Abstract
This study presents artificial induction using tench eggs, Tinca tinca (L.), of androgenetic origin. The oocytes taken from common bream, Abramis brama (L.) and common carp, Cyprinus carpio L. were genetically inactivated using UV irradiation and then inseminated using tench spermatozoa. Androgenetic origin (haploid or diploid embryos) was checked using a recessive colour (blond) and morphological markers. The percentage of hatched embryos in all experimental groups was much lower than in the control groups. All haploid embryos showed morphological abnormalities, which were recorded as haploid syndrome (stunted body, poorly formed retina, etc.). The optimal dose of UV irradiation of common bream and common carp eggs was 3456 J m–2. At this dose, almost 100% of haploid embryos were produced at a hatching rate of over 6%. Lower UV-ray doses affected abnormal embryo development. The highest yield of tench androgenesis (about 2%) was noted when eggs were exposed to thermal shock 30 min after egg activation.
Introduction
Androgenesis is a process in which the nuclear DNA of solely paternal origin is transmitted to the offspring. When possessing gametes of the same species, genetic inactivation of the oocyte is necessary before fertilisation, which means the destruction of its set of chromosomes. This inactivation can be carried out via ion radiation (i.e. UV, gamma, X radiation) (Lin and Dabrowski, Citation1998; Kucharczyk, Citation2002). The application of gamma or X radiation involves technical difficulties, including safety measures (Arai et al., Citation1992) and residual chromosome fragments in the oocyte (Ocalewicz et al., Citation2010). UV irradiation has been used successfully for genetic inactivation of cyprinid oocytes (Bongers et al., Citation1994; Kucharczyk, Citation2001, Citation2002; Kucharczyk et al., Citation2008b, Citation2008c).
The genetically-inactive oocytes were first inseminated with normal spermatozoa and then, in order to duplicate the chromosomes of the zygote, the oocytes were exposed to an environmental shock. In case of androgenesis, the only possible shock is a so-called late shock, which indicates a disorder of the first mitotic division. The duplication of the paternal chromosome set was achieved by the application of environmental shock to suppress the first cleavage in the genetically-inactivated eggs (Rubinshtein et al., Citation1997). A disorder in the process of mitosis can be achieved via a heat-shock by placing the eggs for a few minutes in water at the required temperature. Warm shock is practiced with cold water species, i.e. pike, Esox lucius L., with a temperature of about 34°C (Luczynski et al., Citation1997), and cold shock is used with warm water species, i.e. common carp, Cyprinus carpio L., with the temperature of about 0 to 4°C (Rothbard et al., Citation1999). The duration of the shock should be as short as possible, which means about 1 to 5 min. A pressure shock is yet another method to be used. Fish eggs are placed in special equipment and exposed to high pressure using compressed water for a few minutes. In another (chemical) method, which also causes the disorder of mitosis, the eggs are placed in chemical solutions such as colchicine or cytochalasin B for 5 to 7 min. This is the riskiest method because the substances reach the yolk and influence the later development of the offspring, which will become androgenetic homozygotes. Androgenesis makes it possible to produce groups of fish with defined genetic features. Initially, researchers focused on recovering stocks of breeding fish, although another aim of androgenesis may be the reconstruction of wild fish, which is possible when gene banks store the sperm of the extinct populations (Arai et al., Citation1992; Bongers et al., Citation1994; Flajshans, Citation1998; Kucharczyk, Citation2002). It is also possible to produce androgenetic offspring using eggs from other fish species (Grunina et al., Citation1995; Bercsenyi et al., Citation1998; Kucharczyk et al., Citation2008b, Citation2008c). The common tench Tinca tinca (L.) is a species which has a huge commercial value in many European countries, such as the Czech Republic, Hungary, Italy, Spain and Poland and other continents (Wang et al., Citation2006; Celada et al., Citation2009; Kujawa et al., Citation2011). For developing a culture method, different aspects of artificial reproduction (Kujawa et al. Citation2011; Targońska et al., Citation2012), gamete management (Mamcarz et al., Citation2006), hatchery techniques (Kujawa et al., Citation2010) and larval and juvenile rearing (Mamcarz et al., Citation2011; Nowosad et al., Citation2013) are studied. Since some of the common tench wild and cultured stocks may be extinct, many aspects of biotechnology should be studied, e.g. cryopreservation of sperm (Rodina et al., Citation2007) or genome manipulation like androgenesis.
The aim of this study is to determine the possibility of obtaining androgenotes from common tench, using the eggs of common carp and bream Abramis brama (L.).
Materials and methods
Breeders and reproduction procedure
In the experiment, coloured (yellow) males of tench and common carp breeders were used from the Polish Anglers Association (PZW) Fish Farm at Halinów, near Warsaw (central Poland) and wild fish (tench and common bream) from PZW Olsztyn (northern Poland). Among wild tench, there were 15 females and 10 males, with 5 yellow-coloured tench males. Carp and bream spawners were collected as 5 females and 8 males of each species. The selected fish (0.5 to 1.5 kg and 2.5 to 3.5 kg of body mass for common tench with common bream and common carp, respectively) were transported to the hatchery of the Department of Lake and River Fishery where they were placed in 1000 dm3 containers equipped for thermo-oxygen control (Kujawa et al., Citation1999). All the manipulations with spawners were carried out after having the fish anaesthetised in a solution of 2-phenoxyethanol (Sigma-Aldrich, St. Louis, MO, USA) at a dose of 0.5 cm3 dm–3.
The reproduction of tench, common carp and common bream was stimulated by double injections of Ovopel (Unic-trade, Budapest, Hungary) following the procedure described by Kucharczyk et al. (Citation2005, Citation2008a). In each experiment, milt was collected from several males. The quality of sperm was expressed as the percentage of motile spermatozoa. Motility was estimated by microscopic (500X) observation of sperm activated with 0.5% NaCl. Samples of sperm with 70 to 80% (or more) motile spermatozoa were pooled and used for further treatments. Spawning occurred after 8 to 10 h for tench, 12-14 h for common carp and 14 to 16 h for common bream post-injection at a water temperature of 20 to 22°C. The oocytes were kept in plastic containers before the experiments.
Genetic inactivation of DNA oocytes and induction of androgenesis
Eggs of common carp and common bream during irradiation were kept on Petri dishes in artificial ovarian fluid composed for common carp (Bongers et al., Citation1994) which was also found to be optimal for common bream eggs (Kucharczyk, Citation2002). The dishes with eggs were placed on a rocking table with a cycle of ˜1 s. During stirring, the eggs were able to roll in the fluid. An UV lamp (30 W, 6.4 W m–2) was switched on for at least 30 min before the onset of irradiation. Before irradiation of the oocytes, control samples of eggs (150 to 200 eggs in each sample) were inseminated using yellow-coloured tench spermatozoa with a small volume (0.05 mL) of sperm (control of egg quality: groups K). Non-irradiated eggs treated in ovarian fluid were also fertilised using the same volume of milt (control of ovarian fluid quality: groups D1). Experimental groups of eggs (common carp and common bream) after exposition to the different time of UV irradiation: E1, E3, E6, E8, E9, E10, E12 or E14 min (dose of UV irradiation ranged from 384 to 5376 J m–2) were inseminated with 0.05 mL of yellow-coloured tench sperm. After irradiation, eggs from additional control groups were fertilised (control of treatment in ovarian fluid: groups D2). The eggs were incubated in a laboratory recirculating system at 21°C. All experimental groups were analysed in triplicate. During androgenesis, experimental groups were fertilised with 0.05 mL of yellow tench form sperm after exposition of the oocytes to a 9 min UV irradiation (an UV irradiation dose of 3456 J m–2). Inseminated eggs were exposed to thermal shock from 20 to 60 min after egg activation (40°C; 2 min duration). This constituted E20, E30, E40, E50 and E60 groups, respectively. Eggs treated with UV irradiation, but not exposed to the thermal shock, were fertilised using the yellow form of tench sperm (control of irradiation quality, group I). After the experiment, eggs from other control groups were inseminated (control of treatment in ovarian fluid: groups D2). The whole procedure during both experiments was carried out in darkness to avoid genetic photo-reactivation (Kaastrup and Horlyck, Citation1987). Before the application of thermal shock, the eggs were kept at 21°C. After the experiment, the eggs were incubated in a laboratory recirculating system at 21-22°C, which was found to be the optimum incubation temperature for tench, carp and bream (Kucharczyk et al., Citation1997a, Citation1997b, Citation1998, Citation2005). All experimental groups were analysed in triplicate.
The success of androgenetic development in embryos in present study was determined in few ways: i) haploid syndrome (poorly formed retina, stunted body, etc.); ii) colour marker (wild-dark colour or yellow); and iii) morphological marker: the differences between embryos of pure species and their hybrids (Kucharczyk, Citation2002; Mamcarz et al., Citation2006).
The differences in hatching success and in the survival of tench embryos were analysed using ANOVA and tested by post-hoc Duncan’s multiple range test (P<0.05). All of the values expressed as percentages were arcsine transformed prior to statistical analysis.
Results and discussion
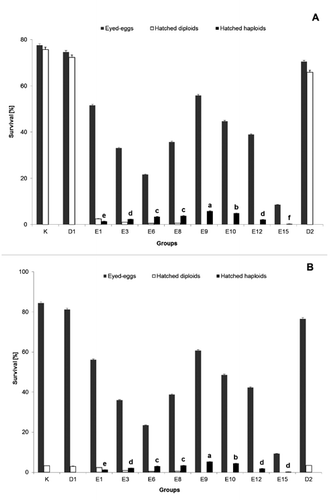
The optimum irradiation dose of eggs for both fish species (bream and carp) was 3456 J m–2 (9 min exposure) ( and ). In these groups, the highest percentage of live embryos at the eyed-egg-stage, as well as the highest hatching rate was observed. All hatched embryos from these groups were yellow-coloured and showed morphological abnormalities, which were recorded as haploid syndrome, i.e. stunted body, poorly-formed retina. The survival of newly-hatched (5 h after hatching) larvae in all experimental groups was statistically lower than in the control groups (groups K and D) ( and ). Such results were in contrast with embryos survival to the eyed-egg-stage, where there were no significant differences between treated and control groups (P>0.05). All hatched larvae in these groups irradiated from 6 (dose 1152 J m–2) to 14 min (dose 5376 J m–2) were yellow, whereas all specimens from control groups were wild (dark) coloured. In groups where the lowest UV doses were applied (1 and 3 min of treatment), dark diploid, aneuploid, as well as a few blond haploid embryos were recognised. Many abnormal dark-coloured embryos, morphologically similar to the typical haploids, were probably aneuploids.
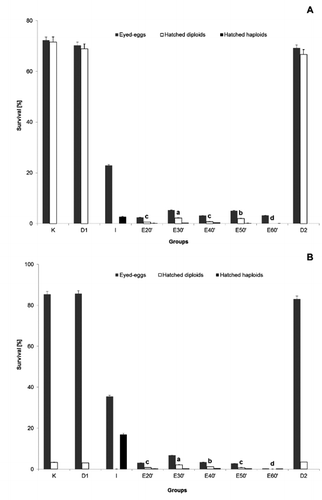
The survival of newly-hatched larvae (5 h after hatching) in all experimental groups involving androgenesis, was significantly lower than in the control groups. In the latter groups, the survival was high, except in the control groups where common carp eggs were used ( and ). Yellow-coloured haploid embryos showed morphological abnormalities. The highest yield of androgenesis (P<0.05) was noted when eggs were exposed to shock 30 min after egg activation. The high level of survival to the eyed-egg-stage in control (D1 and D2) and treated groups suggests that stirring and keeping the eggs in artificial ovarian fluid is not harmful to embryonic development. Diploid androgenotes of tench were morphologically different than embryos from the control groups (K). Haploid larvae were viable only for the next 3 to 4 days after hatching.
During the experimental period, it was observed that one of the negative effects on common tench production is the unpredictable variation of environmental conditions. This situation has prompted a search for ways to increase the tench population or at least maintain the population in Polish lakes. Tench is economically important for Polish inland fisheries and the gradual decline in production has caused serious difficulties, particularly in increasing interspecies competition, rural and industrial origin pollution and overexploitation. One solution to the problem is to apply modern genome engineering. The aquaculture of many more species was dynamically powered to the developing genome engineering (Krasznai and Marian, Citation1986; Goryczko et al., Citation1991; Kucharczyk et al., Citation2008b, Citation2008c; Ocalewicz et al., Citation2010) and the results of the studies are used not only for commercial purposes but are also of scientific interest. The low hatching rate of tench embryos from genetically inactivated oocytes (0-2%) has been observed in many other fish species. Similar data to the present work have been reported by other authors: i.e. Scheerer et al. (Citation1986) and Babiak et al. (Citation2002a, Citation2002b) for rainbow trout (Oncorhynchus mykiss Walbaum 1792), May et al. (Citation1988) for brook trout (Salvelinus fontinalis Mitchill 1814), Arai et al. (Citation1992) for loach (Misgurnus anguillicaudatus Cantor 1842), Bongers et al. (Citation1994) for common carp, Kucharczyk (Citation2002) for common bream, Lin and Dabrowski (Citation1998) for northern pike and Kucharczyk (Citation2001) and Kucharczyk et al. (Citation2008b, Citation2008c) for ide (Leuciscus idus L.) and dace (Leuciscus leuciscus L.). Such low survival rates of androgenotes were probably connected with the synergic effect of a few sublethal manipulations such as irradiation of oocytes and applied temperature shock as well as increased inbreeding.
The high level of survival to the eyed-egg-stage in control (D1 and D2) and treated groups suggests that stirring is not harmful to eggs. Similar observations have been made by Bongers et al. (Citation1994). The obtained results show that UV treatment successfully inactivated the nuclear DNA in common carp and common bream eggs. Androgenetic origin (haploid or diploid embryos) was checked using a recessive colour marker (blond) and a morphological marker. Data published by Mamcarz et al. (Citation2006) showed that newly-hatched hybrids between tench and carp or bream are morphologically different from genetically pure tench specimens. The low survival of hatched diploid embryos in the case of common carp – tench hybrids was also previously reported by Mamcarz et al. (Citation2006). The applied dose of UV irradiation was 3456 J m–2, at which almost 100% haploid embryos were produced at a hatching rate of over 2%. These doses were higher than those described as the optimum UV oocyte treatment for common carp (2500 J m–2) by Bongers et al. (Citation1994) or for northern pike (660 to 1320 Jm–2) by Lin and Dabrowski (Citation1998) and were similar to those reported by Kucharczyk (Citation2002) for common bream and Kucharczyk (Citation2001) for ide (2700 to 3500 J m–2). For doubling chromosomal material during the androgenesis process, heat shocks were usually applied and, in some cases, very intensively (up to 42°C) (Arai et al., Citation1995; Grunina et al., Citation1995; Bercsenyi et al., Citation1998; Pandian and Koteeswaran, Citation1998; Kucharczyk, Citation2002). The survival of larvae of androgenetic origin is variable (usually very low) and depended on many different factors. The data published by Scheerer et al. (Citation1986), Bongers et al. (Citation1994, Citation1995) and by Pandian and Koteeswaran (Citation1998), Rothbard et al. (Citation1999), Babiak et al. (Citation2002a, Citation2002b) showed that if androgenesis is involved in inbred lines, the survival was much higher than in wild ones. The obtained results in the present work showed that the survival of androgenetic-origin larvae was between 0 and 2%. However, the results of the present research were not different from those obtained by Kucharczyk (Citation2002) for pure tench androgenesis. Grunina et al. (Citation1995) and Bercsenyi et al. (Citation1998) suggest that if eggs from other fish species than spermatozoa were used, this usually increased embryo survival. However, the obtained results in the present work did not differ from the data obtained by Kucharczyk (Citation2002) for pure tench androgenesis.
Conclusions
The study found that androgenetic tench production using eggs from common carp and bream is possible. However, the yield of androgenesis is not satisfactorily high, although it is enough for the stock producing of androgenetic tench.
Acknowledgments
The authors wish to acknowledge the funding provided for this project (No. 528-0808-0801) by the University of Warmia and Mazury in Olsztyn, Olsztyn-Kortowo, Poland.
References
- AraiK. IkenoM. SuzukiR. 1995. Production of androgenetic diploid loach Misgurnus anguillicaudatus using spermatozoa of natural tetraploids. Aquaculture 137:131-138.
- AraiK. MasaokaT. SuzukiR. 1992. Optimum conditions of UV irradiation for genetic inactivation of loach eggs. Nippon Suisan Gakk. 58:1197-1201.
- BabiakI. DoboszS. GoryczkoK. KuzminskiH. BrzuzanP. CiesielskiS. 2002a. Androgenesis in rainbow trout using cryopreserved spermatozoa: the effect of processing and biological factors. Theriogenology 57:1229-1249.
- BabiakI. DoboszS. KuzminskiH. GoryczkoK. CiesielskiS. BrzuzanP. UrbanyiB. HorvathA. LahnsteinerF. PiironenJ. 2002b. Failure of interspecies androgenesis in salmonids. J. Fish Biol. 61:432-447.
- BercsenyiM. MagyaryI. UrbanyiB. OrbanL. HorvathL. 1998. Hatching out goldfish from common carp eggs: interspecific androgenesis between two cyprinid fishes. Genome 41:573-579.
- BongersA.B.J. AbarciaJ.B. Zandieh-DoulabiB. EdingE.H. KomenJ. RichterC.J.J. 1995. Maternal influence on development of androgenetic clones of common carp, Cyprinus carpio L. Aquaculture 137:139-147.
- BongersA.B.J. VeldE.P.C. Abo-HasemaK. BremmerI.M. EdingE.H. KomenJ. RichterC.J.J. 1994. Androgenesis in common carp (Cyprinus carpio L.) using UV irradiation in a synthetic ovarian fluid and heat shock. Aquaculture 122:119-132.
- CeladaJ.D. AguileraA. GarciaV. CarralJ.M. Saez-RoyuelaM. GonzalesR. GonzalesA. 2009. Rearing juvenile tench (Tinca tinca L.) under controlled conditions using Artemia nauplii as a supplement to a dry diet. Aquacult. Int. 17:565-570.
- FlajshansM. 1998. Genome manipulation in fish. Czech J. Anim. Sci. 43:387.
- GoryczkoK. DoboszS. MäkinenT. TomasikL. 1991. UV irradiation of rainbow trout sperm as a practical method for induced gynogenesis. J. Appl. Ichthyol. 7:136-146.
- GruninaA.S RecubratskyA.V. EmelyanovaO.V. NeyfakhA.A. 1995. Induced androgenesis in fish: production of viable androgenetic diploid hybrids. Aquaculture 137:149-160.
- KaastrupP. HorlyckV. 1987. Development of single method to optimize the conditions for producing gynogenetic offspring, using albino rainbow trout, Salmo gairdneri Richardson, females as an indicator for gynogenesis J. Fish Biol. 31:29-33.
- KrasznaiZ. MarianT. 1986. Shock induced triploidy and its effect on growth and gonad development of the European catfish, Silurus glanis L. J. Fish Biol. 30:519-527.
- KucharczykD. 2001. Genetic inactivation of Leuciscus idus L. (ide) oocytes using UV irradiation. Cytobios 104:189-195.
- KucharczykD. 2002. Rozród kontrolowany i androgeneza wybranych gatunków ryb karpiowatych. Uniwersytet Warmińsko-Mazurski, Olsztyn, Poland.
- KucharczykD. KujawaR. LuczynskiM. GlogowskiJ. BabiakI. WyszomirskaE. 1997a. Induced spawning in bream, Abramis brama (L.), using carp and bream pituitary extract and hCG. Aquac. Res. 28:139-144.
- KucharczykD. KujawaR. MamcarzA. Targońska-DietrichK. WyszomirskaE. GlogowskiJ. BabiakI. SzaboT. 2005. Induced spawning in bream (Abramis brama L.) using pellets containing GnRH. Czech J. Anim. Sci. 50:89-95.
- KucharczykD. ŁuczyńskiM. KujawaR. CzerkiesP. 1997b. Effect of temperature on embryonic and larval development of bream (Abramis brama L.). Aquat. Sci. 59:214-224.
- KucharczykD. ŁuczyńskiM. KujawaR. KamińskiR. UlikowskiD. BrzuzanP. 1998. Influences of temperature and food on early development of bream (Abramis brama L.). Arch. Hydrobiol. 141:243-256.
- KucharczykD. TargońskaK. HliwaP. GomułkaP. KwiatkowskiM. KrejszeffS. PerkowskiJ. 2008a. Reproductive parameters of common carp (Cyprinus carpio L) spawners during natural season and out-of-season spawning. Reprod. Biol. 8:285-289.
- KucharczykD. TargońskaK. ŁuczyńskiM.J. SzczerbowskiA. KwiatkowskiM. KujawaR. 2008b. Androgenesis of ide, Leuciscus idus (L.), using chub, Leuciscus cephalus (L.), eggs. Arch. Pol. Fish. 16:453-457.
- KucharczykD. TargońskaK. SzczerbowskiA. ŁuczyńskiM.J. RożekW. KujawaR. MamcarzA. 2008c. Genetic inactivation of dace, Leuciscus leuciscus (L), gametes using UV irradiation. Arch. Pol. Fish. 16:437-446.
- KujawaR. KucharczykD. MamcarzA. 1999. A model system for keeping spawners of wild and domestic fish before artificial spawning. Aquacult. Eng. 20:85-89.
- KujawaR. KucharczykD. MamcarzA. 2010. The effect of tannin concentration and egg unsticking time on the hatching success of tench Tinca tinca (L.) larvae. Rev. Fish Biol. Fisher. 20:339-343.
- KujawaR. KucharczykD. MamcarzA. ŻarskiD. TargońskaK. 2011. Artificial spawning of common tench Tinca tinca (Linnaeus, 1758), obtained from wild and domestic stocks. Aquacult. Int. 19:513-521.
- LinF. DabrowskiK. 1998. Androgenesis and homozygous gynogenesis in muskellunge (Esox masquinongy): evaluation using flow cytometry. Mol. Reprod. Dev. 49:10-18.
- LuczynskiM.J. GlogowskiJ. KucharczykD. LuczynskiM. Demska-ZakesK. 1997. Gynogenesis in northern pike (Esox lucius L.) induced by heat shock - preliminary data. Pol. Arch. Hydrobiol. 44:28-32.
- MamcarzA. KucharczykD. KujawaR. 2006. Reciprocal hybrids of tench Tinca tinca (L.) × bream Abramis brama (L.), and tench × carp Cyprinus carpio L., and some characteristics of their early development. Aquacult. Int. 14:27-33.
- MamcarzA. TargońskaK. KucharczykD. KujawaR. ŻarskiD. 2011. Effect of live and dry food on rearing of tench (Tinca tinca L.) larvae under controlled conditions. Ital. J. Anim. Sci. 10:e9.
- MayB. HenleyK.J. KruegerC.C. GlossS.P. 1988. Androgenesis as a mechanism for chromosome set manipulation in brook trout (Salvelinus fontinalis). Aquaculture 75:57-70.
- NowosadJ. ŻarskiD. BiłasM. DrylK. KrejszeffS. KucharczykD. 2013. Dynamics of ammonia excretion in juvenile common tench, Tinca tinca (L.), during intensive rearing under controlled conditions. Aquacult. Int. 21:629-637.
- OcalewiczK. DoboszS. KuzminskiH. NowosadJ. GoryczkoK. 2010. Chromosome rearrangements and survival of androgenetic rainbow trout (Oncorhynchus mykiss). J. Appl. Genet. 51:309-317.
- PandianT.J. KoteeswaranR. 1998. Ploidy level and sex control in fish. Hydrobiologia 384:167-243.
- RodinaM. GelaD. KocourM. Hadi AlaviS.M. HulakM. LinhartO. 2007. Cryopreservation of tench, Tinca tinca, sperm: Sperm motility and hatching success of embryos. Theriogenology 67:931-940.
- RothbardS. RubinshteinI. DavidL. 1999. Ploidy manipulations aimed to produce androgenetic Japanese ornamental (koi) carp, Cyprinus carpio L. Isr. J. Aquacult.-Bamid. 51:26-39.
- RubinshteinI. RothbardS. SheltonW.L. 1997. Relationships between embryological age, cytokinesis-1 and the timing of ploidy manipulations in fish. Isr. J. Aquacult.-Bamid. 49:99-110.
- ScheererP.D. ThorgardG.H. AllendorfF.W. KnudsenK.L. 1986. Androgenetic rainbow trout produced from inbred and outbred sperm sources show similar survival. Aquaculture 57:289-298.
- TargońskaK. PerkowskiT. ŻarskiD. KrejszeffS. MamcarzA. KujawaR. KucharczykD. 2012. Method of evaluation of wild common tench, Tinca tinca (L.), female suitability for artificial reproduction during the spawning season. Ital. J. Anim. Sci. 11:e30.
- WangJ. MinW. GuanM. GongL. RenJ. HuangZ. ZhengH. ZhangJ. LiuH. HanY. 2006. Tench farming in China: present status and future prospects. Aquacult. Int. 14:205-208.