Abstract
We evaluated the effect of monochromatic and combined light emitting diode (LED) light colour on performance, ovarian morphology, and reproductive hormone and biochemical blood parameters in laying hens. A total of 600 Hy-line Brown pullets, 12 weeks of age, were divided (25×4×6 = birds × replications × treatments) as follows: red (R), green (G), blue (B), and combinations of R → G and R → G → B treatments. Fluorescent white light (W) was the control. The results showed that higher egg production was found under the monochromatic R and combination R → G treatments, and that heavier eggs were laid by the B and G treatments (P<0.05). Consequently, better feed conversion ratio was attained in the R → G treatment. Serum follicle stimulating hormone and 17 -estradiol levels were significantly higher in the R and R → G treatments. B treated birds came into production 15 days later than those treated with R light. Organ weight (ovary and stroma) and ovarian follicle numbers (1-3 and 4-6 mm) were significantly higher in R treated birds, as well as serum glucose and triglyceride contents. Serum IgG concentrations and the heterophil to lymphocyte ratio were not influenced by light colour. In these laying hens, 14 h R with 2 h G light in the later part of the day increased reproductive hormone levels, ovarian weight, and follicle number and hence increased egg production. Thus, these results suggest that a combination of R → G light may be comparable with monochromatic R light to enhance egg production in laying hens.
Introduction
The eye of the chicken is more sensitive to particular light wavelengths than that of humans. Therefore, artificial light-based poultry rearing systems have been developed worldwide. The wavelength of light or colour has become of interest as it may influence growth and reproduction in domestic fowl. Both retinal and extra-retinal photoreceptors are responsive to red light, which stimulates gonadal development (Woodard et al., Citation1969; Pyrzak et al., Citation1987). Retinal cone cells contain coloured oil droplets that filter light and pass the signal to photoreactive pigments (Bowmaker and Knowles, Citation1977). These cone cells respond maximally to violet, blue, green, and yellow (Dartnall et al., Citation1983). Longer wavelength light penetrates the skin and skull of a chicken to stimulate the pineal and pituitary glands, which control the secretion of reproductive hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), and 17 -estradiol (E2); these hormones enhance the growth and number of ovarian follicles (Mitchell, Citation1970). Gilbert et al. (Citation1983) assumed that the large yellow follicles (LYF) are recruited from the pool of small yellow and large white follicles in the ovary, which may be associated with the long sequences of laying. Therefore, light is a fundamentally important factor to increase the ovarian follicular populations, which influence laying rate. Renema et al. (Citation1998) reported that early maturing birds have a greater number of LYFs and more multiple LYF hierarchies compared to those in later maturing birds.
The European Union has prohibited incandescent lighting since 2012, and the Korean government has introduced a new energy-saving target of achieving 100% public-utility light emitting diode (LED) lighting by 2020. Thus, LED lights are becoming popular worldwide. However, most researchers have utilized monochromatic LED light colour to evaluate the performance of laying hens (Kim et al., Citation2012; Rozenboim et al., Citation1998), broiler breeders (Mobarkey et al., Citation2010), Japanese quail (Woodard et al., Citation1969), and turkey (Scott and Payne, Citation1937). Therefore, few studies are available that have examined a combination of light colours on ovarian morphology, reproductive hormones, and biochemical blood parameters in laying hens.
In this study, we evaluated the effects of different LED light colours on the performance, ovarian morphology, reproductive hormones, and biochemical blood parameters in Hy-line Brown laying hens.
Materials and methods
Bird management
A total of 600 Hy-line Brown pullets (12 weeks of age) were obtained from a commercial farm. The pullets were randomly housed in 24 environmental lightproof rooms (six treatments and each treatment replicated four times with 25 birds in each room). The birds were reared following the Hy-line Brown guidelines and were given access to feed (ME, 2750 Kcal/kg, CP 16%, Ca 3.9%, and AP 0.275%) and water throughout the study. The light schedule provided was 10L:14D starting from 12 weeks of age and increased stepwisely each week to reach 16 h light at the birds’ age of 23 weeks. The light treatments were as follows: i) red LED light (R), at a peak wavelength of 620 nm, half band width between 618 and 635 nm; ii) green light (G) at a peak wavelength of 520 nm, half band width between 515 and 535 nm; iii) blue light (B) at a peak wavelength of 460 nm, half band width between 455 and 470 nm; iv) control [compact fluorescent white (W), 400-770 nm]; v) R → G (red light was provided for 14 h in the morning 6.00 AM to 8.00 PM and then switched to G for 2 h from 8.00 PM to 10.00 PM daily); vi) R → G →B, in which R was provided for 12 h (6.00 AM to 6.00 PM) and then G for 2 h at 6.00 PM to 8.00 PM and switching to blue for 2 h at 8.00 AM to 10.00 AM daily). The LED plate (38×24 cm, Taejong C. & I. Co., Seoul, Korea) contained six lines and each line had 12, 8, and 7 lamps for the R, G, and B lights, respectively; and were installed at the top of each light-proof room. The electric voltages of the R, G, and B lamp devices were 2.0V, 3.2V, and 3.2V, respectively; all were using the same forward current of IF =60 mA. Temperature (21-23°C) and humidity (65-70%) were maintained by a ventilator, and an automatic lighting software program controlled daily light colour changes. Light intensity was measured at eight locations and had an average intensity of 0.1 w/m2 at the level of the birds’ head. The light plate was cleaned and intensity was adjusted fortnightly with an illuminance meter T-10 (Konica Minolta, Tokyo, Japan). Each pen was covered with rice husks (approximately 10 cm deep), a perch, and nest boxes, and density was maintained at 5 birds/m2.
Laying performance and egg quality
Egg production, egg weight, and feed consumption in each treatment were recorded daily. Consequently, egg mass (percentage of egg production × egg weight/100) and feed conversion (g of feed/g of egg mass) were calculated based on egg production, egg weight, and feed consumption. At the end of experiment, 30 eggs were randomly picked from each experimental group to assess egg quality parameters. Eggshell breaking strength was measured with an egg multi-tester instrument (QC-SPA, TSS, Cambridge, UK) and expressed as units of compressive force exposed to the unit eggshell surface area (kg/cm2). Then, the eggs were individually weighed and carefully broken on a glass plate and eggshell colour, albumin (ALB) height, Haugh units, and yolk colour were measured with egg quality equipment (QCM+System, TSS).
Ovarian morphology and reproductive hormone measurements
Pullets were killed humanly by neck dislocation on the first day of oviposition, and oviduct, ovary, and stromal weights were recorded. Deepest care was performed in handling, to avoid ovarian damage caused by excessive post-mortem movement. The weight (g) parameters measured included the body, breast, liver, ovary, stroma, and oviduct. The number of LYF was recorded, and LYF were counted, sorted by size, and weighed individually; the number of small yellow follicles in the stroma was also recorded. The remainder of the ovary was briefly rinsed in saline (9 g NaCl/L) and placed in 10% buffered formalin. The healthy follicles were sized after the yolk had hardened (2-3 days fixation). Subsequently, FSH and E2 levels were measured by homologous radioimmunoassay when the birds were 22 weeks of age, (Krishnan et al., Citation1993) assay kit (Coat-A-Count, DPC, Los Angeles, CA, USA).
Serum immunoglobulin-G, biochemical blood parameters, and heterophil:lymphocyte ratios
Peripheral blood samples (n=10 per treatment) were obtained at 22 weeks of age by wing vein puncture and allowed to clot for 2 h at room temperature, centrifuged at 1500 rpm for 15 min at 4°C, and the sera were collected and stored at -80°C until analyses. The serum samples were used to measure serum immunoglobulin-G (IgG) concentrations, using chicken IgG enzyme-linked immunosorbent assay quantitation kits (Bethyl Laboratories, Montgomery, TX, USA), as described by Perez-Carbajal et al. (Citation2010). Biochemical blood parameters including alanine aminotransaminase (ALT, U/L), glucose (GLU, mg/dL), total protein (TP, g/dL), ALB (g/dL), high density lipoprotein (mg/dL), and triglycerides (mg/mL) content were measured on a Konelab 20 analyser (Thermo Fisher Scientific Inc., Vantaa, Finland) using a turbidimetric method as described by the manufacturer. Blood (n=10 per treatment) was collected from the wing vein at 22 weeks of age, smears were prepared, and lymphocytes were counted at ×40 (oil immersion lens). Heterophil:lymphocyte (H/L) ratio was calculated according to Wall et al. (Citation2008).
Statistical analysis
All data were analysed using analysis of variance in the GLM program of SAS (Citation2005). Posthoc testing was performed with Duncan’s multiple range test (Steel and Torrie, Citation1980) and the statistical significance between the treatment intervals was determined at the 5% level.
Results
Egg production, feed intake and feed conversion ratio
The performance of the laying hens during the full trial period (22-57 weeks) is summarized in . The egg production rate in the different laying phase significantly (P<0.05) differed amongst the monochromatic and combined light treatments. Egg production increased under R light compared to that under the W, G, and B treatments. Average egg production increased linearly from 87.79 to 88.99% when the lighting treatment was switched from monochromatic R to the combined R → G treatment. However, further switching the light treatment from R → G to R → G → B had no additional effect on egg production. Thereafter, egg weight increased remarkably due to the G and B light. While egg mass was not significantly affected by the light colour, it was numerically increased by the R → G light. The similarity of feed intake among the light treatment and the beneficial effect of R → G light on egg production influenced more efficiently the feed conversion of laying hen (P<0.05).
Egg quality in laying hens
Eggshell breaking strength, albumin height, Haugh units, and yolk colour were not influenced by either the monochromatic or the combined light colour treatments but eggshell colour did differ by light colour (). Hens reared under the monochromatic R light showed increased shell colour.
Reproductive hormones and ovarian follicles
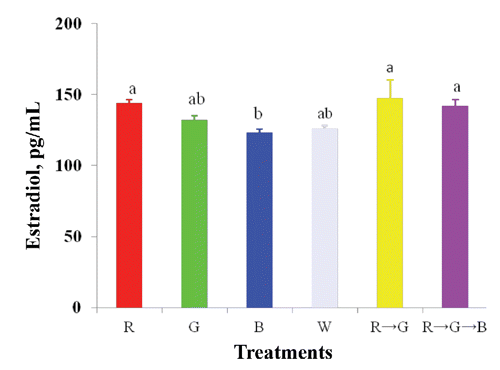
FSH and E2 concentrations increased significantly under the R and R → G light treatments ( and ). Light colour also influenced (P<0.05) body weight, age at sexual maturity, breast muscle, ovarian, and stromal weight and follicle number (). A higher body weight was obtained in the B treatment but birds matured 15 day later compared to those under R light (). Consequently, ovarian and stromal weights increased significantly under the R and R → G light treatments but were lower under the B and G treatments. The number of ovarian follicles also increased under the R and R → G treatments. In contrast, oviduct weight, total large follicle weight, and unexplained post-ovulatory follicle weight were not affected by the light treatments. The B and G treatments resulted in increased breast muscle weight compared to that in birds under the R treatment, but liver and spleen weights did not respond to the light treatments.
Blood properties in laying hens
Monochromatic or combined light colour did not affect serum TP, ALB, total cholesterol, or ALT content (), but significantly increased serum GLU and triglyceride concentrations in laying hens. Serum GLU increased under the combined R → G → B treatment, while it decreased under the B treatment, whereas higher triglyceride concentrations were found under the R treatment.
A higher serum IgG concentration was found under the B light treatment (). No difference in the H:L ratio was observed among the light-treated hens ().
Discussion
This experiment was conducted to identify the effects of combinations of light colour on performance, egg quality, blood parameters, ovarian morphology, and reproductive hormones in laying hens. Most researchers have focused on the effects of monochromatic light colour on laying hens (Kim et al., Citation2012, Rozenboim et al., Citation1998) and limited information is available on the effect of light combinations. Our results showed that R light increased egg production compared to that of W, G, and B light, which correspond with previous findings (Kim et al., Citation2012; Rozenboim et al., Citation1998). The increased average egg production under the 14 h R light with 2 h G light (R → G) treatment may have been due to the increase in serum FSH and E2 concentrations, as well as the increase in ovarian follicle number and, thus, enhanced egg production. Due to a lack of studies on the effects of light colour combinations on laying hens, a direct comparison was difficult.
Broiler breeder hens were provided R and G lighting for 6 of 14 h during the light period for 1 week (Mobarkey et al., Citation2010). Then, the G light was turned off in three rooms, and hens were exposed to R light for 14 h. In other three rooms, the R light was turned off and G lighting continued for 14 h. The results showed that the R light increased egg production compared to that in broilers under the G light (Mobarkey et al., Citation2010). These results are not consistent with our results, which might be due to differences in photoperiod or time of exposure to the different light combinations. Egg weight increased under the G and B light treatment due to the rate of egg production. Green and B light stimulated growth and enhanced body size and, thus, increased egg size. Similar results were reported by McGinnis et al. (Citation1986), Pyrzak et al. (Citation1987) and Kim et al. (Citation2012). However, W light increased egg weight in laying hens from other experiments (Er et al., Citation2007; Kim et al., Citation2012), and R light either increases egg weight in turkey hens (Pyrzak and Siopes, Citation1986) or light treatment has no effect (Rozenboim et al., Citation1998; Lewis et al., Citation2007). Harrison et al., (Citation1969) reported that the difference in egg weight is a consequence of egg production rate rather than the light treatment, which is consistent with the present results. The present study indicates that layers under R → G light were efficient in mean feed conversion due to the sensitivity of the light wavelengths. Rathinam and Kuenzel (Citation2005) mentioned that photoreceptors were located in the eyes (retina), pineal gland and deep brain tissue. However, Saldanha et al., (Citation2001) reported that brain photoreceptors communicate directly with GnRH neurons that activate the reproductive axis. Therefore, the shift from R to G in the later part of the day stimulate the reproductive axis and thus reflected by a significant increase of egg production and mean FCR of laying hen. Eggshell colour increased significantly in hens under the monochromatic R treatment. This meaning of this result is unclear. One possible reason might be that the combination light treatment enhanced the epithelial cell lining of the surface of the shell gland, which synthesizes and accumulates biliverdin-IX pigments (Butcher and Miles, Citation2003). Serum FSH concentration increased under the R and R → G treatments and E2 concentration increased under the R, R → G and R → G → B treatments. These results suggest that the long wavelength stimulated extra retinal photoreceptors, which reflect on the pituitary gland and deep brain and influence gonadal hormone (FSH, LH, and E2) secretion and subsequent rapid development of the oviduct (Hartwig and Veen, Citation1979). Therefore, FSH is responsive to follicular recruitment and growth of smaller follicles (Palmer and Bahr, Citation1992; Mobarkey et al., Citation2010). Higher body weight was found in hens exposed to the B treatment, and these hens matured 15 day later compared to those under R light. This may have occurred due to the higher circulating FSH levels and faster rate of follicular recruitment under the R light treatment; thus, stimulating sexual maturity (Lewis et al., Citation1998). In contrast, Harrison et al. (Citation1969) and Lewis et al., (Citation2007) reported that pullets mature significantly earlier under short wavelength (440-540 nm) than those under long wavelength (560-660 nm) light; nevertheless, Pyrzak et al. (Citation1986) found no differences in sexual maturity. These differences may have been due to differences in light sources.
Ovarian and stromal weights were significantly higher in the R and R → G light-treated birds but lower in the B and G light-treated groups due to the larger size and greater number of LYF present in the R group. Consequently, follicle number (1-3 mm and 4-6 mm in size) increased in the R and R → G groups. The small yellow follicles and LWFs provide a constant supply of growing follicles for the hierarchy (Gilbert et al., Citation1983) and these developed follicles form yellow-yolky follicles in the ovary and finally influence lay rate. However, Pyrzak et al. (Citation1986) did not find a difference in the rate of gonadal maturation in pullets exposed to B, G, R, or W monochromatic light. Breast muscle weight is significantly higher under B and G treatments but lower under the R treatment due to faster growth rate and body size (Rozenboim et al., Citation2004).
Blood GLU content was remarkably higher under the combined R → G → B treatments but lower under the B treatment. Although the mechanism involved in this result remains to be investigated, it could be related to metabolic adaptation for gluconeogenesis (Corzo et al., Citation2005). In contrast, triglycerides were significantly higher in birds under R light but lower in those under the B treatment, which may have been due the calming effect of the B treatment on chicken activity (Prayitno et al., Citation1997), which would decrease blood pressure and reflect on blood GLU and triglyceride levels. IgG concentration and H:L were not significantly affected by most of the light treatments but did increase under B light, which corresponded to the results of Xie et al., (Citation2008).
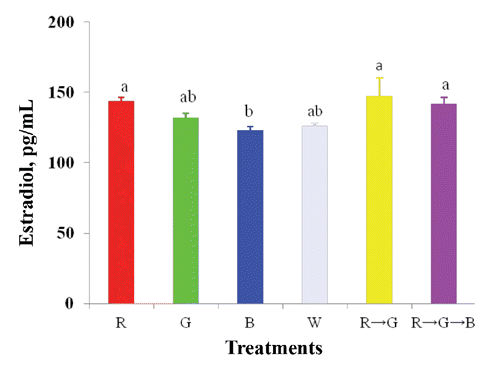
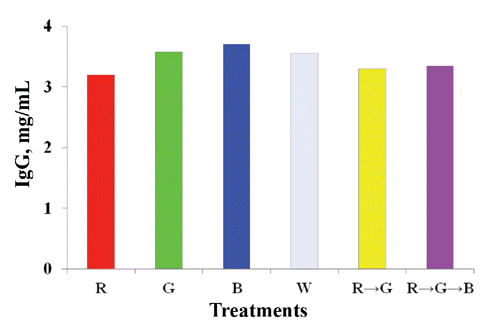
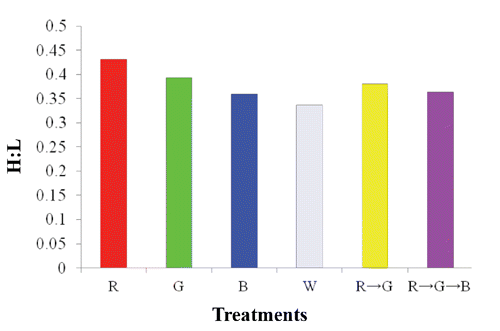
Table 1. Effect of LED light colours on the performance (22-57 weeks) of laying hens.
Table 2. Effect of different LED light colours on egg quality in laying hens at 24 weeks of age.
Table 3. Effect of different LED light colours on ovarian parameters of laying hens at 22 weeks of age.
Table 4. Effect of LED light colours on the biochemical blood parameters of laying hens at 22 weeks of age.
Conclusions
In conclusion, light colour influenced egg production, which was enhanced by combinations of light colour during the rearing period. Applying 14 h R with 2 h G light (R → G) at the latter part of the day resulted in higher reproductive hormone levels, ovarian weight, and follicle number, which increased egg production. Thus, the present result suggests that a combination of R and G light may be comparable to R light in laying hens. A further followup study is needed on the influence of LED lights on stress and immune responses of laying hens.
References
- BowmakerJ.K. KnowlesA. 1977. The visual pigments and oil droplets of the chicken retina. Vision Res. 17:755-764.
- ButcherG.D. MilesR.D. 2003. Factors causing poor pigmentation of brown shelled eggs. Available from: http://edis.ifas.ufl.edu/vm047
- CorzoA. MoranE.T. HoehlerD. LemmeA. 2005. Dietary tryptophan need of broiler males from 42-56 days of age. Poultry Sci. 84:226-231.
- DartnallH.J.A. BowmakerJ.K. MollonJ.D. 1983. Human visual pigments: microspectrophotometric results from the eyes of seven persons. P. Roy. Soc. Lond. B Bio. 220:115-130.
- ErD. WangZ CaoJ. ChenY. 2007. Effect of monochromatic light on the egg quality of laying hens. J. Appl. Poultry Res. 16:605-612.
- GilbertA.B. PerryM.M. WaddingtonD. HardieM.A. 1983. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus). J. Reprod. Fertil. 69:221-227.
- HarrisonP. McGinnisJ. ShumaierG. LauberJ. 1969. Sexual maturity and subsequent reproductive performance of white leghorn chickens subjected to different parts of the light spectrum. Poultry Sci. 48:878-883.
- HartwigH.G. VeenT. 1979. Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors. J. Comp. Physiol. A 130: 277-282.
- KimM.J. HossanM.S. NazmaA. JaeC.N. HanB.T. HwanK.K. DongW.K. HyunS.C. HeeC.C. OkS.S. 2012. Effect of monochromatic light on sexual maturity, production performance and egg quality of laying hens. Avian Biol. Res. 5:69-74.
- KrishnanK.A. ProudmanJ.A. BoltD.J. BahrJ.M. 1993. Development of an homologous radioimmunoassay for chicken follicle-stimulating hormone and measurement of plasma FSH during the ovulatory cycle. Comp. Biochem. Physiol. Comp. Physiol. 105A:729-734.
- LewisP.D. CastonL. LeesonS. 2007. Green light during rearing does not significantly affect the performance of egg-type pullets in the laying phase. Poultry Sci. 86:739-743.
- LewisP.D. MorrisT.R. 1998. Responses of domestic poultry to various light sources. World. Poultry Sci. J. 154:21-25.
- McGinnisJ. RamirezH. BoydJ. LauberJ.K. 1986. Effect of using coloured light during the rearing and laying periods on performance of hens. Poultry Sci. 45:1104 ( abstr.).
- MitchellM.E. 1970. Treatment of hypophysectomised hens with partially purified avian FSH. J. Reprod. Fertil. 2:223-241.
- MobarkeyN. AvitalN. HeiblumR. RozenboimI. 2010. The role of retinal and extra-retinal photostimulation in reproductive activity in broiler breeder hens. Domest. Anim. Endocrin. 38:235-243.
- PalmerS.S. BahrJ.M. 1992. Follicle stimulating hormone increases serum oestradiol-17, number of growing follicles and yolk deposition in aging hens (Gallus domesticus) with decreased egg production. Brit. Poultry Sci. 33:403-414.
- Perez-CarbajalC. CaldwellD. FarnellM. StringfellowK. PohlS. CascoG. Pro-MartinezA. Ruiz-FeriaC.A. 2010. Immune response of broiler chickens fed different levels of arginine and vitamin E to a coccidiosis vaccine and Eimeria challenge. Poultry Sci. 89:1870-1877.
- PrayitnoD.S. PhillipsC.J.C. StokesD.K. 1997. The effects of colour and intensity of light on behaviour and leg disorders in broiler chickens. Poultry Sci. 76:1674-1681.
- PyrzakR. SiopesT.D. 1986. The effect of light colour on egg quality hens in cages. Poultry Sci. 65:1262-1267.
- PyrzakR. SnapirN. GoodmanG. PerekM. 1987. The effect of light wavelength on the production and quality of eggs of the domestic hen. Theriogenology 28:947-960.
- RathinamT. KuenzelW. J. 2005. Attenuation of gonadal response to photostimulation following ablation of neurons in the lateral septal organ of chicks. Brain Res. Bull. 64:455-461.
- RenemaR.A. RobinsonF.E. MelnychukV.L. ClassenH.L. 1998. Reproductive organ morphology and carcass traits in naturallymating female bronze turkeys at onset of lay. Can. J. Anim. Sci. 78:181-187.
- RozenboimI. BiranI. ChaisehaY. YahavS. RosenstrauchA. SklanD. HalevyO. 2004. The effect of a green and blue monochromatic light combination on broiler growth and development. Poultry Sci. 83:842-845.
- RozenboimI. ZilbermanE. GvaryahuG. 1998. New monochromatic light source for laying hen. Poultry Sci. 77:1695-1698.
- SaldanhaC.J. SilvermanA.J. SilverR. 2001. Direct innervation of GnRH neurons by encephalic photoreceptors in birds. J. Biol. Rhythm. 16:39-49.
- SAS, 2005. User’s guide, ver. 9.1. SAS Inst. Inc., Cary, NC, USA.
- ScottH.M. PayneL.F. 1937. Light in relation to the experimental modification of the breeding season of turkeys. Poultry Sci. 16:90-96.
- SteeleR.G.D. TorrieJ.H. 1980. Principles and Procedures of statistics: a biometrical approach. 2nd ed. McGraw-Hill Publ., New York, NY, USA.
- WallH. TausonR. ElwingerK. 2008. Effects of litter substrate and genotype on layers’ use of litter, exterior appearance, and heterophil: lymphocyte ratios in furnished cages. Poultry Sci. 87:2458-2465.
- WoodardA.E. MooreJ.A. WilsonW.O. 1969. Effect of wave length of light on growth and reproduction in Japanese quail (Coturnix coturnix japonica). Poultry Sci. 48:118-123.
- XieD. WangZ.X. DongY.L. CaoJ. WangJ.F. ChenJ.L. ChenY.X. 2008. Effects of monochromatic light on immune response of broilers. Poultry Sci. 87:1535-1539.