Abstract
Plants engineered with genes encoding for the antigenic proteins of various microorganisms have shown to correctly express the proteins that elicit the production of antibodies in mammalian hosts. In livestock, plant-based vaccines could represent an innovative strategy for oral vaccination, especially to prevent infection by enteric pathogens. The aim of this study was to evaluate tobacco plants as a seed-specific expression system for the production of the flgK flagellar hook-associated protein from a wild type Salmonella typhimurium strain, as a model of an edible vaccine. The flgK gene is the principal component of bacterial flagella and is recognised as virulence factor by the innate immune system. It was isolated from the Salmonella typhimurium strain by PCR. The encoding sequence of flgK was transferred into a pBI binary vector, under control of soybean basic 7S globulin promoter for the seed-specific. Plant transformation was carried out using recombinant EHA 105 Agrobacterium tumefaciens. A transgenic population was obtained made up of independently kanamycin-resistant transgenic plants, which had a similar morphological appearance to the wild-type plants. Molecular analyses of seeds confirmed the integration of the gene and the average expression level of flgK was estimated to be about 0.6 mg per gram of seeds, corresponding to 0.33% of the total amount of soluble protein in tobacco seeds. This study showed that the foreign flgK gene could be stably incorporated into the tobacco plant genome by transcription through the nuclear apparatus of the plant, and that these genes are inherited by the next generation.
Introduction
Plants are interesting expression systems for the effective and financially viable production of recombinant proteins for therapeutic and medical purposes (Streatfield et al., Citation2001; Daniell et al., Citation2009). Plant systems represent a relatively inexpensive way for the expression and large-scale production of proteins rather than using industrial methods (fermentation of bacteria, yeast, cultured animal or human cell lines) and have the ability to carry out post-translational modifications similar to naturally-occurring systems (Streatfield, Citation2006). Several studies have shown that plants that have been engineered with genes encoding for antigenic proteins of various microorganisms correctly express the proteins that elicit the production of antibodies in mammalian hosts (Lamphear et al., Citation2002; Rossi et al., Citation2003a, Citation2003b; Pinotti et al., Citation2003). Plant-based vaccines offer different advantages (Salazar-Gonzales et al., Citation2013). The risk of contamination from potential animal pathogens is minimised, as plants are not hosts for mammalian infectious agents (Giddings et al., Citation2000). Plant-based vaccines offer a convenient way of storage: they do not need to be maintained in a cold chain, as the plant parts expressing the vaccine or plant extracts can be stored and transported at room temperature. In addition, if orally administered by feeding, production costs can be reduced by almost 90%, thus avoiding purification from plants, and eliminating the syringes and needles in the delivery of vaccines. In livestock, plant-based vaccines could represent an innovative strategy for oral vaccination, especially to prevent infection by enteric pathogens. Furthermore, edible vaccines could be an efficient way to reduce antibiotic treatments, in compliance with EC Regulation 1831/2003. In fact, an effective oral vaccination could increase the likelihood of a local immune response at the site of infection, thus avoiding systemic symptoms.
Tobacco plays an important role in the advancement of plant biotechnology. It has been found to be extremely versatile for genetic manipulation and tissue culture research (Ganapathi et al., Citation2004). Several recombinant proteins have been expressed in transgenic tobacco as bioreactors for the production of commercially important pharmaceutical molecules (Shoji et al., Citation2012). Many immunogenic proteins have been synthesised in tobacco and several plant-based vaccine candidates have demonstrated efficacy against a large number of human and animal pathogens after oral administration (Daniell et al., Citation2001). However, antigenic proteins expressed in tobacco require a very thorough purification process, which represents the main cost involved in recombinant protein production, because of the presence of nicotine in leaves, which is about 200 mg/kg. Previous studies have shown that tobacco seeds, which have less than 2 μg/kg of nicotine, can be integrated into livestock feeding without affecting the palatability (Rossi et al., Citation2007) or metabolic parameters. A recent study showed that tobacco seed cake, a by-product obtained after oil extraction, could be used as a cost-effective protein source in piglet nutrition (Rossi et al., Citation2013b). It is also included in the EU feed material register (number 00753-IT). Enteric diseases represent a serious problem in the pig industry and are responsible for significant economic losses (Rossi et al., Citation2012).
We focused on Salmonella typhimurium, which is responsible for considerable economic losses in pigs and which represents a major zoonotic pathogen (Foley and Lynne, Citation2008). The transmission of Salmonella typhimurium in swine occurs mainly through the faecal-oral route, with invasion through the intestinal wall and Peyer’s patches. Although invasion of the intestinal epithelium is a required virulence factor of all salmonellae, invasiveness may be limited to certain times and host sites during pathogenesis (Fedorka-Cray et al., Citation1995). The most commonly studied virulence factors are flagella, which play a pivotal role in the motility and invasion of the mucosal surface. Flagella of S. typhimurium have been shown to stimulate both the innate and adaptative immune system (Olsen et al., Citation2013). Flagellins, component of bacterial flagella, are recognised by the innate immune system in mammals (Hayashi et al., Citation2001) and induce systemic and mucosal immune responses after intranasal immunisation (Strindelius et al., Citation2004). Non-flagellated serotype Salmonella typhimurium mutants have been shown to cause less inflammation than their isogenic parents after infection of bovine ligated ileal loops (Macnab, Citation2003). In particular, flgK, a hook-associated protein, appeared to be the most immunoreactive protein (Kwang and Littledike, Citation1995). In swine, salmonellosis is a serious problem responsible for enterocolites of a variable severity, followed by a carrier state that can last up to 28 weeks (Rementeria et al., Citation2009; Gebreyes and Altier, Citation2002). This carrier status maintains the infection in the farms involved, and is thus an important public health risk (Boyen et al., Citation2009). According to an EFSA report, the prevalence of Salmonella-positive slaughter pigs was as high as 10.3%, although this figure varied among member states, including both primary infections and surface contamination of carcasses. Of particular concern is the increasing number of infections with antimicrobial drug-resistant Salmonella, including the recent emergence of drug-resistant Salmonella enterica serotype typhimurium (Helms et al., Citation2002). Novel methods are required to control salmonellosis in livestock, and vaccination is an effective way to prevent the disease. An efficient vaccination strategy could also reduce antibiotic treatments, as suggested by EC Regulation 1831/2003.
The aim of this study is to evaluate tobacco plants as a seed-specific expression system for the production of flgK gene – the principal component in bacterial flagella and recognised as a virulence factor by the innate immune system – from a wild type Salmonella typhimurium strain, as a model of an edible vaccine.
Materials and methods
Isolation of flgK gene from Salmonella typhimurium strain
The Salmonella typhimurium strain, isolated from a piglet that had died from salmonellosis, was grown in triptone soy broth (Oxoid, Basingstoke, UK) at 37°C for 24 h. A 1 mL aliquot of enrichment broth was centrifuged (2500 x g for 10 min) and the pellet was resuspended in 474 μL of TE (10 mM Tris-HCl pH 8, 1 mM Na2EDTA), 25 μL 10% SDS and 1.25 μL proteinase K (20 mg/mL). After incubation at 55°C for 30 min, 500 μL of phenol-chloroform pH 8 (1:1) was added, mixed vigorously, and the samples were centrifuged (10,000 x g, 10 min). The aqueous phase was transferred to a fresh micro-tube, and the DNA was precipitated with 3 M sodium acetate and ice-cold isopropanol for 30 min. Samples were centrifuged (16,000 x g for 10 min) and the pellet was washed with 90% ethanol. The final pellet was resuspended in 50 mL of TE, and was then stored at 4°C until PCR was performed. The primer pair was designed on the flgK gene sequence (Genebank, Accession Number: X51738), codifying for the flgK flagellar protein made up of 552 amino acids, in order to detect the entire gene from start to stop codon (81 to 1742). Oligonucleotide primers (5’ggatccatgtccagcttgattaatcacgcc and 3’ aaactacgcaataacttataagcgattctcgag) included unique cloning sites for specific endonucleases (BamHI - 5’, SacI - 3’) to facilitate direct sub-cloning of the fragments in the plant transformation vector. A polymerase chain reaction was performed in order to obtain one DNA fragment of 1662 pb corresponding to the flgK gene. An aliquot of 5 μL of bacterial genomic DNA was used as the template DNA in the PCR. Negative controls were represented by the PCR mix (without DNA template) and genomic DNA extracted from the BL21 E. coli strain. The PCR mixture consisted of 5 μL of amplification buffer (Promega, Fitchburg, WI, USA), 1.5 mM MgCl2, 200 mM (each) of the four deoxyribonucleoside triphosphates, 1 mM (each) of primer pairs, 1.25 U of Taq polymerase (Promega), and distilled H2O, which was added to make a total volume of 50 μL. The parameters for the amplification cycles were as follows: 25 cycles of 1 min of denaturation at 94°C, 1’ 20” of annealing at 55°C, and 1’ 30” of extension at 72°C. Aliquots of PCR products were electrophoresed in a 0.8% agarose gel. After staining with ethidium bromide, the amplified DNA fragments in the gel were visualised and photographed under UV illumination. The amplified flgK gene, purified from agarose gel (Geneclean; Q-BioGene, Irvine, CA, USA), were inserted by ligation into a high copy number plasmid vector (pGEM-T Easy; Promega) in order to obtain a pGEM-T Easy-flgK chimeric construct. pGEM-T Easy-flgK was cloned into (TetR) XL1B E. coli strain by electroporation for preliminary clone selection. Restriction analyses were carried out on plasmidic DNA extracted from XL1b E. coli strains growth on LB medium containing tetracycline and ampicillin as markers of transformation. Restriction analyses were carried out with EcoRI, BamHI and SacI in order to evaluate the correct insertion of the flgK gene.
Expression of recombinant flgK through pET system
E. coli BL21 competent cells (Novagen, Madison, WI, USA) were transformed with the expression plasmid pET-28a containing the flgK gene and selected on LB-Kan plates (kanamycin 30 μg/mL). A single recombinant colony was inoculated with 5 mL of the LB medium containing kanamycin (37°C, o/n). A total of 1 mL of the pre-inoculum was added to 100 mL of the LB medium in a 1 L flask, and incubated with shaking (175 rpm at 37°C) until the OD600 reached 0.6. Isopropyl- beta-D-thiogalactoside (IPTG) was added to the culture to a final concentration of 0.1 mM and the incubation was continued for another 2 h. The culture was incubated for an additional 3 h and then harvested by centrifugation. The soluble protein fraction and inclusion body proteins were eluted separately in columns with a specific resin. The proteins were resolved by 10% polyacrylamide gel electrophoresis (PAGE). Detection was performed by Coomassie blue staining. The purified flgK protein was concentrated in a 4 M urea elution buffer with a centrifugal filter (10 kD molecular weight cut-off) (Centricon; Millipore, Billerica, MA, USA). The flgK was evaluated on a sodium dodecyl sulphate (SDS)-PAGE. The purified proteins were stored at -80°C. The protein concentration of the crude samples was determined by the Bradford’s (Bio-Rad) method with bovine serum albumin as a standard. A total of 250 μg of purified flgK in 1 mL of sterile PBS was emulsified with an equal volume of complete Freund’s adjuvant and injected into a New Zealand white rabbit subcutaneously on the dorsal site. Subsequent immunisations were given with incomplete Freund’s adjuvant at 20-day intervals. After three immunisations, the rabbit was bled, and the titre of antibodies in the serum samples was evaluated and stored at -20°C.
Construction of the expression cassette
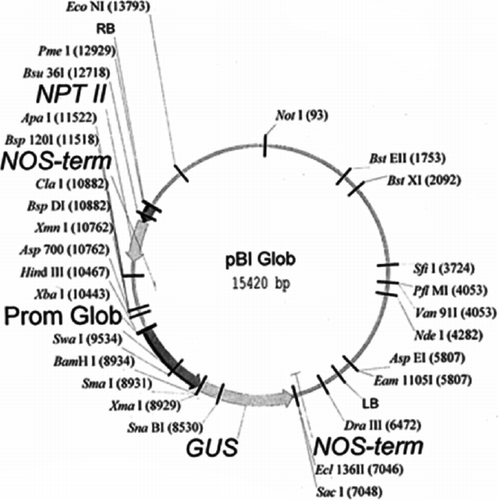
The flgK gene obtained by SacI/BamHI digestion of pGEM-T Easy-flgK, after purification from the agarose gel, was separately subcloned in a pBIpGLOB vector (Patent WO0004146) using ligation. The soybean basic 7S globulin promoter (GLOB) (DDBJ Accession number: AX006477) was used for the seed-specific expression of antigenic proteins according to Reggi et al. (Citation2005). The pBIpGLOB vector was linearised by the BamHI-SacI restriction enzyme digestion, obtaining, after elimination of the GUS gene, a plasmid of 13938 pb. A total of 100 ng of the pBIpGLOB vector (linearised by SacI-BamHI digestion), 17 ng of the flgK SacI-BamHI fragment, 1 L of the ligase buffer 10 X and 1 U of T4 DNA ligase (Promega) were incubated with nuclease-free water (to final volume of 10 μL) at 4°C overnight. In the expression cassette, the flgK gene was inserted under the control of GLOB promoter in order to induce the seed specific expression (Appendix). The ligation reaction was used to transform competent cells of the XL1B E. coli strain by electroporation, maintained in a selective medium containing kanamycin for clone selection. The chimeric constructs pBIpGLOB-flgK was evaluated by restriction analyses.
Tobacco transformation and regeneration
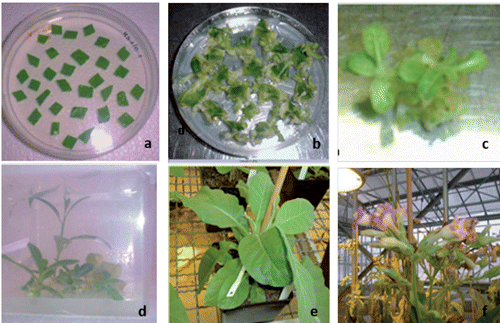
Agrobacterium tumefaciens EHA 105 competent cells were transformed with the chimeric construct pBIpGLOB-flgK by electroporation. Recombinant Agrobacterium strains were screened on a selective medium and using a PCR assays in order to evaluate the presence of the flgK gene. For the infection of 100 tobacco leaf disks with recombinant Agrobacterium, we used an overnight culture of bacterium, whose optical density at 600 nm (OD600) was about 0.6. We then put a tobacco leaf disk (Nicotiana tabacum L., cv. Xanthi) on Murashige Skog 10 (MS10) medium for two weeks to allow callus shaping. The kanamycin that was incorporated into the medium enabled the transformed callus tissue to be selected, as previously described by Rossi et al. (Citation2003a). Regenerated shoots were separated from the callus and cultured on a rooting medium MS with 30 mg/L of kanamycin. The regenerated plants were finally transferred to the soil and grown to maturity in a greenhouse in homogeneous environmental conditions.
The plants were analysed for the presence of foreign DNA using internal primers of the flgK sequence by PCR, using, for each sample 80 ng of genomic DNA extracted from young leaves of the regenerated plants as a template, according to Doyle and Doyle (Citation1987). The mRNA was evaluated by northern blot analysis on the immature seeds (12 days after pollination) of all PCR-positive plants. Northern blot analysis was carried out using DIG-labelled RNA probes hybridised with total RNA extracted with one volume of 50 mM Tris buffer pH 7.5 containing 150 mM NaCl, 5 mM ethylendiaminetetracetic acid (EDTA), 1% sodiumdodecilsolphate (SDS), 150 mM B-mercaptoethanol and one volume of phenol:chloroform (1:1). Electrophoresis, blotting and hybridisation were performed as described previously, and the specific RNA detection was performed using CDP-star (Boehringer, Mannheim, Germany) according to the manufacturer’s instructions. The total proteins were extracted from all the mature transformed tobacco lines by homogenisation with liquid N2 with the solubilisation buffer (50 mM Tris, pH 8, 5 mM EDTA, 200 mM NaCl, 0.1% Tween 20) in a mortar. Protein content was estimated by a Bradford assay (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as the standard. The expression of flgK in the total protein sample was evaluated by western blotting with specific polyclonal antibodies obtained from New Zealand rabbits, after immunisation with flgK expressed through pET-system (Novagen) in a BL21 E. coli strain. Samples (80 μg total protein) were loaded in a 10% polyacrylamide gel together with the Precision Standards (Bio-Rad) and a positive control represented by 200 ng of flgK protein obtained through pET-system (Novagen) in BL21 E. coli strain. The proteins were transferred to an Immobilon-PSQ membrane (Millipore) with the Trans-Blot SD apparatus (Bio-Rad); the filters were incubated overnight with rabbit polyclonal anti-flgK serum (1:5000). After incubation for 1 h with HRP-conjugated secondary antibody (1:10,000), chemiluminescence was developed using the SuperSignal West Pico Trial Kit (Thermo Fisher Scientific, Rockford, IL, USA). The best positive producing lines were selected and self-pollinated. The R1, and then, R2 generations were propagated in a greenhouse. R1 and R2 tobacco leaf samples were collected in order to evaluate by PCR, using the same experimental conditions previously described, the stable integration of flgK into tobacco genome.
Results and discussion
Transgenic plants offer an attractive alternative to microorganisms and animals for the production of novel vaccine antigens. Edible vaccines represent an interesting challenge for the control of various human and animal diseases. The aim of our experimental design was to engineer tobacco plants for the seed-specific expression of the flgK protein, which would induce, upon oral administration, the production of specific mucosal antibodies against Salmonella typhimurium. The loss or inactivation of flgK by specific antibodies leads to a reduction in the pathogenicity of Salmonella typhimurium. The flgK flagellin, a hook-filament junction protein of Salmonella typhimurium, was chosen as an antigen. In fact, flgK is recognised as an important virulence factor by an innate immune system and contributes to intestinal inflammation. The development of a mucosal vaccination system is an innovative way to produce specific antibodies in the mucosal, where the major pathogens gain access to the body.
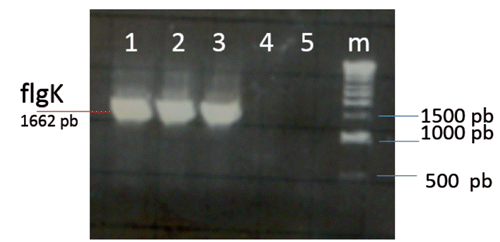
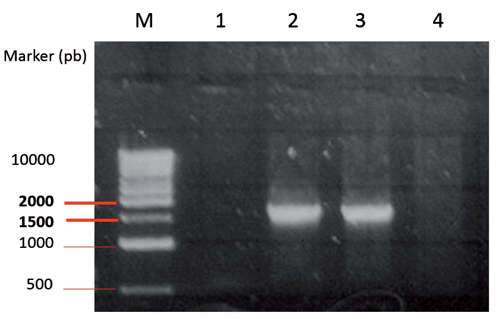
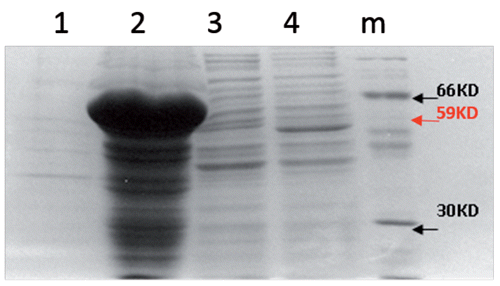
The first step was to isolate the flgK gene from Salmonella typhimurium wild type. The flgK flagellin, electrophoresis of PCR products for the detection of the flgK gene on agarose gel identified flgK genes as bands with a length of 1662 (). Negative controls did not show bands. Since the inserted products showed the expected size in the agarose gel, they were recovered from the gel, diafiltrated against water and ligated into plasmid T-vectors for restriction analyses. The flgK gene was identified by BamHI/SacI restriction analysis of positive clones, grown on a selective medium. The results confirmed the correct insertion of flgK gene and the match of the obtained gene sequence with the GenBank. The pET expression system was used for the cloning and expression of recombinant proteins in E. coli. The flgK gene was cloned in pET-28a plasmid under the control of strong bacteriophage T7 transcription and expression. Our results showed that T7 RNA polymerase was effectively induced by IPTG and flgK proteins were significantly detected in the total E. coli protein. The flgK proteins were accumulated as insoluble aggregates defining inclusion bodies (). This meant that the recombinant proteins were easily isolated by centrifugation and yielded highly concentrated and relatively pure protein. In addition, it is known that the formation of inclusion bodies protects the protein from citoplasmatic proteolytic attack. Signals corresponding to flgK proteins were not detected in soluble protein fractions, no differences were observed between soluble proteins with IPTG induction and without IPTG induction. A total of 2 mg of purified flgk, concentrated through a Centricon ultrafiltration set-up, were stored at -20°C. Obtained flgK proteins, expressed by pET expression system, were used as positive controls and for the production of specific rabbit serum used in subsequent western blot assays.
The third step involved the evaluation of tobacco plants as a seed-specific expression system of the isolated flgK gene. Thus, the flgK gene was subcloned in the binary vector pBIpGLOB instead of the gusA gene. After the engineered vector had been mobilised to A. tumefaciens EHA 105, tobacco transformation was carried out following standard procedures (Rossi et al., Citation2013a). Several shoots, surviving levels of kanamycin, antibiotic selective agents, were obtained (). A transgenic population consisted of 15 independent kanamycin-resistant transgenic plants, which had a similar morphological appearance to the wild-type plants. About 80% of the lines of transformed tobacco plants, screened for the presence of genes by PCR on DNA from young leaves, harboured transgenes. Samples containing transgenes were identified by the presence of an amplified product of 1.6 Kb, representing the gene encoding flgK fimbriae. Molecular analyses confirmed the integration and the expression of the flgK gene. Northern blot analyses were performed on tobacco seeds in order to select transcription positive transformants as well as to verify that the mRNA had been processed correctly and to estimate RNA abundance, depending on the position effects of the integrated transgene. Northern blot analyses showed signals corresponding to flgK mRNA in seven plants, corresponding to 60% of the PCR positive flgK plants. Different lines were compared for transgene transcription and lines with the strongest signals were selected for subsequent plant generations. Western blot analysis, carried out on plants positive for flgK mRNA, detected flgK signals in all samples. By comparison with a positive control (200 ng of flgK protein expressed by pET system in BL21 E. coli strain), the best producing lines () showed an amount of flgK protein of 600 ng per 80 μg of total soluble protein (0.75%), corresponding to 1.5 mg per gram of seeds (considering that the crude proteins in tobacco seeds are about 20%). The lowest observed level of expression was amounted to 0.125% of total soluble proteins. The different level of expression of regenerated plants is caused by Agrobacterium-mediated transformation at random positions in the tobacco genome. Nevertheless, the average amount of flgK was estimated to be about 0.6 mg per gram of seeds, corresponding to 0.33% of the total soluble protein in tobacco seeds. The obtained number of antigens (per gram of seeds), according to other authors, is sufficient for subsequent trials focusing on the in vivo evaluation of the oral administration of tobacco seeds transformed for flgK seed-expression (Lamphear et al., Citation2002; Streatfield et al., Citation2001). No cross-reacting proteins were identified in any of the wild-type seed extracts. No traces of degradation products were apparent in any of the transformed samples. The best lines of tobacco-regenerated plants were selected, propagated in vitro and then grown in a greenhouse. Obtained data showed the inheritance of transgenes in the R0, R1 and R2 generations () and the stable integration of flgK gene into tobacco genome. The production of antigens in seeds has the advantage of a long storage period and a natural encapsulation in the tissues of the expression host. This encapsulation offers the potential for the antigen to be protected against rapid and complete degradation and to be gradually released as host tissues are digested (Rossi et al., Citation2011). As previously discussed, the inclusion of tobacco seeds in the piglet diet was able to cover the nutritional requirements without affecting growth, feed efficiency and metabolic parameters and can thus be used as a protein source (Rossi et al., Citation2013b). In order to reduce the volume of vaccine seeds, tobacco seed cake obtained through cold pressing can be used, which would be an effective way to obtain a higher concentration of heterologous proteins.
Conclusions
This study has shown that the foreign flgK gene, derived from a wild type Salmonella typhimurium strain, can be stably incorporated into the tobacco plant genome by transcription through the nuclear apparatus of the plant for specific expression in the seeds, and that these genes are inherited by the next generation. Tobacco seeds could then be used as an effective system for flgK flagellin expression.
References
- BoyenF. PasmansF. Van Immerseel F. Donné E. MorganE. DucatelleR. HaesebrouckF.,2009. Porcine in vitro and in vivo models to assess the virulence of Salmonella enterica serovar Typhimurium for pigs. Lab Animal 43 :46-52.
- DaniellH. Singh N.D. MasonH. Streatfield S.J.,2009. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 14 :669-679.
- DaniellH. Streatfield S.J. WycoffK.,2001. Medical molecular farming: production of antibodies, biopharmaceutical and edible vaccines in plants. Trends Plant Sci. 6 :219-226.
- Doyle J.J. Doyle J.L.,1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19 :11-15.
- Fedorka-Cray P.J. Collins Kelley L. Stabel T.J. Gray J.T. Laufer J.A.,1995. Alternate routes of invasion may affect pathogenesis of Salmonella typhimurium in swine. Infect. Immun. 63 :2658-2664.
- Foley S.L. Lynne A.M. 2008. Food animal-associated Salmonella challenges: pathogenicity and antimicrobial resistance. J. Anim. Sci. 86(Suppl.14):E173-E187.
- Ganapathi T.R. SuprasannaP. Rao P.S. Bapat V.A.,2004. Tobacco (Nicotiana tabacum L.) – a model system for tissue culture intervention and genetic engineering. Indian J. Biotechnol. 3 :171-184.
- Gebreyes W.A. AltierC.,2002. Molecular characterization of multidrug-resistant Salmonella enteric subsp. enterica serovar typhimurium isolates from swine. J. Clin. Microbiol. 40 :2813-2822.
- GiddingsG. AllisonG. BrooksD. CarterA.,2000. Transgenic plants as factories for biopharmaceuticals. Nat. Biotechnol. 18 :1151-1154.
- HayashiF. Smith K.D. OzinskyA. ThomasR. Hawn T.R. Yi E.C. Goodlett D.R. JimmyK. Eng J.K. AkiraS. Underhill D.M. AderemA.,2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410 :1099-1103.
- HelmsM. VastrupP. Gerner-SmidtP. Mølbak K.,2002. Excess mortality associated with antimicrobial drug-resistant Salmonella typhimurium. Emerg. Infect. Dis. 8 :490-495.
- KwangJ. Littledike E.T.,1995. Production and identification of recombinant proteins of Salmonella typhimurium and their use in detection of antibodies in experimentally challenged animals. FEMS Microbiol. Lett. 130 :25-30.
- Lamphear B.J. Streatfield S.J. Jilka J.M. Brooks C.A. Barker D.K. Turner D.D. Delaney D.E. GarciaM. WigginsB. Woodard S.L. Hood E.E. Tizard I.R. LawhornB. Howard J.A.,2002. Delivery of subunit vaccines in maize seed. J. Control. Release 85 :169-180.
- Macnab R.M.,2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57 :77-100.
- Olsen J.E. Hoegh-Andersen K.H. CasadesusJ. Rosenkrantz J.T. Chadfield M.S. Thomsen L.E.,2013. The role of flagella and chemotaxis genes in host pathogen interaction of the host adapted Salmonella enterica serovar Dublin compared to the broad host range serovar S. typhimurium. BMC Microbiol. 13 :1-11.
- PinottiL. RossiL. RebucciR. Dell’OrtoV. BaldiA.,2003. La sicurezza delle colture GM: criteri di valutazione. Tec. Molit. 38-45.
- ReggiS. MarchettiS. PattiT. De Amicis F. CariatiR. BembiB. FogherC.,2005. Recombinant human acid β-glucosidase stored in tobacco seed is stable, active and taken up by human fibroblasts. Plant Mol. Biol. 57 :101-113.
- RementeriaA. Vivanco A.B. RamirezA. Hernando F.L. BikandiJ. Herrera-LeonS. EcheitaA. GaraizarJ.,2009. Characterization of monoclonal antibody directed against Salmonella enterica serovar typhimurium and serovar [4,5,12:i:–]. Appl. Environ. Microb. 75 :1345-1354.
- RossiL. BaldiA. Dell’OrtoV. FogherC., 2003a. Antigenic recombinant proteins expressed in tobacco seeds as a model for edible vaccines against swine oedema. Vet. Res. Commun. 27(Suppl.1):659-661.
- RossiL. Di Giancamillo A. ReggiS. DomeneghiniC. BaldiA. SalaV. Dell’OrtoV. CoddensA. CoxE. FogherC., 2013a. Expression of verocytotoxic Escherichia coli antigens in tobacco seeds and evaluation of gut immunity after oral administration in mouse model. J. Vet. Sci. 14 :263-270.
- RossiL. FusiE. BaldiG. FogherC. CheliF. BaldiA. Dell’OrtoV., 2013b. Tobacco seeds by-product as protein source for piglets. Open J. Vet. Med. 3 :73-78.
- RossiL. ReggiS. Di Giancamillo A. DomeneghiniC. PinottiL. FogherC. BaldiA., 2003b. Oral administration of tobacco seeds expressing antigenic proteins in mice balb-c: a model of edible vaccines for edema disease. Ital. J. Anim. Sci. 2(Suppl.1):7-9.
- RossiL. ReggiS. VagniS. FogherC. BaldiA.,2011. Evaluation of gastric degradability of antigenic protein expressed in tobacco seeds. Ital. J. Anim. Sci. 10(Suppl.1):19.
- RossiL. SelminiG. CheliF. FusiE. FogherC.,2007. Potential use of tobacco seed cake in piglet diet. Large Anim. Rev. 13 :211-215.
- RossiL. VagniS. PolidoriC. Alborali G.L. BaldiA. Dell’OrtoV.,2012. Experimental induction of Escherichia coli diarrhoea in weaned piglet. Open J. Vet. Med. 2 :1-8.
- Salazar-González, J.A. Rosales-MendozaS.,2013. A perspective for atherosclerosis vaccination: is there a place for plant-based vaccines? Vaccine 31 :1364-1369.
- ShojiY. Farrance C.E. BautistaJ. BiH. MusiychukK. HorseyA. Park H.W. JajeJ. Green B.J. ShamloulM. SharmaS. Chichester J.A. MettV. YusibovV.,2012. A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respir. Viruses 6 :204-210.
- Streatfield S.J.,2006. Mucosal immunization using recombinant plant-based oral vaccines. Methods 38 :150-157.
- Streatfield S.J. Jilka J.M. Hood E.E. Turner D.D. Bailey M.R. Mayor J.M. Woodard S.L. Beifuss K.K. Horn M.E. Delaney D.E. Tizard I.R. Howard J.A.,2001. Plant-based vaccines: unique advantages. Vaccine 19 :2742-2748.
- StrindeliusL. FillerM. Sjöholm, I.,2004. Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice. Vaccine 22 :3797-3808.
APPENDIX
Plant bioreactors for the antigenic hook-associated flgK protein expression
Luciana Rossi, Luciano Pinotti, Alessandro Agazzi, Vittorio Dell’Orto, Antonella Baldi
Dipartimento di Scienze Veterinarie per la Salute, la Produzione Animale e la Sicurezza Alimentare, Università di Milano, Italy