Lactoferrin is a key element in the host innate defense system with its antimicrobial properties, which include iron sequestration, direct lytic activities, and the ability of the molecule to impair the binding of microbes to host cells (Kutila et al., Citation2003; Valenti and Antonini, Citation2005). Therefore, a less vulnerability to mastitis in buffalos might be partially due to the high level of Lfe in milk. A strong association between milk Lfe and stage of lactation and daily milk yield, and no association with parity have been observed in the present study. Cheng et al.(Citation2008) found similar results in dairy cows.
Our study indicated that milk Lfe concentration tended to be higher when SCC increased. In contrast, the behaviour of Lfe and SCS during lactation did not match. In fact, Lfe concentration increased significantly during the second phase of lactation reaching the maximum levels at the end of lactation. In contrast, SCS did not increase in this phase. In previous study carried out on buffalo Piccinini et al.(Citation2005) observed a significant increase in the concentration of Lfe in milk with SCC greater than 400,000 cells/mL. Cheng et al.(Citation2008) in dairy cows reported that Lfe concentration tended to be significantly higher when SCC exceeded 141,000 cell/mL. Moreover, in camels significant increase was observed with SCC value greater than 30,000 cells/mL (Al-Majali et al., Citation2007). Those studies have been demonstrated a slight elevation of Lfe with subclinical mastitis, while Lfe was significantly elevated in clinically affected ones. In goats, Lfe has been found to be elevated during mastitis and a close relationship between SCC and Lfe was reported, too (Chen et al., Citation2004). In mid-lactating goats, Hiss et al.(Citation2008) observed that subjects with SCC higher than 430,000 cell/mL had higher Lfe milk concentration. Those authors considered the increased concentrations of Lfe towards the end of lactation, most likely as a physiological alterations rather than innate pathogen responses. Considering results of our and other studies, the increase of milk Lfe concentrations, proportional to somatic cells count qualify Lfe as an acute phase protein in milk. On the other hand, the increase of Lfe concentration during lactation may be due to mammary gland involution as reported in cows (Shamay et al., Citation2003) and in goats (Hiss et al., Citation2008) and to dilution effect.
Somatic cells count comprises cellular components that are recruited from the blood stream, whereas Lfe is synthesized in the mammary gland itself (Molenaar et al., Citation1996) by the epithelial cells and it is also present in the granules of neutrophils. Increase in milk somatic cells are initiated by chemoattractants, e.g. cell wall components or metabolites of bacteria but also by endogenous components including cytokines or complement components (Kehrli and Shuster, Citation1994). Such cytokines are upregulated during physiological processes and may affect Lfe secretion. Shen et al.(Citation2007) reported that transforming growth factor 1, an important local regulator on mammary tissue involution, has an inhibitory effect on neutrophil degranulation and thus on Lfe secretion. Baumrucker (Citation2005) suggests that the high regulation by mammary cells provides the opportunity to define the cellular route and potential intracrine role of Lfe. The relationships between Lfe concentrations and SCC in buffalo is difficult to analyse; this is due to the differential data for buffalo milk SCC that leads to uncertainty about the level of SCC in buffalo milk that can be used to define the presence of an inflammation (Dhakal et al., Citation1992; Mahendra and Ludri, Citation2001; Ceron-Munoz et al., Citation2002; Pasquini et al., Citation2003).
In a study carried out in Sri Lanka, it was reported that total SCC in normal buffalo milk varied from 50,000 to 375,000/mL (Silva and Silva, Citation1994). Tripaldi et al.(Citation2010) reported that in buffalo as in other species, total SCC is a valid indicator of udder inflammation and a value of 200,000 cells/mL should be used as the threshold value for early identification of an animal affected by subclinical mastitis. In addition, a total SCC value above that threshold value was associated with significantly decreased milk yield and with changes in milk composition and coagulating properties. Our results indicate that milk Lfe concentration increased significantly when the levels of SCC were up to 100,000 cells/mL and the highest concentrations were observed when SCC increased up to 200,000 cells/mL. Moreover, milk Lfe concentration was positively correlated with SCS (r=0.21) and negatively related (r=-0.31) to lactose. Sharma et al.(Citation2011) reported that elevated SCC was usually associated with a decrease in lactose, because elevated SCC reduced synthetic activity of the mammary tissue. These results combined with our results, may indicate that Lfe might be used as a possible indicator of subclinical mastitis in buffalo as in dairy cattle. Early detection of mastitis in buffaloes could be important for most dairy farmers to reduce production losses and to enhance prospects of recovery.
Conclusions
In conclusion, this is the first investigation on the levels of Lfe in buffalo milk in reference to daily milk yield, lactation stage, parity and SCC. The stage of lactation and daily milk yield contributed to the most of Lfe concentration in the milk from normal lactating buffaloes, whereas parity showed no association. Further studies are needed to better clarify the relationship between Lfe and SCC in buffalo milk and the possible role of Lfe as indicator of intramammary infection in buffalo.
References
- Al-MajaliA.M.IsmailZ.B.Al-HamiY.NourA.Y. 2007. Lactoferrin concentration in milk from camels (Camelus dromedarius) with and without subclinical mastitis. Int. J. Appl. Res. Vet. Med. 5:120-124.
- BartonM.H.HurleyD.NortonN.HeusnerG.CostaL.JonesS.ByarsD.WatanabeK. 2006. Serum lactoferrin and immunoglobulin G concentrations in healthy or ill neonatal foals and healthy adult horses. J. Vet. Int. Med. 20:1457-1462.
- BaumruckerC.R. 2005. Intracrine signalling in the mammary gland. Livest. Prod. Sci. 98:47-56.
- BaynesR.D.BezwodaW.R. 1994. Lactoferrin and the inflammatory response. Adv. Exp. Med. Biol. 357:133-141.
- CampanellaL.MartiniE.PintoreM.TomassettiM. 2009. Determination of lactoferrin and Immunoglobulin G in animal milks by new immunosensors. Sensors 9:2202-2221.
- Ceron-MunozM.TonhatiH.DuarteJ.OliveiraJ.Munoz-BerrocalM.Jurado-GomezH. 2002. Factors affecting somatic cell counts and their relations with milk and milk constituent yield in buffaloes. J. Dairy Sci. 85:2885-2889.
- ChenP.W.MaoF.C. 2004. Detection of lactoferrin in bovine and goat milk by enzymelinked immunosorbent assay. J. Food Drug Anal. 12:133-139.
- ChengJ.B.WangJ.Q.BuD.P.LiuG.L.ZhangC.G.WeiH.Y.ZhouL.Y.WangJ.Z. 2008. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 91:970-976.
- DhakalI.P.KapurM.P.AnshuS. 1992. Significance of differential somatic cell counts in milk for the diagnosis of subclinical mastitis in buffaloes using foremilk and stripping milk. Indian J. Anim. Health 31:39-42.
- El-AgamyE.I.NawarM.A. 2000. Nutritive and immunological values of camel milk: a comparative study with milk of other species. In: Proc. 2nd Int. Camelid Conf. Agroeconomics of Camelid Farming, Almaty, Kazakhstan, pp 33-45.
- HagiwaraS.KawaiK.AnriA.NagahataH. 2003. Lactoferrin concentrations in milk from normal and subclinical mastitic cows. J. Vet. Med. Sci. 65:319-323.
- HarmonR.J.SchanbacherF.L.FergusonL.C.SmithK.L. 1975. Concentration of lactoferrin in milk of normal lactating cows and changes occurring during mastitis. Am. J. Vet. Res. 36:1001-1007.
- HissS.MeyerT.SauerweinH. 2008. Lactoferrin concentrations in goat milk throughout lactation. Small Ruminant Res. 80:87-90.
- KehrliM.E.ShusterD.E. 1994. Factors affecting milk somatic cells and their role in health of the bovine mammary gland. J. Dairy Sci. 77:619-627.
- KonuspayevaG.FayeB.LoiseauG.LevieuxD. 2007. Lactoferrin and immunoglobulin contents in camel’s milk (Camelus bactrianus, Camelus dromedarius, and Hybrids) from Kazakhstan. J. Dairy Sci. 90:38-46.
- KutilaT.PyöräläS.SaloniemiH.KaartinenL. 2003. Antibacterial effect of bovine lactoferrin against udder pathogens. Acta Vet. Scand. 44:35-42.
- LaemmliU.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685.
- LeeN.Y.KawaiK.NakamuraI.TanakaT.KumuraH.ShimazakiK. 2004. Susceptibilities against bovine lactoferrin with microorganisms isolated from mastitic milk. J. Vet. Med. Sci. 66:1267-1269.
- LiuG.ZhangC.WangJ.BuD.ZhouL.ZhaoS.LiS. 2010. Canonical correlation of milk immunoglobulins, lactoferrin concentration and dairy herd improvement data of Chinese Holstein cows. Livest. Sci. 128:197-200.
- LonnerdalB.IyerS. 1995. Lactoferrin: molecular structure and biological function. Ann. Rev. Nutr. 15:93-110.
- MahendraS.LudriR.S. 2001. Somatic cells counts in Murrah buffaloes during different stages of lactation, parity and season. Asian Austral. J. Anim. Sci. 14:189-192.
- MalacarneM.MartuzziF.SummerA.MarianiP. 2002. Protein and fat composition of mare’s milk: some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 12:869-877.
- MolenaarA.J.KuysY.M.DavisS.R.WilkinsR.J.MeadP.E.TweedieJ.W. 1996. Elevation of lactoferrin gene expression in developing, ductal, resting, and regressing parenchymal epithelium of the ruminant mammary gland. J. Dairy Sci. 79:1198-1208.
- NewmanK.A.Rajala-SchultzP.J.LakritzJ.DeGravesF.J. 2009. Lactoferrin concentrations in bovine milk prior to dry-off. J. Dairy Res. 76:426-432.
- PalmanK.P.ElgarD.F. 2002. Detection and quantitation of lactoferrin in bovine whey samples by reversed-phase high- performance liquid chromatography on polystyrene-ivinylbenzene. J. Chromatogr. A 947:307-311.
- PasquiniM.TommeiB.MattiiS. 2003. Buffalo milk: proteins electrophoretic profile and somatic cell count. Ital. J. Anim. Sci. 2(Suppl.1):299-301.
- PiccininiR.MiarelliM.FerriB.TripaldiC.BelottiM.DapràV.OrlandiniS.ZecconiA. 2006. Relationship between cellular and whey components in buffalo milk. J. Dairy Res. 73:129-133.
- PotenaAZicarelliL.NapolanoR.IovaneG.CampanileG.GasparriniB.Di PaloR. 2007. Milk and curd characteristics depending on farm and production level. Ital. J. Anim. Sci. 6(Suppl. 2):1104-1107.
- RiechelP.WeissT.WeissM.UlberR.BuchholzH.ScheperT. 1998. Determination of the minor whey protein bovine lactoferrin in cheese whey concentrates with capillary electrophoresis. J. Chromatogr. A 817:187-193.
- RosatiA.Van VleckL.D. 2002. Estimation of genetic parameters for milk, fat, protein and mozzarella cheese production for the Italian river buffalo Bubalus bubalis population. Livest. Prod. Sci. 74:185-190.
- ShamayA.ShapiroF.LeitnerG.SilanikoveN. 2003. Infusions of casein hydrolyzates into the mammary gland disrupt tight junction integrity and induce involution in cows. J. Dairy Sci. 86:1250-1258.
- SharmaN.SinghN.K.BhadwalM.S. 2011. Relationship of somatic cell count and mastitis: an overview. Asian Austral. J. Anim. Sci. 24:429-438.
- ShenL.SmithJ.M.ShenZ.ErikssonM.SentmanC.WiraC.R. 2007. Inhibition of human neutrophil degranulation by transforming growth factor-1. Clin. Exp. Immunol. 149:155-161.
- SilvaT.D.SilvaK.F.S.T. 1994. Total and differential cell counts in buffalo milk. Buffalo J. 2:133-137.
- SmithK.L.SchanbacherF.L. 1977. Lactoferrin as a factor of resistance to infection of the bovine mammary gland. JAVMA-J. Am. Vet. Med. A. 170:1224-1227.
- StelwagenK.CarpenterE.HaighB.HodgkinsonA.WheelerT.T. 2009. Immune components of bovine colostrum and milk. J. Anim. Sci. 87:3-9.
- TripaldiC.PalocciG.MiarelliM.CattaM.OrlandiniS.AmatisteS.Di BernardiniR.CatilloG.R. 2010. Effects of Mastitis on Buffalo Milk Quality. Asian Austral. J. Anim. Sci. 23:1319-1324.
- ValentiP.AntoniniG. 2005. Lactoferrin: An important host defense against microbial and viral attack. Cell. Mol. Life Sci. 62: 2576-2587.
- WeltyF.K.SmithK.L.SchanbacherF.L. 1976. Lactoferrin concentration during involution of the bovine mammary gland. J. Dairy Sci. 59:224-231.
- ZicarelliL. 2004. Buffalo milk: its properties, dairy yield and mozzarella production. Vet. Res. Commun. 28:127-135.