Abstract
An experiment was carried out with 630 one day-old chicks to evaluate the effects of organic acids when birds were experimentally inoculated with Salmonella Typhimurium. Liver damage and the persistence of the bacterium in the organs were evaluated as well. Broilers were distributed in a completely randomised experimental design in a 3×2 factorial arrangement of six treatments with seven replicates of 15 birds each. Birds were inoculated with saline solution or the bacterium via gavage at 1 day of age, or were offered a feed containing or not the organic acid blend for the period of 7 to 14 days of age. A dose of 5.0x102 colony-forming units (CFU)/0.5 mL of Salmonella Typhimurium was used for inoculation both via gavage and feed. The parameters evaluated are weight, liver histopathology, liver and serum biochemistry, and bacteriological analyses of the caeca, crop, spleen, and liver and heart pool. At 21 and 28 days of age, the liver of the non-inoculated groups was significantly lighter as compared to the other treatments. Birds fed organic acids presented lower bacterial isolation rates in all organs tested. Birds inoculated in the crop and treated with organic acids presented lower E. coli CFU counts (P<0.05). Birds inoculated with Salmonella presented significant changes (P<0.05) in liver enzymes, as detected by serum biochemistry, and in liver histopathology. It was concluded that organic acids effectively controlled Salmonella Typhimurium and did not cause any liver damage.
Introduction
Salmonella enterica is one of the most important public health pathogens and may be acquired by the consumption of poultry products (Beal et al., Citation2004). Salmonella enterica serovars have been increasingly implicated in foodborne diseases in industrialised countries. The most common serotype implicated in these conditions is Salmonella Typhimurium, a globally distributed foodborne serotype (Olsen et al., Citation2001; Mukesh and Mukesh, Citation2002). This bacterium may persistently infect the gastrointestinal tract of poultry, but clinical signs are commonly absent in birds infected after the first week of life (Beal et al., Citation2004). Salmonella Typhimurium may cause local inflammation, diarrhea, and may progress to systemic infection (Baucheron et al., Citation2005; Cardinale et al., Citation2005).
One of the main systemic organs affected by Salmonella is the liver. As liver is involved in several metabolic functions, any factor that changes its physiology may induce liver damage, consequently affecting the entire body of the individual. Biochemical tests are used to detect several liver abnormalities (Minafra et al., Citation2009), including necrosis, hepatocyte damage, and changes in liver functions. Liver enzymes increase in the blood stream when the cells producing them are damaged. The two main enzymes used to indicate liver function status are aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Histopathology findings can also be used to detect liver abnormalities, thus aiding disease diagnosis. In acute infections by bacteria such as Salmonella, the liver parenchyma is infiltrated by heterophils, as described by Barcelos (Citation2005), with evident lesions in acute and severe disease.
Therefore, infections by Salmonella Typhimurim must be controlled mainly for public safety reasons, but also due to the significant financial losses in the poultry industry caused by mortality of young birds, medications costs, low-quality chicks, and the implementation of eradication measures (Hafez, Citation2005).
Dunkley et al. (Citation2009) suggested some strategies to limit the presence of Salmonella in the gastrointestinal tract of the poultry. Organic acids can be used to control (i.e. reduce or eliminate) these pathogens, although their concentration levels may differ for controlling the growth of pathogenic bacteria (Rezende et al., Citation2008). The antimicrobial activity of organic acids is related to pH reduction and the dissociation capacity of their carboxyl groups. In their undissociated form, organic acids passively penetrate the microbial cell, and once inside the cell, they dissociate due to the neutral pH of the intracellular medium, releasing H+ cations, thereby decreasing intracellular pH. The export of these protons by the cells to maintain neutral internal pH leads to energy depletion, and consequent microbial death (Russel, Citation1992; Bellaver and Scheuermann, Citation2004).
Taking into consideration the consequences of the presence of Salmonella in poultry production relative both to public and poultry health, the present study aimed at evaluating the efficacy of a novel association of organic acids for the control of Salmonella Typhimurium in poultry. The persistency of Salmonella Typhimurium in different organs and liver function parameters were studied in 1- to 28-day-old broilers.
Materials and methods
Six hundred thirty one-day-old broiler chicks were distributed in six treatments with seven replicates of 15 birds each (). A completely randomised experimental design in a 3×2 factorial arrangement, evaluating inoculating agents (saline solution at 0.85%, Salmonella Typhimurium via gavage or feed) and organic acids (0 or 4 kg/tonne) was applied. The concentration adopted referred to the need to simulate situations of low concentration contamination and not to promote lesions that could be confused with those likely promoted by organic acids. The inoculum was prepared with Salmonella Typhimurium isolated from broiler samples, as referred to by Rezende et al. (Citation2006), and was obtained according to the method described by Fernández et al. (Citation2001). Birds were fed a mash diet based on ground corn and soybean meal, with no antibiotic growth promoters or anticoccidials agents. The feed was formulated according to the composition and nutritional requirements proposed by Rostagno et al. (Citation2005). Feed and water were offered ad libitum. The organic acid blend, containing benzoic (22.44%), fumaric (41.34%) and 2-hydroxy-4-methylthio-butanoic acid (HMTBa) (28.40%), was supplied in the feed all through the experimental period at 0.4%.
At 14 and 28 days of age, the blood of one bird per replicate was collected, the serum was prepared, and stored at- 20°C for up to two weeks to determine ALT and AST values. Analyses were carried out by kinetic method using a commercial kit (Labtest®, Lagoa Santa, Brazil). At 7, 14, 21, and 28 days of age, birds were fasted for 2 to 3 h to promote emptying of the digestive tract. One bird per replicate was sacrificed, and submitted to necropsy. Their livers were collected, and weighed, and their weights relative to bird live weight were calculated following Grieves (Citation1991). Sections of the collected livers from 14- and 28-day-old broilers were collected for histopathological examination in order to observe possible lesions caused by Salmonella or the organic acids. The sections were placed in buffered formalin at 10% for further processing, and the remaining liver was submitted to bacteriologic analysis. The liver sections (n=84) were processed according to the conventional methodology described by Luna (Citation1968). The following parameters were considered in the histopathological evaluation: liver irrigation, hepatocyte integrity, and presence of lymphocyte infiltrate. Caecal content samples, crop swabs, spleen, liver and heart pools of the control and infected birds were collected at necropsy at days 7, 14, 21, and 28 for bacteriological analysis and isolation of Salmonella Typhimurium. Birds put to death by cervical dislocation during the experiment were also submitted to necropsy, and their yolk sac, liver, and spleen were collected for the detection of the bacterium.
Salmonella Typhimurium detection was carried out according to the methods proposed by the Georgia Poultry Laboratory (Citation1997) and the Ministério da Agricultura, Pecuária e Abastecimento (Citation2003), with some modifications as described below. After preparation, organ samples were enriched in cystine selenite broth (CS) in 10 mL aliquots, and placed in an oven at 37°C for 24 h. Each culture was then seeded in Brilliant Green agar, Hektoen agar, and XLT4 agar, and again incubated at 37°C for 24 h. Three to five colonies appearing as Salmonella in each plate were reseeded in triple sugar iron (TSI) agar and incubated at 37°C for 24 h. Bacterial samples presenting features compatible with the genus Salmonella were submitted to the following biochemical tests: indol and H2S production, urease, lysine decarboxylation, methyl red (MR), malonate use, and motility. Biochemical analyses test tubes were incubated at 37°C for up to four days, with daily readings, for the tests that presented negative results until the first 48 h of incubation and for the amino-acid decarboxylation or deamination tests. Samples biochemically confirmed as being Salmonella were submitted to serologic tests using anti-O polyvalent sera. Samples confirmed as being positive both by biochemical and serologic tests were submitted to the Osvaldo Cruz Institute (FIOCRUZ-RJ) for serotyping. Enumeration of Escherichia coli (CFU/g) was carried at 14 and 21 days by taking samples of the final portion of the large intestine from each bird of T1, T2, T5 and T6. For the sake of analysis, 0.5 grams of the final portion of the large intestine of bird/plot were weighed and tubes containing 4.5 mL of buffered saline solution 0.1% were used. From this initial dilution made with the aid of automatic pipettes, serial dilutions (104 and 106) were made and 0.1 mL of these last were transferred to two Petri dishes containing agar Eosin Methylene Blue (EMB), thus becoming plating on the surface. The typical and atypical colonies, according to the Ministry of Agriculture, Husbandry, and Supply (2003), were transplanted into triple sugar iron agar (TSI) and TSI isolates were biochemically confirmed for the presence of E. coli by production of indole and methyl red, no motility, H2S, urease reaction, and by using Simmons citrate.
Biometric, mortality, and blood serum biochemistry results were submitted to analysis of variance, and means were compared by Tukey’s test at 5% probability, using SAS® (Citation2001) statistical package. Salmonella Typhimurium colonisation and liver histopathology results were submitted to descriptive test, considering only the frequency of the parameters.
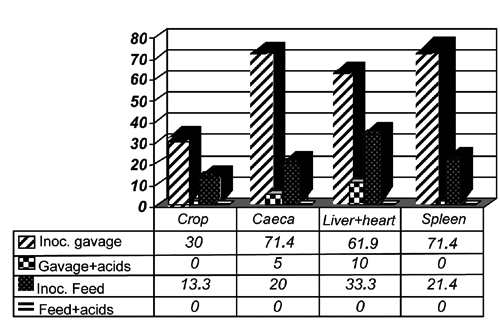
Table 1. Description of treaments.
Table 2. Enumeration of Escherichia coli in the final portion of the large intestine of 14- to 21-day-old broilers inoculated with Salmonella Typhimurium via gavage and feed and treated with organic acids or not.
Table 3. Relative liver weight in 7, 14, 21 and 28-day-old broilers inoculated with Salmonella Typhimurium via gavage or feed and treated with organic acids or not.
Table 4. Serum levels of the liver enzymes aspartate aminotransferase and alanine aminotransferase of 14- and 28-day-old broilers inoculated with Salmonella Typhimurium via gavage or feed and treated with organic acids or not.
Table 5. Histopathological liver changes in 14- and 28-day-old broilers inoculated with Salmonella Typhimurium via gavage or feed and treated with organic acids or not.
Results and discussion
Birds were observed daily, and any clinical sign was recorded. Some birds presented watery excreta and vent plugging. Some Salmonella enterica serovars may cause digestive system dysfunctions, which are characterised by accumulation of feed and fluids around the vent in young birds. There was no difference (P>0.05) in mortality rate among treatments during the experimental period. This suggests that Salmonella Typhimurium was not pathogenic and also that organic acids did not present toxicity that could cause death (Viola and Vieira, Citation2007). Also, no effects of an organic acid blend on broiler mortality were found. The yolk sac, spleen, liver, and heart of the birds that died during the experiment were collected and submitted to bacteriological analyses. All three birds (100%) of the positive control group (inoculated with Salmonella Typhimurium and not treated with organic acids) that were found dead were positive for the presence of this bacterium both by bacteriological analyses and serotyping (FIOCRUZ). The organ samples of the five (100%) negative-control birds (not inoculated with Salmonella Typhimurium and not treated with organic acids) and of the two birds (100%) inoculated with the bacteria and treated with organic acids were negative for the presence of that pathogen. Seven apparently healthy birds from each treatment were weighed and sacrificed cervical dislocation at 7, 14, 21, and 28 days of age, and crop and caecal content swabs, spleen, liver, and heart samples were collected. The results of the bacteriological analyses of the collected tissues and of contaminated feed are shown in . The feed was positive for the presence of Salmonella Typhimurium, whereas the bacterium was not detected in the tissue samples of the birds not inoculated with Salmonella Typhimurium and not treated with organic acids (negative control). The graph also shows that the experimental dose of 5.0×102 CFU/0.5 mL/bird of Salmonella Typhimurium used as challenge promoted the invasion of organs and colonisation of the digestive system of the inoculated birds. The high levels of Salmonella detected in the organs may be explained by its dissemination in the body. After oral ingestion, Salmonella colonises the intestinal tract, particularly the caeca, and then penetrates the epithelial mucosa (Desmidt et al., Citation1997). Salmonella is able to replicate inside the phagosomes, survive inside the macrophages, and thereby spread into internal organs, such as liver and spleen, thus establishing a systemic infection (Bohez et al., Citation2008). However, if the bird has already developed the gut-associated lymphoid tissues (GALT), the pathogen may be eliminated (Desmidt et al., Citation1997; Henderson et al., Citation1999).
Despite the lower level of Salmonella recovery in the crop as compared to other organs in birds contaminated by gavage in the present study, the crop should be considered as a possible site of carcass contamination during processing. The acid pH of the crop helps to protect the digestive system against pathogens, providing a natural protection barrier. Consistently with the findings of the present experiment, Brito et al. (Citation1995), who inoculated 1-day-old chicks with Salmonella Typhimurium via feed or gavage, also observed that the colonisation of the digestive system and visceral organs was faster and more intense in birds inoculated by gavage. also shows that the groups treated with organic acids, independently of the route of Salmonella Typhimurium inoculation, presented lower isolation frequency in all evaluated organs as compared to those that did not receive the organic acids. Organic acids reduce the number of acid-intolerant bacteria, such as Salmonella spp., promoting better energy and protein digestibility possibly due to the reduction of bacterial load.
The results for Escherichia coli enumeration (CFU/g) are shown in . The lowest Escherichia coli count of 14 to 21 days may be related to competition with Salmonella Typhimurium inoculated. It is possible to observe that between the two groups that received organic acids, that inoculated with Salmonella Typhimurium by gavage and feed presented the lowest (P<0.05) E. coli CFU values. Escherichia coli is part of the normal enteric microbiota of mammals and birds (Cardoso et al., Citation2002). Machado (Citation2000) observed that bacteria colonise the intestinal tract through two main mechanisms: attachment or close association with the intestinal epithelium, or staying in the intestinal lumen for those bacteria unable to attach to the mucosa. In the present study, the inoculated Salmonella probably competed with E. coli for attachment sites in the intestine, consequently reducing E. coli counts in the excreta. In addition, the birds inoculated with Salmonella Typhimurium via gavage and treated with organic acids presented lower E. coli counts as compared to those also inoculated by gavage, but not receiving organic acids. In the experiment carried out by Izat et al. (Citation1990), the addition of graded levels of organic acids caused lower E. coli colonisation in the small intestine. Some authors also observed that feed supplementation with organic acids may reduce the proliferation of pathogenic bacteria, such as E. coli, in the digestive system of monogastric animals (Richards et al., Citation2005). In addition, Li et al. (Citation2008), studying the action of fumaric and benzoic acids and HMTBa on the intestinal microbiota, observed a trend of lower E. coli ileal counts in pigs fed organic acids as compared to those receiving a basal diet with no organic acids. The liver of one bird per replicate was collected at 7, 14, 21, and 28 days of age, and its weight relative to bird live weight was calculated as well. Significant effects of the interaction (P<0.05) between inoculation and treatment with organic acids was observed on this parameter when birds were 7, 21, and 28 days of age ().
It was observed that 7-day-old broilers inoculated with saline solution and treated with organic acids presented lower liver weight (P<0.05) than those that received the saline solution (0.85%), but no organic acids. The same effect was also observed in 21-day-old birds inoculated with Salmonella and treated or not with organic acids. Li et al. (Citation2008) also found lower liver weight when feeding piglets fumaric and benzoic acids and HTMBa at 0.5 and 1% as compared to the control group. It was also observed that the group inoculated with saline solution presented lower relative weight than those inoculated at 7, 21 and 28 days. Higher liver weight in Salmonella-inoculated groups was probably caused by the dissemination of the bacterium in systemic organs. This hypothesis is supported by Barrow (Citation1999), who found that Salmonella is able to disseminate in internal organs, such as liver, thus establishing a systemic infection condition. Xie et al. (Citation2000) also verified an increase (P<0.05) in relative liver weight in all broilers inoculated with Salmonella Typhimurium’s lipopolysaccharide. According to Van Hemert et al. (Citation2006), CFU number increased in the liver samples of analysed broilers up to 7 days post-inoculation. At 14 and 28 days of age, blood was collected from one bird per replicate, and the liver enzymes AST and ALT were analysed in the serum using commercial kits. These analyses have been used to aid the diagnosis of diseases in livestock, but there are few studies on their reference levels in poultry, possibly due to the infrequent use of laboratory exams in poultry pathology.
Salmonella Typhimurium inoculation influenced (P<0.05) ALT values of 28-day-old broilers, with higher values in those inoculated by gavage as compared to those inoculated via feed, and those fed organic acids (). This increase in serum ALT levels may be attributed to liver dysfunction caused by hepatocyte necrosis or by changes in cell membrane permeability (ALT, AST) (Borsa et al., Citation2006). Only the group inoculated by gavage and treated with organic acids presented lower AST levels (P<0.05) as compared to those inoculated by gavage but not fed organic acids. Considering the above mentioned assertion of Borsa et al. (Citation2006), this is a positive result, as organic acids probably prevented the tissue damages caused by Salmonella and also suggests that the organic acids were not toxic to the liver. The enzyme aspartate aminotransferase (AST) is present in the cytoplasm and mitochondria of tissues, such as the liver and skeletal and cardiac muscles, of all domestic species and it is highly active in the liver. The changes observed in the present experiment confirm that acute or chronic liver damages increase AST activity (Franciscato, Citation2006). The enzyme aspartate aminotransferase serum level changes can be explained by changes in hepatocyte membrane permeability or hepatocyte death, releasing the enzyme in the blood stream, and are related to the extent of liver damage (Butkeraitis, Citation2003). Along with serum biochemistry, histopathological findings can also be used to detect liver abnormalities, aiding disease diagnosis in poultry. Fragments of the liver of one bird per replicate were collected at days 14 and 28, and the results of the histopathological examination are shown in .
At both ages, birds in the negative control group and those fed organic acids and not inoculated with Salmonella did not present any significant liver lesions, again indicating that the organic acid blend used did not produce any toxic effect. On the other hand, the liver of birds inoculated with Salmonella Typhimurium, regardless of the organic acid treatment, presented focal necrosis. The lesions of birds inoculated via gavage were more severe, with predominance of diffuse necrosis (1/7 birds) and with peri-portal lymphocyte infiltrate (1/7 birds). At 28 days of age, the groups inoculated with Salmonella Typhimurium and treated with organic acids presented hepatocyte lesions on the order of 66.6% and 50%, respectively, in comparison with the inoculated groups not fed organic acids (71.4 and 100%). Howeve, these results are higher as compared to those of the groups not inoculated with Salmonella. The high ALT levels observed in birds of the same age not treated with organic acids may be related to this high frequency of liver damage, as explained above. At 28 days of age, birds fed the inoculated feed and not treated with organic acids presented massive liver necrosis, whereas those inoculated by gavage also presented higher frequency of liver damage as compared to the other treatments, with one replicate (1/7) presenting multiple foci of lymphocyte infiltrate (granuloma), and one replicate (1/7) presenting periportal infiltrate.
Despite the diversity of lesions, multifocal necrosis with lymphocyte infiltration of the liver parenchyma is a common microscopic finding in acute bacterial contamination (Barcelos, Citation2005). In the experiment of Brito et al. (Citation1995), 1-day-old chicks were inoculated with Salmonella Typhimurium via feed and gavage, and the histopathological exam showed several areas of hepatocyte necrosis, and mononuclear and heterophil infiltration. Desmidt et al. (Citation1997) argued that birds infected with Salmonella may present granulomas that, once reactivated, may cause infection in broilers.
Conclusions
Organic acids (benzoic, fumaric, and 2-hydroxy-4-methylthio-butanoic acid) supplied at 0.4% in the feed efficiently controlled the infection of Salmonella Typhimurium in broilers, as determined by lower frequency of bacterial isolation in the organs and absence of liver cell damage as compared to birds not fed these organic acids.
Acknowledgments
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for its financial support.
References
- BarcelosA.S. 2005. Avaliação macroscópica, histopatológica e bacteriológica de fígados de frangos (Gallus gallus) condenados no abate pela inspeção sanitária. Degree Diss., Universidade Federal de Santa Maria, Brazil.
- BarrowP.A. 1999. Experimental infection of chickens with Salmonella enteritidis. Avian Pathol. 20:145-153.
- BaucheronS. MoulineC. PraudK. Chaslus-DanclaE. CloeckaertA. 2005. TolC but not AcrB is essential for multidrug-resistant Salmonella enterica serotype Typhimurium colonization of chicks. J. Antimicrob. Chemoth. 55:707-712.
- BealR.K. WigleyP. PowersC. HulmeS.D. BarrowP.A. SmithA.L. 2004. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunop. 100:151-164.
- BellaverC. ScheuermannG. 2004. Aplicações dos ácidos orgânicos na produção de aves de corte. Available from: http://www.cnpsa.embrapa.br/sgc/sgc_publicacoes/publicacao_h6n45p3z.pdf
- BohezL. GantoisI. DucatelleR. PasmansF. DewulfJ. HaesebrouckF. ImmerseelF.V. 2008. The Salmonella pathogenicity island 2 regulator ssrA promotes reproductive tract but not intestinal colonization in chickens. Vet. Microbiol. 126:216-224.
- BorsaA. KohayagawaA. BorettiL.P. SaitoM.E. KuibidaK. 2006. Níveis séricos de enzimas de função hepática em frangos de corte de criação industrial clinicamente saudáveis. Arq. Bras. Med. Vet. Zoo. 58:675-677.
- BritoJ.R.F. XuY. HintonM. PearsonG.R. 1995. Pathological findings in the intestinal tract and liver of chicks after exposure to Salmonella serotypes Typhimurium or Kedougou. Brit. Vet. J. 151:311-323.
- ButkeraitisP. 2003. Efeitos da fumonisina B1 em codornas poedeiras (Coturnix coturnix japonica). Degree Diss., Universidade de São Paulo, Brazil.
- CardinaleE. Gros-ClaudeJ.D.P. RivoalK. RoseV. TallF. MeadG.C. SalvatG. 2005. Epidemiological analysis of Salmonella enterica spp. enterica serovars Hadar, Brancaster and Enteritidis from humans and broiler chickens in Senegal using pulsed-field gel electrophoresis and antibiotic susceptibility. J. Appl. Microbiol. 99:968-977.
- CardosoA.L.S.P. TessariE.N.C. CastroA.G.M. ZanattaG.F. 2002. Avaliação da susceptibilidade a antimicrobianos de cepas de Escherichia coli de origem aviária. Arq. Inst. Biol. (Sao Paulo) 69:1-5.
- DesmidtM. DucaletteR. HaesebrouckF. 1997. Pathogenesis of Salmonella Enteritidis phage types four after experimental infection of young chickens. Vet. Microbiol. 56:99-109.
- DunkleyK.D. CallawayT.R. ChalovaV.I. McReynoldsJ.L. HumeM.E. DunkleyC.S. KubenaL.F. RickeS.C. 2009. Foodborne Salmonella ecology in the avian gastrointestinal tract. Anaerobe 15:26-35.
- FernándezA. LaraC. LosteA. CalvoS. MarcaM.C. 2001. Control of Salmonella enteritidis phage type 4 experimental infection by fosfomycin in newly hatched chicks. Comp. Immunol. Microb. 24:207-216.
- FranciscatoC. 2006. Avaliação dos minerais séricos e da função hepática de frangos de corte experimentalmente intoxicados com aflatoxina e submetidos a diferentes concentrações de montmorilonita sódica na dieta. Degree Diss., Universidade Federal de Santa Maria, Brazil.
- Georgia Poultry Laboratory, 1997. Monitoring and detection of Salmonella in poultry and poultry environments. Georgia Poultry Laboratory ed., Oakwood, GA, USA.
- GrievesD.B. 1991. Inmunología aviar y aplicaciones prácticas. pp 1-16 in Proc. 20th Int. Latin American Congr. on Poultry, Quito, Equador.
- HafezH.M. 2005. Perspectiva global de enfermidades emergentes e re-emergentes em aves. pp 123-138 in 18th Nat. Conf. of the Brazilian Association of Producers of Pinto Court on Poultry Science and Technology, Santos, Brazil.
- HendersonS.C. BounousD.I. LeeM.D. 1999. Early events in the pathogeneses of avian salmonellosis. Infect. Immun. 67:3580-3586.
- IzatA.L. TidweelN.M. ThomasR.A. ReiberM.A. AdamsM.H. ColbergM. WaldroupP.W. 1990. Effects of a buffered propionic acid in diets on the performance of broiler chickens and on the microflora of the intestine and carcass. Poultry Sci. 69:818-826.
- LiZ. YiG. YinJ. SunP. LiD. KnightC. 2008. Effects of organic acids on growth performance, gastrointestinal pH, intestinal microbial populations and immune responses of weaned pigs. Asian Austral. J. Anim. 21:252-261.
- LunaL.G. 1968. Manual of histologic staining methods of the armed forces institute of pathology. 3rd rev. ed. McGraw-Hill Publ., New York, NY, USA.
- MachadoJ.N. 2000. Tendências atuais e futuras no uso de colonizadores bacterianos intestinais na avicultura industrial. pp 18-27 in Proc. Int. Meet. Avian Sciences, Uberlândia, Brazil.
- MinafraC.S. MoraesG.H.K. LopesC.O.Jr. VieitesF.M. Minafra-RezendeC.S. ViuM.A.O. 2009. Balanço eletrolítico e proteico dietéticos sobre as aminostransferases hepáticas, renais e séricas e teores séricos de magnésio e cloro de frangos de corte. Cienc. Anim. Bras. 10:425-437.
- Ministério da Agricultura, Pecuária e Abastecimento, 2003. Instrução normativa n° 62, de 26 de agosto de 2003. Métodos analíticos oficiais para análises microbiológicas para controle de produtos de origem animal e água. In: Official Journal, Section 1, 18/9/2003, p 14.
- MukeshD.S. MukeshG.S. 2002. Serotypic and antibiotic susceptibility pattern of Salmonella species isolated from cases of gastroenteritis at infectious disease hospital (IDH), Delhi from 1997-2000. J. Commun. Disord. 34:237-244.
- OlsenS.J. BishopR. BrennerF.W. RoelsT.H. BeanN. TauxeR.V. SlutskerL. 2001. The changing epidemiology of Salmonella: trends in serotypes isolates from human in the States, 1987-1997. J. Infect. Dis. 183:753-761.
- RezendeC.S.M. MesquitaA.J. AndradeM.A. LinharesG.F.C. MesquitaA.Q. MinafraC.S. 2006. Ácidos orgânicos em rações experimentalmente contaminadas com Salmonella Enteritidis e Salmonella Typhimurium. Degree Diss., Universidade Federal de Goiás, Brazil.
- RezendeC.S.M. MesquitaA.J. AndradeM.A. StringhiniJ.H. ChavesL.S. MinafraC.S. LageM.E. 2008. Ácido acético em rações de frangos de corte experimentalmente contaminadas com Salmonella Enteritidis e Salmonella Typhimurium. Rev. Bras. Saude Prod. Anim. 9:516-528.
- RichardsJ.D. GongJ. LangeC.F.M. 2005. The gastrointestinal microbiota and its role in monogastric nutrition and heath with an emphasis on pigs: current understanding, possible modulations, and new technologies for ecological studies. J. Anim. Sci. 85:421-435.
- RostagnoH.S. AlbinoL.F.T. DonzeleJ.L. GomesP.C. OliveiraR.F. LopesD.C. FerreiraA.S. BarretoS.L.T. EuclidesR.F. 2005. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. Universidade Federal de Viçosa ed., Brazil. Available from: http://www.lisina.com.br/arquivos/Geral%20Português.pdf
- RussellJ.B. 1992. Another explanation for the toxicity of fermentation acids at low pH: anion accumulation versus uncoupling. J. Appl. Bacteriol. 73:363-370.
- SAS, 2001. User’s guide statistics, 10th ed. SAS Inst. Inc., Cary, NC, USA.
- Van HemertS. HoekmanA.J.W. SmitsM.A. RebelJ.M.J. 2006. Gene expression responses to a Salmonella infection in the chicken intestine differ between lines. Vet. Immunol. Immunop. 114:247-258.
- ViolaE.S. VieiraS.L. 2007. Suplementação de acidificantes orgânicos e inorgânicos em dietas para frangos de corte: desempenho zootécnico e morfologia intestinal. Rev. Bras. Zootecn. 36:1097-1104.
- XieH. RathN.C. HuffG.R. HuffW.E. BalogJ.M. 2000. Effects of Salmonella typhimurium lipopolysaccharide on broiler chickens. Poultry Sci. 79:33-40.