Abstract
The aim of this study was to evaluate whether the developmental speed affects the cryotolerance of in vitro produced buffalo embryos. In Experiment 1, abattoir-derived oocytes were in vitro matured, fertilized and cultured. The embryos produced by Say 7 of culture were vitrified at the tight morula (TM), early blastocyst (EBL), blastocyst (BL), expanded-blastocyst (XBL) and hatched-blastocyst (HBL) stage. The embryos were vitrified by cryotop in 16.5% ethylene glycol (EG) and 16.5% dimethyl sulfoxide (DMSO) and 0.5 M sucrose. Embryos were warmed in 0.25 M sucrose for 1 min and then in 0.15 M sucrose for 5 min and cultured in vitro for 24 h, to evaluate post-culture viability. In Experiment 2, ovum pick-up (OPU) was carried out on lactating buffaloes to produce embryos that were vitrified-warmed and transferred into synchronized recipients. The lowest (P<0.01) survival rates were recorded with TM (22.4%) and the highest (P<0.01) with HBL (84.5%), whereas intermediate results were observed with EBL, BL and XBL (54.5, 64.7, 67.9%, respectively). Pregnancy rate on both Days 25 and 45 was significantly higher (P<0.05) for faster (XBL and HBL) developing embryos (75.0% and 62.5%, respectively) than for the slower (TM, EBL and BL) developing counterparts (36.4% and 0, respectively). Interestingly, pregnancies to term were only recorded when HBL were transferred (20%). In conclusion, it was demonstrated that the chronology of development is a major factor affecting the cryotolerance of in vitro produced buffalo embryos.
Introduction
In the last two decades interest in buffalo breeding has tremendously increased worldwide, due to the critical role played by this species both in developing countries in tropical and sub-tropical environments and in developed countries, as an important source of high quality animal proteins, both milk and meat (Campanile et al., Citation2010). In the current scenario, the competitiveness of buffalo breeding highly depends on genetic improvement.
In buffalo the ovum pick-up (OPU) and in vitro embryo production (IVEP) technology are the best tools to improve the genetic merit through the maternal lineage. Despite the high efficiency in blastocyst rate (Gasparrini et al., Citation2006), the diffusion in the field of IVEP technology in buffalo species is still hampered by two major factors: the low number of oocytes that can be recovered per donor and the low pregnancy rate after transfer of cryopreserved in vitro produced (IVP) embryos (Gasparrini, Citation2002). While the first factor is not easy to improve, arising from physiological features of the species (Kumar, Citation1997; Gasparrini, Citation2002), the improvement of embryo cryopreservation efficiency is currently critical. Indeed, buffalo IVP embryos are very sensitive to cryopreservation, likely due to their high lipid content (Gasparrini, Citation2002). Nevertheless, they have been successfully cryopreserved by vitrification, as demonstrated by their survival following in vitro culture (Gasparrini et al., Citation2001) and development to term after embryo transfer (ET) (Hufana-Duran et al., Citation2004; Neglia et al., Citation2004). However, efficiency still needs to be improved for the diffusion of OPU-IVEP technologies in the field.
Over the years the efficiency of vitrification has improved by developing innovative methods based on the utilization of minimum volumes and direct contact with liquid nitrogen. Buffalo IVP embryos have been successfully vitrified in pointed-shaped open straw (Hufana-Duran et al., Citation2004) and in open pulled straws (De Rosa et al., Citation2006). The cryotop vitrification (CTV) method, that allows to reach very high cooling and warming rates, has been proven feasible to cryopreserve nuclear transfer hatched buffalo blastocysts (Laowtammathron et al., Citation2005). Furthermore, a a preliminary trial suggested that this method can be used for IVP buffalo embryos (De Rosa et al., Citation2007).
It is known that embryo quality is the major factor affecting the resistance to cryopreservation and that the chronology of development is a reliable indicator of embryo quality (Bavister, Citation1995). Buffalo embryos in vitro develop approximately 12 to 24 h earlier than cattle embryos (Galli et al., Citation2000). On Day 6 of culture it is possible to find embryos in advanced stages of development, including hatched blastocysts but most embryos reach the blastocyst stage on Day 7 (Gasparrini, Citation2002). A certain proportion of embryos are delayed, reaching the blastocyst stage on Day 8 and later but their quality and viability is poor (Gasparrini et al., Citation2001). Interestingly, also within Day 7, assumed usually as the end of culture in our laboratory, we observe transferable embryos at various stages of development, indicating a different developmental speed. It is worth reminding that another important factor limiting the feasibility of the IVEP technology in buffalo is the low number of oocytes (Kumar, Citation1997) and, hence of embryos that can be produced per donor/session (Gasparrini, Citation2002). This avoids to operate a strict selection of the best quality embryos prior to cryopreservation, as currently occurs for other species.
Therefore, the aim of this study was to evaluate to which extent the chronology of development affects the cryotolerance of IVP buffalo embryos vitrified by CTV. In particular, this was evaluated by assessing survival rates after in vitro culture of vitrified-warmed embryos (Experiment 1) and pregnancy rates after transfer of vitrified-warmed embryos into synchronized recipients (Experiment 2).
Materials and methods
Experimental design
In Experiment 1, abattoir-derived oocytes (n=1193; over 12 replicates) were used to produce 358 transferable embryos that were vitrified-warmed and cultured to evaluate the survival and the development rates after 24 h in vitro culture post-warming. Prior to vitrification, embryos were categorized as slow developing embryos, i.e. the tight morulae (TM), early blastocysts (EBL), blastocysts (BL), and as fast developing embryos, i.e. those that reached the stages of expanded blastocysts (XBL) and hatched blastocysts (HBL) by the end of culture (Day 7). In order to verify whether the developmental speed affects the resistance to cryopreservation, embryos that reached by Day 7 different developmental stages (TM=49; EBL=55; BL=51; XBL=106; HBL=97) were compared.
In Experiment 2, OPU-derived oocytes (n=189) were used to produce 38 embryos, that were vitrified-warmed and transferred into synchronized recipients to assess pregnancy rates. In this case, due to the limited number of cases, the in vivo survival rates of slow developing embryos, i.e TM (n=4), EBL (n=10) and BL (n=8), and fast developing embryos, i.e. XBL (n=6) and HBL (n=10) were compared. The institutional Ethical Animal Care and Use Committee of the University of Naples Federico II approved the experimental design and animal treatments (Prot 2012/0084952).
Unless otherwise stated, reagents were purchased from Sigma Chemical Company (Milano, Italy).
Oocyte source and in vitro maturation
In Experiment 1, buffalo ovaries were collected from a local abattoir, transported to the lab within 3 to 4 h after slaughter at 30°C to 35°C in physiological saline supplemented with 150 mg/L kanamycin. Cumulus-oocyte complexes (COCs) were recovered by aspiration of 2 to 8 mm follicles using an 18G needle under vacuum (40 to 50 mmHg). The COCs were evaluated on the basis of their morphology and Grades A and B COCs, that are considered suitable for IVEP (Di Francesco et al., Citation2011), were selected, washed in 25 mM Hepesbuffered TCM199 (H199) + 10% fetal calf serum (FCS), and allocated to 50 µL drops under mineral oil (10 per drop) of IVM medium, i.e. TCM199 buffered with 25 mM sodium bicarbonate (B199) and supplemented with 10% FCS, 0.2 mM sodium pyruvate, 0.5 µg/mL FSH, 5 µg/mL LH, 1 µg/mL 17 -estradiol, 50 µg/mL kanamycin, 50 µM cysteamine and 0.3 mM cystine (Gasparrini et al., Citation2006).
In Experiment 2, ovum pick-up was performed twice/week on 10 lactating pluriparous buffalo cows, over 10 sessions, as previously described (Neglia et al., Citation2004). The donors were under controlled nutrition, barn-housed, and the farm was in appropriate sanitary condition. They were restrained in a squeeze chute and given an epidural administration of 4 to 6 mL 2% lidocaine (Farmaceutici Gellini Spa, Aprila, RM, Italy) at the moment of the oocyte retrieval session. During the study, buffalo cows did not show any behavioral modification and the OPU treatment did not cause any adverse effect. COCs were searched immediately after follicular aspiration by using proper filters (Emcon Technologies, Columbus, IN, USA), and Grade A, B and C COCs were placed in H199 supplemented with 10% FCS, 0.5 mg/mL FSH, 5 mg/mL LH, 1 mg/mL 17β-estradiol, 0.3 mM cystine and 50 µM cys-teamine in a portable incubator at 38.5°C and moved to the lab within 4 to 6 h, where they were transferred into 50 µL droplets of the final in vitro maturation (IVM) medium. The IVM was carried out at 38.5°C for 22 h in a controlled gas atmosphere of 5% CO2 in humidified air.
In vitro fertilization and culture
Spermatozoa were prepared from frozen-thawed semen, obtained from a bull previously tested for the in vitro fertilization (IVF) in our laboratory. Sperm were treated by swim-up procedure in Sydney IVF Gamete Buffer (Cook, Nova Milanese, MI, Italy) medium for 1 h and the pellet obtained after centrifugation of the supernatant, was re-suspended to a final concentration of 2×106 mL1 in the IVF medium, that was Tyrode albumin lactate pyruvate (Lu et al., Citation1987) supplemented with 0.2 mM penicillamine, 0.1 mM hypotaurine and 0.01 mM heparin. Insemination was performed in 50 µL drops of IVF medium under mineral oil (5 oocytes/drop) at 38.5°C under humidified 5% CO2 in air. Twenty hours after IVF zygotes were stripped of cumulus cells by gentle pipetting, washed in HEPES-buffered synthetic oviduct fluid (SOF) and allocated (10 per drop) to 20 µL drops of in vitro culture (IVC) medium, that was SOF including essential and non-essential amino acids and 8 mg/mL bovine serum albumin (Tervit et al., Citation1972). Culture was carried out under humidified 5% CO2, 7% O2 and 88% N2 at 38.5°C. On day 5 (Day 0=IVF) cleavage rate was assessed and the embryos were transferred into fresh medium for further 2 days. The embryos obtained by the end of culture (Day 7) were scored for quality, on the basis of morphological criteria (Robertson and Nelson, Citation1998), and for developmental stage, as previously described.
Vitrification and warming
The IVP embryos were vitrified by the Cryotop method, previously described (Kuwayama et al., Citation2005) for human oocytes, and used for buffalo embryos (De Rosa et al., Citation2007). After washing in HEPES-buffered SOF, the embryos were incubated in 200 l drops of equilibration solution, consisting in 7.5% DMSO and 7.5% of EG in H199 + 20% FCS for 3 minutes. Subsequently, the embryos were transferred in 20 µl drops of the final vitrification solution, consisting of 16.5% DMSO, 16.5% of EG and 0.5 M sucrose in H199 + 20% FCS. Embryos were then placed in <0.1 µL of the solution on the end of a cryotop that was immersed in LN2 within 20 to 25 sec. For warming, the cryotop was taken from LN2 and the tip was immediately immersed in 1 mL of a 0.5 M solution of sucrose in H199 + 20% FCS for 1 minute. The recovered embryos were moved in 0.25 M sucrose solution for 5 minutes, washed in HEPES-buffered SOF and either cultured for 24 h as previously described (Experiment 1) or loaded in straws for embryo transfer (ET, Experiment 2).
In vitro assessment of embryo survival
After 24 h culture, post-warming survival rates were evaluated with morphological criteria, on the basis of the integrity of the embryo membrane and the zona pellucida (with the exception of hatched blastocysts), and reexpansion of the blastocoele. Furthermore, the percentages of surviving embryos that resumed their development and reached a more advanced developmental stage after 24 h culture were recorded (development rate). Obviously, this parameter was not considered in case of HBL as they had already reached the most advanced stage before vitrification.
Embryo transfer and pregnancy diagnosis
The recipient animals were synchronized by Ovsynch program, consisting in the administration of a GnRH agonist (buserelin acetate, 12 mg; Receptal®, Intervet) on Day 0, a PGF2 analogue (luprostiol, 15 mg; Prosolvin®, Intervet) on Day 7, and GnRH agonist (12 mg) again on Day 9. The day 7 embryos were transferred on Day 15 recipients, corresponding to 6.5 days after the last GnRH treatment. Embryonic development was assessed on day 25 by trans-rectal ultrasonography, with an Aloka SSD-500 unit equipped with a 5.0 MHz linear array probe (Aloka Co., Tokyo, Japan). Pregnancy diagnosis was confirmed on Day 45 by rectal palpation. Buffaloes pregnant on Day 25 but not on Day 45 were considered to have undergone late embryonic mortality (LEM). The pregnant buffaloes were then monitored up to term to evaluate calving rate.
Statistical analysis
Differences in survival and developmental rates after vitrification-warming among the stages of development were analyzed by the c2-test. Differences in pregnancy rates between slow and fast developing embryos were also analyzed by χ2-test.
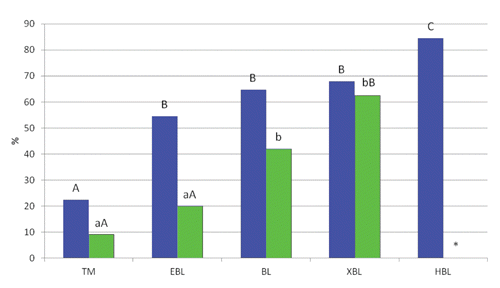
Table 1. Pregnancy and embryonic mortality rates after embryo transfer of in vitro produced buffalo embryos with different developmental speed, i.e. embryos that reached by the end of culture (Day 7) the stages of tight morula, early blastocyst, blastocyst, expanded blastocyst and hatched blastocyst.
Results and discussion
In Experiment 1, the average cleavage rate was 65.4% and the percentage of transferable embryos, i.e. tight morulae and blastocysts (TMBL) was 30.0%. Regardless of the stage of development, the survival and developmental rates of buffalo embryos vitrified by CTV after 24 h culture were 63.7% and 31.3%, respectively. However, differences in both parameters were recorded among the stages of development. As shown in , the lowest (P<0.01) survival rates were recorded with TM (22.4%) and the highest (P<0.01) with HBL (84.5%), whereas intermediate results were observed with EBL, BL and XBL (54.5, 64.7 and 67.9%, respectively). A similar pattern was found when we considered the development rate, i.e. the percentage of embryos that over 24 h culture developed to a further stage of development (). Similar development rates were recorded with TM (9.1%) and EBL (20.0%), that were lower than those obtained with BL (42.4%; P<0.05) and XBL (62.5%; P<0.01). Also if we compare the slow developing embryos (TM+EBL+BL) with the fast developing counterparts (XBL+HBL), the latter show a clear improvement of both survival rates (75.9% vs 47.7%; P<0.01) and development rates (59.1% vs 28.4%; P<0.01). The increased resistance to cryopreservation of faster developing embryos was previously observed in cattle (Nedambale et al., Citation2004). In contrast, an earlier study reported high hatching rates after 72 h culture for buffalo embryos of all developmental stages, including morulae (Hufana-Duran et al., Citation2004). It is not clear, however, whether in that study the embryos of different stages had the same age of culture.
The results of Experiment 1 demonstrated that CTV is an efficient method to cryopreserve advanced IVP embryos in buffalo species, as indicated by the higher survival rates compared to those previously reported employing both the traditional straws (Neglia et al., Citation2004) and open pulled straw (De Rosa et al., Citation2006). Similar survival rates were reported for buffalo nuclear transfer HBL cryopreserved by CTV in an earlier work (Laowtammathron et al., Citation2005).
In Experiment 2, the average (± SE) number of follicles, oocytes and viable oocytes observed per buffalo per session were 6.9±0.3, 2.8±0.2 and 1.2±0.1. Cleavage and TMBL rates were 59.7 and 31.6%, respectively. The results of Experiment 2 indicated that the developmental speed also affects the in vivo survival of cryopreserved IVP buffalo embryos after ET (). Indeed, an improvement of pregnancy rate was recorded for fast developing embryos on day 25. Furthermore, all the slow developing embryos underwent LEM between 25 and 45 days, whereas limited embryonic loss was observed in case of fast developing embryos. This temporal window is particularly critical in buffalo because in this species the highest incidence of LEM is observed between 25 and 45 days after natural mating and artificial insemination (AI) (Campanile et al., Citation2010). Interestingly, pregnancy rate on day 25 was higher than in all previous trials; in fact, regardless of the stage of the transferred embryos, it was over 50%, to reach 75% in the case of fast developing embryos. Furthermore, in the latter case, pregnancies were maintained at high levels (62.5%) also on Day 45. However, the overall pregnancy to term was only 5.3%, reaching 12.5% in case of faster developing embryos. Therefore, it is evident that in case of ET of IVP embryos, fetal mortality also occurs. Interestingly, the two pregnancies to term obtained in this trial came from the transfer of HBL, i.e. the embryos showing the highest developmental speed.
The overall pregnancy rate here recorded was similar to that previously reported in another study after ET of vitrified embryos (Hufana-Duran et al., Citation2004). On the contrary, it was slightly lower than that we previously obtained in buffalo heifers in an earlier study, in which embryos were vitrified with 3.4 M glycerol and 4.6 M EG in French straws (Neglia et al., Citation2004).
The results of this study clearly demonstrated that the in vitro chronology of development is a major factor affecting the cryotolerance of IVP buffalo embryos, assessed as both in vitro and in vivo survival rates. It is worth reminding that, as in this study the endpoint of culture for all the embryos was Day 7, it is likely that the improved freezability of the advanced stages here reported was due to their quality, indicated by the faster developmental speed. However, we cannot rule out that stage-related ultrastructural/physico-chemical properties also played a role. Indeed, many studies demonstrated in different species that earlier developmental stages are less resistant to cryopreservation due to intrinsic structural differences (Vajta et al., Citation1996), such as the higher lipid content (Dobrinsky and Johnson, Citation2004), the lower resistance of cell membranes to osmotic and toxic effects, due to the larger size of the blastomeres (Tachikawa et al., Citation1993), and the greater damages to the cytoskeleton (Overstrom et al., Citation1993). Despite the evidence in other species of a stage-specific response to cryopreservation, we cannot attribute the lower freezability of TM and EBL to characteristics intrinsic to the stage because in this study the earlier stages considered were delayed and hence less viable. It would be, therefore, necessary to compare embryos of different stages showing a normal growth pattern in vitro to rule out an additional stage effect. The results of an earlier trial, however, suggest that additional days of culture will not improve the freezability of delayed embryos. Indeed, a lower survival rate after vitrification was found for buffalo IVP embryos that reached the blastocyst stage on day 8 rather than on days 6 or 7, confirming that embryos that develop later to the blastocyst stage are less viable (Gasparrini et al., Citation2001).
The importance of the developmental speed is strengthened by the observation that early cleaving buffalo embryos give higher blastocyst production along with a higher cell number (Totey et al., Citation1996). Furthermore, an altered expression profile of developmentally important genes has been recently reported in slower-developing buffalo embryos (Rajhans et al., Citation2010).
Conclusions
In conclusion, it was demonstrated that CTV is a reliable method for cryopreserving buffalo IVP embryos, as indicated by the high in vitro survival rates and by the establishment of pregnancy. It was also proven that fast developing embryos are less sensitive to the cryopreservation injuries. The results of this study suggest that it is advisable to select the IVP buffalo embryos to be cryopreserved and transferred, even in relation to their developmental speed. On the other hand, a strict selection will decrease the number of transferable embryos, leading to an unfavorable cost-benefit ratio of IVEP. Therefore, as the embryo quality is somewhat due to the oocyte quality but mainly to the culture conditions, the optimization of the culture system is compulsory in order to improve the efficiency of embryo cryopreservation in buffalo species.
Acknowledgments
the authors gratefully acknowledge Dr. Serena Di Francesco for critical reading of the manuscript.
This research was supported by the MIURPRIN/2008 Project “Strategies aimed to improve the efficiency of reproductive biotechnologies in buffalo species”.
References
- BavisterB.D. 1995. Culture of preimplantation embryos: facts and artifacts. Hum. Reprod. Update 1:91-148.
- CampanileG. BaruselliP.S. NegliaG. VecchioD. GasparriniB. GimenesL.U. ZicarelliL. D’OcchioM.J. 2010. Ovarian function in the buffalo and implications for embryo development and assisted reproduction. Anim. Reprod. Sci. 121:1-11.
- De RosaA. AttanasioL. BocciaL. VecchioD. CampanileG. GasparriniB. 2007. Cryotop vitrification for in vitro produced bovine and buffalo (Bubalus bubalis) embryos at different stages of development. Ital. J. Anim. Sci. 6(Suppl.2):747-750.
- De RosaA. Di PaloR. AttanasioL. MonacoE. CampanileG. GasparriniB. 2006. Open pulled straw vitrification for in vitro produced buffalo (Bubalus bubalis) embryos. Reprod. Fertil. Dev. 18:153.
- Di FrancescoS. NovoaM.V. VecchioD. NegliaG. BocciaL. CampanileG. ZicarelliL. GasparriniB. 2011. Ovum pick-up and in vitro embryo production (OPU-IVEP) in Mediterranean Italian Buffalo performed in different seasons. Theriogenology 77:148-154.
- DobrinskyJ.R. JohnsonL.A. 2004. Cryopreservation of porcine embryos by vitrification: A study of in vitro development. Theriogenology 42:25-35.
- GalliC. CrottiG. NotariC. TuriniP. DuchiR. LazzariG. 2000. Embryo production by ovum pick-up from live donors. Theriogenology 55:1341-1357.
- GasparriniB. 2002. In vitro embryo production in buffalo species: state of the art. Theriogenology 57:237-256.
- GasparriniB. BocciaL. MarchandiseJ. Di PaloR. GeorgeF. DonnayI. ZicarelliL. 2006. Enrichment of in vitro maturation medium for buffalo (Bubalus bubalis) oocytes with thiol compounds: effects of cystine on glutathione synthesis and embryo development. Theriogenology 65:275-287.
- GasparriniB. NegliaG. Caracciolo di BrienzaV. CampanileG. Di PaloR. ZicarelliL. 2001. Preliminary analysis of vitrified in vitro produced embryos. in Proc. 27th Int. Conf. IETS, Omaha, NE, USA. Theriogenology 55:307.
- Hufana-DuranD. PedroP.B. VenturinaH.V. HufanaR.D. SalazarA.L. DuranP.G. CruzL.C. 2004. Post-warming hatching and birth of live calves following transfer of in vitro-derived vitrified water buffalo (Bubalus bubalis) embryos. Theriogenology 61:1429-1439.
- KumarA. SolankiV.S. JindalS.K. TripathiV.N. JainG.C. 1997. Oocytes retrieval and histological studies of follicular population in buffalo ovaries. Anim. Reprod. Sci. 47:189-195.
- KuwayamaM. VajtaG. KatoO. LeiboS.P. 2005. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod. Biomed. Online 11:300-308.
- LaowtammathronC. LorthongpanichC. Ketudat-CairnsM. HochiS. ParnpaiR. 2005. Factors affecting cryosurvival of nuclear-transferred bovine and swamp buffalo blastocysts: effects of hatching stage, linoleic acid-albumin in IVC medium and Ficoll supplementation to vitrification solution. Theriogenology 64:1185-1196.
- LuK.H. GordonI. GallagherM. Mc GovernH. 1987. Pregnancy established in cattle by transfer of embryos derived from in vitro fertlization of oocytes matured in vitro. Vet. Rec. 121:259-260.
- NedambaleT.L. DinnyésA. YangX. TianX.C. 2004. Bovine blastocyst development in vitro: timing, sex, and viability following vitrification. Biol. Reprod. 71:1671-1676.
- NegliaG. GasparriniB. Caracciolo di BrienzaV. Di PaloR. ZicarelliL. 2004. First pregnancies to term after transfer of buffalo vitrified embryos entirely produced in vitro. Vet. Res. Commun. 28(Suppl.1): 233-236.
- OverstromE.W. DubyR.T. DobrinskyJ.R. RoblJ.M. BaguisiA. LonerganP. DuffyP. WalshJ.H. RocheJ.F. BolandM.P. 1993. Cytoskeletal damage in vitrified and frozen embryos. Theriogenology 39:276.
- RajhansR. KumarG.S. DubeyP.K. SharmaG.T. 2010. Effect of timing of development on total cell number and expression profile of HSP-70.1 and GLUT-1 in buffalo (Bubalus bubalis) oocytes and preimplan-tation embryos produced in vitro. Cell. Biol. Int. 34:463-468.
- RobertsonI. NelsonR.E. 1998. Certification and identification of the embryo. In: StringfellowD.A. SeidelS.M. ( eds.) Manual of the International Embryo Transfer Society, 3rd ed. IETS Publ., Savoy, IL, USA, pp 103-134.
- TachikawaS. OtoiT. KondoS. MachidaT. KasaiM. 1993. Successful vitrification of bovine blastocysts, derived by in vitro maturation and fertilization. Mol. Reprod. Dev. 34:266-271.
- TervitH.R. WhittinghamD.G. RowsonL.E. 1972. Successful culture in vitro of sheep and cattle ova. J. Reprod. Fertil. 30:493-497.
- ToteyS.M. DaliriM. Appa RaeK.B.C. PawsheC.H. TanejaM. ChillarR.S. 1996. Differential cleavage and developmental rates and their correlation with cell number and sex ratios in buffalo embryos generated in vitro. Theriogenology 45:521-533.
- VajtaG. HolmP. GreveT. CallesenH. 1996. Factors affecting survival rates of in vitro produced bovine embryos after vitrification and direct in straw-straw rehydration. Anim. Reprod. Sci 45:191-200.