Abstract
In this study, we investigated the role of liver X receptor (LXR) in regulating cholesterol concentration in goose primary hepatocytes. Intracellular and extracellular cholesterol concentration, mRNA expression levels of genes related to phosphatidylinositol 3-kinase (PI3K) pathway, sterol regulatory element-binding protein 2 (SREBP-2), and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) were measured in primary goose hepatocytes. We found that the intracellular cholesterol concentration showed an uptrend manner when the TO901317 concentration was under 1 μmol/L; compared with 1 μmol/L TO901317, 10 μmol/L TO901317 had an inhibiting effect (P<0.05). The regulation mode of SREBP-2 and HMGR gene expression is similar with that of intracellular cholesterol concentration. These data suggested that LXR activation may stimulate the cholesterol biosynthesis by activating expression of SREBP-2 and HMGR. Interestingly, the mRNA levels of genes related with PI3K pathway increased in the presence of TO901317, which suggested that LXR activation could promote PI3K signalling activity.
Introduction
Cholesterol is a central component of lipid rafts, specialised microdomains of the plasma membrane that serve as organising centers for the assembly of signalling molecules (Zhao and Dahlman-Wright, Citation2009; Guo et al., Citation2011). Sterol regulatory element-binding protein 2 (2SREBP-2) is a transcription factor that regulates many genes involved in cholesterol synthesis and uptake in response to cellular cholesterol levels (Shimano et al., Citation1997), whose nuclear fragments are released from membranes by controlling proteolysis. When cells are overloaded with sterols, SREBP-2 remains bound to the endoplasmic reticulum membranes, and consequently, transcription of its target genes declines (Horton et al., Citation2002). Many studies in recent years have significantly enhanced our understanding of the molecular mechanisms of liver X receptor (LXR) signalling as an important global regulator of cholesterol homeostasis involved in the efflux, transport, and excretion of cholesterol (Peet et al., Citation1998; Repa and Mangelsdorf, Citation2000; Tontonoz and Mangelsdorf, Citation2003). One recent study showed that SREBP-2 positively regulates transcription of the cholesterol efflux gene ABCA1, by generating oxysterol ligands for LXR (Wang et al., Citation2006).
Cholesterol synthesis is normally tightly regulated by a feedback mechanism to maintain the appropriate level. Cholesterol synthesis is catalysed by a group of microsomal enzymes, including rate-limiting enzymes HMG-CoA synthase and reductase. Studies in vivo and in vitro have shown that 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) is highly regulated at the transcriptional level by SREBP-2 (Goldstein et al., Citation2006; Wong et al., Citation2006). Activation of SREBP-2 is dependent on the cholesterol status of the cell, and when cholesterol concentration drops in the endoplasmic reticulum (ER), the SREBP-2 transcription factor is released and binds to a sterol-response element (SRE) located on the HMGR promoter. This leads to an increased transcription of the HMGR gene, stimulating the cholesterol biosynthesis and safeguarding the adequate cholesterol concentration within the cell (Goldstein et al., Citation2006). A common link between SREBP-2 and LXR-mediated processes is their regulatory response to certain oxidised cholesterol derivatives (oxysterols). Certain oxysterols inhibit SREBP-2 activation, but serve as ligands for LXR (Venkateswaran et al., Citation2000; Janowski et al., Citation2001; Abildayeva et al., Citation2006). A role for SREBP-2 in the positive regulation of LXR-target genes has been implied in two previous studies (Forman et al., Citation1997; DeBose-Boyd et al., Citation2001). A recent study showed that LXR activation up-regulates the expression of SREBP-2 and its regulatory genes in astrocytes (Abildayeva et al., Citation2006). However, Wang et al. demonstrated that LXR knock-down would result in an increased cholesterol content in HepG2 cells by regulating the expression of SREBP-2 and its target genes (Wang et al., Citation2008). All these data showed that both LXR and SREBP-2 play an important role in regulating the cholesterol metabolism, and in different cell types can exhibit different effects. Interestingly, recent studies have well established that the phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway is involved in the regulation of cholesterol metabolism (Emerling and Akcakanat, Citation2011; Laplante et al., Citation2012). The PI3K-AKT downstream target gene mTOR, to a large extent, acts through SREBP-2 transcription factor that controls the expression of genes involved in cholesterol synthesis. Mammalian target of rapamycin appears to regulate SREBP-2 function through several mechanisms, including 4E-BP1 and S6K (Porstmann et al., Citation2008; Li et al., Citation2011; Wang et al., Citation2011). In some cell types (such as hepatocellular and carcinoma cells), S6K was a key regulator of cholesterol biosynthesis (Düvel et al., Citation2010). These findings suggested that mTOR inhibition would reduce mRNA expression level of SREBP-2.
The present work was, thus, undertaken to determine whether SREBP-2 plays a regulatory role in LXR activation-induced cholesterol synthesis, and to investigate how LXR activation affect PI3K pathway. Primary hepatocytes were isolated from Sichuan white geese (Anser cygnoides) and we investigated the effect of a synthetic LXR agonist, TO901317, on the intracellular cholesterol level and extracellular cholesterol level, and the effect of LXR activation on mRNA level and enzyme activity of factors involved in the PI3K/AKT/mTOR pathway. This was the first time to detect a novel role of SREBP-2 and PI3K signalling pathways in response to LXR agonist, and it is significant to understand cholesterol metabolism in the liver.
Materials and methods
Isolation and culture of goose primary hepatocytes
Hepatocytes were isolated from three 30-day-old Sichuan White geese from the Experimental Farm for Waterfowl Breeding at Sichuan Agricultural University using a modification of the two-step procedure described by Seglen (Seglen, Citation1976). The protocol for bird treatment was in accordance with the Canadian Council on Animal Care guidelines (1994). The method differed from that of Seglen in that the liver was removed before the preperfusion step. Cellular viability was greater than 90%, as assessed by the trypan blue dye exclusion test. Freshly isolated hepatocytes were diluted to 1×106 cells/mL. The culture medium was composed of DMEM (containing 4.5 g/L glucose; Life Technologies, Carlsbad, CA, USA) with 100 IU/L insulin (Sigma Aldrich, St. Louis, MO, USA), 100 IU/mL penicillin (Sigma Aldrich), 100 g/mL streptomycin (Sigma Aldrich), 2 mmol/L glutamine (Sigma Aldrich), and 100 mL/L fetal bovine serum. The hepatocytes were then either plated in 60-mm culture dishes at 3×106 cells per dish for total RNA isolation or plated in 24-well plates at 1×106 cells per well to measure cholesterol concentrations. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2, and the media was renewed after 3 h followed by the addition of serum-free media after 24 h. After another 24 h, cells were separately treated with serum-free media supplemented with 0.01, 0.1, 1, or 10 μmol/L TO901317 and incubated for 48 h, while the control sample cells were cultured with serum-free media for 48 h (serum-free media was renewed every 24 h).
Isolation of total RNA and real-time polymerase chain reaction
Total RNA was isolated from cultured cells using Trizol (Invitrogen, Carlsbad, CA, USA) and reverse-transcribed using the PrimerScriptTM RT system kit for real-time PCR (TaKaRa, Otsu, Japan) using oligo dT primer and random primer according to the manufacturer’s instructions. The quantitative real-time PCR reaction contained the newly generated cDNA template, SYBR Premix Ex TaqTM, sterile water, and primers of target genes. Real-time PCR was performed on the Cycler system (one cycle of 95°C for 10 s, followed by 40 cycles of 95°C for 5 s, and 60°C for 40 s). An 80-cycle melt curve was performed, starting at 55°C and increasing by 0.5 degrees every 10 s, to determine primer specificity. Specific primers are listed in , and were designed according to the goose gene sequences, PI3K: GenBank No. KF011500; AKT: GenBank No. KF011501; mTOR: GenBank No. KC424580; S6k: GenBank No. KC424581; SREBP-2: GenBank No. EF579754; HMGR: GenBank No. EF635218; 18S: GenBank No. L21170; β-actin: GenBank NO: M26111.1.
Amplicons corresponding to each target were examined by agarose gel to confirm the presence of a unique band of the expected size. Negative controls, which consisted of PCR amplifications with non-reverse transcribed RNA, did not generate any signal. All samples were amplified in duplicate, with the same PCR-mixture and in the same 96-well plate. The cycle threshold variation observed between duplicates was on average 0.12±0.1, thus demonstrating a high level of intra-assay reproducibility. Each sample was also repeated in another 96-well plate. The variation of Ct between the two independent plates was 0.28±0.22, showing a fair level of inter-assay reproducibility as well. PCR products were then diluted 16-fold and were used to generate the calibration curve and the amplification rate for each gene (PI3K, AKT, mTOR, S6K, SREBP-2, HMGR, and 18S). For each experimental sample, a normalised target gene level (Exp), corresponding to the target gene expression level relative to 18S (housekeeping genes) expression levels was determined by the 2-∆∆Ct method.
For the target gene expression analyses, the normalised target gene expression level for each sample was compared to the positive control sample. Therefore, the final results were expressed as N-fold differences in the normalised target gene expression level between each treated and control sample.
Measurement of intracellular cholesterol concentration
Samples of cultured cells for each treatment were sharked for 1h using an ultrasonic processor after washed three times with ice-cold phosphate-buffered saline. Total cholesterol was measured by a cholesterol oxidase method using CHOD-PAP assay Kit (BioSino, Beijing, China). Analyses were performed three times.
Extracellular triglyceride, total cholesterol, total protein and albumin levels
Primary goose hepatocytes were plated at a density of 3×106 cells/well in a 6-well culture dish. After 3 h, the serum-rich medium was refreshed, followed by serum-free media after 24 h. Then, cells were separately treated with culture medium supplemented with 0.01, 0.1, 1, or 10 μmol/L TO901317 and incubated for another 48 h, while the control sample cells were cultured with serum-free media (serum-free media was renewed every 24 h). After the 48 h incubation, the culture media were cooled on ice and collected for the analysis of extracellular triglyceride (TG) and total cholesterol (TC) concentration in the media, as well as total total protein (TP) and albumin (ALB) concentration. The commercial biochemical reagents kits (MinDray, Shenzhen, China) and BS-400 auto chemistry analyser (MinDray) were used for measuring these parameters.
Enzyme activity assay
The assay for enzyme activity of PI3K and mTOR enzymes was performed according to Engvall and Perlmann (Citation1972), by using Geese PI3K and mTOR ELISA kit. Primary cultures of goose hepatocytes were plated at a density of 0.5×104 cells/well in a 96-well culture dish. After 3 h, the serum-rich medium was refreshed, followed by serum-free media after 24 h. Then, cells were separately treated with culture medium supplemented with 0.01, 0.1, 1, or 10 μmol/L TO901317 and incubated for 48 h, while the control sample cells were cultured with serum-free media for 48 h (serum-free media was renewed every 24 h). Then the assay procedures were followed as the manufacturer instructions at 490 nm. All samples were analysed in triplicates.
Statistical analysis
The results were presented as means±SD. Statistical analyses were conducted by the 2-tailed Student-t test with the use of SAS 6.12 package (SAS Institute Inc., Cary, NC, USA). The difference between 2 sets of values was considered significant when the P value was <0.05.
Results and dicussion
Effect of TO901317 on extracellular total protein and albumin accumulation
To identify the normal cell function after the treatment with TO901317 in goose primary hepatocytes, we measured the extracellular TP and ALB accumulation. The results showed that treatment with the LXR agonist TO901317 induced extracellular TP accumulation in a dose-dependent manner from 0.01 to 10 μmol/L. Compared with the control group, TO901317 at 0.01, 0.1, 1, or 10 μmol/L all had a significant up-regulation effect (P<0.05). Similar with the result of TP, after the treatment with 0.01, 0.1, 1, or 10 μmol/L TO901317, ALB level all existed the significant increase (P<0.05) (). These data indicated that TO901317 could promote primary hepatocytes protein synthesis, and the cells had the normal function.
Effect of TO901317 on intracellular and extracellular cholesterol concentration
To understand the role of LXR in cholesterol homeostasis, we investigated the effect of LXR agonist on the concentration of intracellular and extracellular cholesterol. As shown in , there was an up regulation manner of extracellular cholesterol accumulation when culture with 0, 0.01, 0.1, 1, and 10 μmol/L TO901317. The intracellular cholesterol concentration only showed an uptrend manner when the TO901317 concentration was under 1 μmol/L, and it decreased when TO901317 reached 10 μmol/L (P<0.05).
Liver X receptor agonist TO901317 treatment regulated hepaticellar expression of phosphatidylinositol 3-kinase signalling pathway
presented the effect of TO901317 on the mRNA expression of PI3K, AKT, mTOR and S6K by quantitative real-time PCR analysis. TO901317 at 0.01 μmol/L did not have a significant effect on gene expression level of mTOR (P>0.05). However, the treatment with 0.1, 1, or 10 μmol/L TO901317 all had significant effects on the mRNA level of these genes (P<0.05), and the up-regulation of gene expression in response to TO901317 was dose-dependent. To determine whether LXR agonist affects PI3K and mTOR translation, goose hepatocytes were exposed to the LXR agonist TO901317 for 48 h. As shown in , After incubation with 0.01, 0.1, 1, or 10 μmol/L TO901317, the PI3K and mTOR enzyme activity were up-regulated by all dose of TO901317 (P<0.05).
Effect of TO901317 on mRNA expression level of sterol regulatory element-binding protein 2 and 3-hydroxy-3-methylglutaryl-CoA reductase
To confirm the roles of SREBP-2 in mediating cholesterol biosynthesis induced by TO901317 in hepatocytes, we studied the effect of TO901317 on mRNA expression level of SREBP-2 and HMGR in goose hepatocytes. As shown in , the gene expression levels of SREBP-2 and HMGR were regulated by TO901317 at 0.01, 0.1 and 1 μmol/L in an uptrend manner, but when the TO901317 concentration reached 10 μmol/L, the effect decreased. Compared with the effects of TO901317 at other concentrations, the effect of 1 μmol/L TO901317 was most evident.
Effect of TO901317 on extracellular triglyceride concentration
Based on these data, we demonstrated that SREBP-2 and its target gene HMGR play a regulatory role in LXR-induced cholesterol synthesis. In order to understand whether changes LXR activity affects TGs content, we tested the TG content of hepatocytes. As shown in , the extracellular content of TG had a rising trend with the increase of TO901317.
General remarks
In our previous study, we found that overfeeding to geese induced an increase of the plasma cholesterol concentrations and the cholesterol content in their liver, accompanied with the formation of hepatic steatosis (Han et al., Citation2008). Thus, changes of hepatic cholesterol synthesis may have a close relation with the goose hepatic steatosis. In liver, intracellular cholesterol levels are finely controlled by SREBP-2, a transcription factor whose nuclear fragments are released from membranes by controlled proteolysis. To our knowledge, it has not yet been investigated whether the activation of LXR influences the expression of SREBP-2 and its target genes in liver. During the last several years, one study has provided experimental evidences supporting the notion that the activation of LXR can result in lipogenesis in goose hepatocytes (Han et al., Citation2009). Aravindhan et al. demonstrates that incubation with synthetic LXR agonists increased cholesterol synthesis in HepG2 cells (Aravindhan et al., Citation2006).
In this study, we found an up regulation manner of extracellular cholesterol accumulation after treatment with 0, 0.01, 0.1, 1, and 10 μmol/L TO901317, and the intracellular cholesterol concentration only showed an uptrend manner when the TO901317 concentration was under 1 μmol/L, but 10 μmol/L TO901317 had an inhibiting effect (P<0.05) (). Compared with the effect of 1 μmol/L TO901317, the mRNA expression levels of SREBP-2 and HMGR in goose hepatocytes decreased after incubation with 10 μmol/L TO901317, which is similar with the change of intracellular cholesterol concentrations. This result clearly demonstrates that SREBP-2 and its target gene HMGR play a regulatory role in LXR-induced cholesterol synthesis. This is similar with a previous report that SREBP-2 and LXR together response to cellular cholesterol status through regulating the mRNA expression of their target genes (Tamehiro et al., Citation2007).
We also found that treatment with TO901317 at all dose increased evidently the extracellular TP and ALB levels in this study. So it was obvious that the decrease of the intracellular cholesterol level induced-by the high concentration of TO901317 at 10 μmol/L was not because of hepatic dysfunction. On one hand, the treatment of high dose TO901317 (10 μmol/L) could increase the intracellular total TG accumulation (), but decrease the intracellular cholesterol level (). We propose the synthesised intracellular cholesterol induced by 10 μmol/L TO901317 could active the de novo lipogenesis. On the other hand, the treatment of high dose TO901317 (10 μmol/L) could increase the extracellular cholesterol accumulation. It was predicted that the LXR activation increases the cholesterol efflux from the hepatocytes, and a similar result reported by Zanotti et al., showed that in vivo stimulation of LXR by TO901317 increase the efflux of cholesterol from peritoneal macrophages (Zanotti et al., Citation2008). Another previous study showed that LXR agonist TO901317 has been shown to induce the expression of ABCA1 and other LXR-responsive genes. However, in primary human fibroblasts an increase in cellular cholesterol efflux in response to TO901317 was not demonstrated (Sparrow, Citation2002). We assumed that the ndifference results may due to the cell types and species differences.
Previous studies confirmed that SREBP-2 is also regulated by mTOR. Similarly, our study showed that treatment with TO901317 in goose hepatocytes promoted mRNA expression of S6K. Otherwise, one previous study suggested that cholesterol trafficking is required for mTOR activation in endothelial (Xu et al., Citation2010), which confirmed the role of mTOR in cholesterol biosynthesis. Huwait et al. showed that the LXR agonists-induced ABCA1 promoter activity was inhibited by the expression of dominant negative forms of the components of the PI3K pathways (Huwait et al., Citation2011). These results suggested that LXR and PI3K/AKT/mTOR pathway is closely related with cholesterol metabolism. Interestingly, in our study we found that LXR agonists at all dose could increase the mRNA level of mTOR in goose hepatocytes. And the mRNA expression levels of PI3K and AKT also have similar increase trend. These results suggest that LXR agonists may regulate mTOR activation by controlling the upstream gene translation of one or more proteins involved in mTOR processing. Another possibility is that PI3K gene transcription is coordinately regulated by LXR, because the results showed that TO901317 treatment also increased PI3K enzyme activity. Similarly, previous research has shown that in vitro primary cortical neuron culture TO901317 treatment significantly increased the p-PI3K and p-AKT activity (Chen et al., Citation2009). Otherwise, we observed when TO901317 concentration reached 10 μmol/L, the expression of PI3K/AKT/mTOR remained a significant high level, but the SREBP-2 mRNA level and the intracellular cholesterol concentration decreased. Recently, by using ATP-site mTOR inhibitor PP242 in HCT15 cells and murine NIH 3T3 cells, Wang et al. confirmed that mTOR significantly decreases the expression of SREBP-2 and target genes HMGCR and DHCR24 (Wang et al., Citation2011). Thus we concluded that the dual system driven by LXR and mTOR regulates the hepatic SREBP-2 expression and mediates a unique response to cellular cholesterol status in liver cells. However, the co-ordinated mechanism of LXR and mTOR regulating hepatic SREBP-2 expression is not clearly.
Conclusions
To conclude, our current work provided a new evidence suggesting an additional role for LXR in positively regulating the SREBP-2 expression. Particularly, we have demonstrated that LXR agonists could stimulate PI3K/AKT/mTOR pathway, this suggested that PI3K/AKT/mTOR pathway seems to contribute to TO901317-induced cholesterol homoeostasis. These findings indicate that LXR plays an important role in the regulation of cholesterol biosynthesis, meanwhile, this nuclear receptor also contacts with PI3K/AKT/mTOR pathway in cholesterol biosynthesis.
Acknowledgements
The work was supported by the National Natural Science Funds of China (No. 31101712).
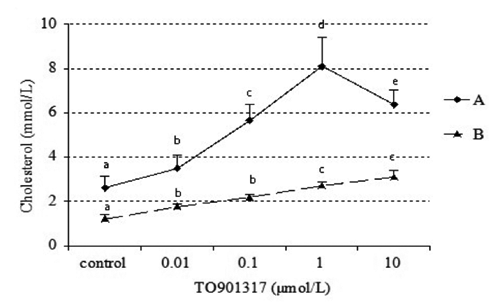
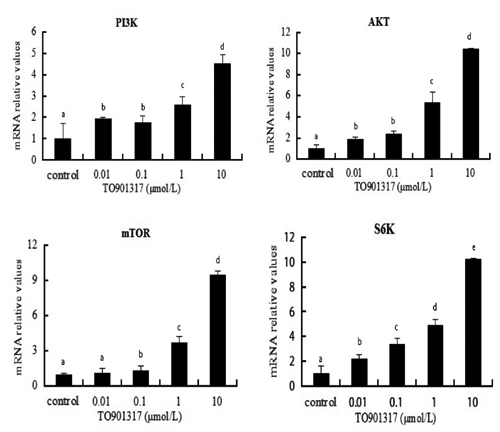
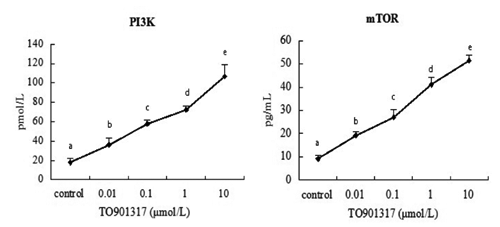
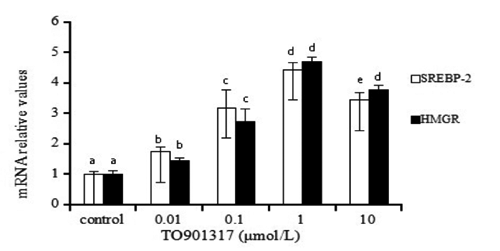
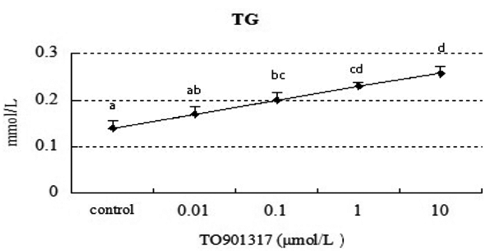
Table 1. Sequence of polymerase chain reaction primers.
Table 2. Total protein and albumin accumulation of goose primary hepatocytes after TO901317 treatment.
References
- AbildayevaK. Jansen P.J. Hirsch-ReinshagenV. Bloks V.W. Bakker A.H. Ramaekers F.C. de Vente J. Groen A.K. Wellington C.L. KuipersF. MulderM.,2006. 24(S)-hydroxycholesterol participates in a liver X receptor-controlled pathway in astrocytes that regulates apolipoprotein E-mediated cholesterol efflux. J. Biol. Chem. 281 :12799-12808.
- AravindhanK. Webb C.L. JayeM. GhoshA. Willette R.N. Di Nardo N.J. Jucker B.M.,2006. Assessing the effects of LXR agonists on cellular cholesterol handling: a stable isotope tracer study. J. Lipid Res. 47 :1250-1260.
- ChenJ. ZacharekA. CuiX. ShehadahA. JiangH. RobertsC. LuM. ChoppM.,2009. Treatment of stroke with a synthetic liver X receptor agonist, TO901317, promotes synaptic plasticity and axonal regeneration in mice. J. Cerebr. Blood F. Met. 30 :102-109.
- DeBose-Boyd R.A. OuJ. Goldstein J.L. Brown M.S.,2001. Expression of sterol regulatory element-binding protein 1c (SREBP-1c) mRNA in rat hepatoma cells requires endogenous LXR ligands. P. Natl. Acad. Sci. USA 98 :1477-1482.
- DüvelK. Yecies J.L. MenonS. RamanP. Lipovsky A.I. Souza A.L. TriantafellowE. MaQ. GorskiR. CleaverS. Vander Heiden M.G. Mac Keigan J.P. Finan P.M. Clish C.B. Murphy L.O. Manning B.D.,2010. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 39 :171-183.
- Emerling B.M. AkcakanatA.,2011. Targeting PI3K/mTOR signaling in cancer. Cancer Res. 71 :7351-7359.
- EngvallE. PerlmannP.,1972. Enzyme-linked immunosorbent assay, Elisa 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J. Immunol. 109 :129-135.
- Forman B.M. RuanB. ChenJ. Schroepfer G.J.Jr. Evans R.M.,1997. The orphan nuclear receptor LXRα is positively and negatively regulated by distinct products of mevalonate metabolism. P. Natl. Acad. Sci. USA 94 :10588-10593.
- Goldstein J.L. DeBose-Boyd R.A. Brown M.S.,2006. Protein sensors for membrane sterols. Cell 124 :35-46.
- GuoD. ReinitzF. YoussefM. HongC. NathansonD. AkhavanD. KugaD. Amzajerdi A.N. SotoH. ZhuS. BabicI. TanakaK. DangJ. IwanamiA. GiniB. DejesusJ. Lisiero D.D. Huang T.T. Prins R.M. Wen P.Y. Robins H.I. Prados M.D. Deangelis L.M. Mellinghoff I.K. Mehta M.P. James C.D. ChakravartiA. Cloughesy T.F. TontonozP. Mischel P.S.,2011. An LXR agonist promotes glioblastoma cell death through inhibition of an EGFR/AKT/SREBP-1/LDLR-dependent pathway. Cancer Discov. 1 :442-456.
- Han C.C. WangJW. LiL. WangL. ZhangZ.,2009. The role of LXRα in goose primary hepatocyte lipogenesis. Mol. Cell. Biochem. 322 :37-42.
- Han C.C. Wang J.W. Xu H.Y. LiL. Ye J.Q. JiangL. Zhuo W.H.,2008. Effect of overfeeding on plasma parameters and mRNA expression of genes associated with hepatic lipogenesis in geese. Asian Austral. J. Anim. 21 :590-595.
- Horton J.D. Goldstein J.L. Brown M.S.,2002. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109 :1125-1131.
- Huwait E.A. Greenow K.R. Singh N.N. Ramji D.P.,2011. A novel role for c-Jun N-terminal kinase and phosphoinositide 3-kinase in the liver X receptor-mediated induction of macrophage gene expression. Cell. Signal. 23 :542-549.
- Janowski B.A. ShanB. Russell D.W.,2001. The hypocholesterolemic agent LY295427 reverses suppression of sterol regulatory element-binding protein processing mediated by oxysterols. J. Biol. Chem. 276 :45408-45416.
- LaplanteM. Sabatini D.M.,2012. mTOR signaling in growth control and disease. Cell 149 :274-293.
- LiS. OgawaW. EmiA. HayashiK. SengaY. NomuraK. HaraK. YuD. KasugaM.,2011. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem. Bioph. Res. Co. 412 :197-202.
- Peet D.J. Janowski B.A. Mangelsdorf D.J.,1998. The LXRs: a new class of oxysterol receptors. Curr. Opin. Genet. Dev. 8 :571-575.
- PorstmannT. Santos C.R. GriffithsB. CullyM. WuM. LeeversS. Griffiths J.R. Chung Y.L. SchulzeA.,2008. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8 :224-236.
- Repa J.J. Mangelsdorf D.J.,2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Gene. Dev. 14 :2819-2830.
- Seglen P.O.,1976. Preparation of isolated rat liver cells. Method. Cell. Biol. 13 :29-83.
- ShimanoH. ShimomuraI. Hammer R.E. HerzJ. Goldstein J.L. Brown M.S. Horton J.D.,1997. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J. Clin. Invest. 100 :2115-2124.
- Sparrow C.P.,2002. A potent synthetic LXR agonist is more effective than cholesterol loading at inducing ABCA1 mRNA and stimulating cholesterol efflux. J. Biol. Chem. 277 :10021-10027.
- TamehiroN. Shigemoto-MogamiY. KakeyaT. Okuhira K.I. SuzukiK. SatoR. NagaoT. Nishimaki-MogamiT.,2007. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282 :21090-21099.
- TontonozP. Mangelsdorf D.J.,2003. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17 :985-993.
- VenkateswaranA. Laffitte B.A. Joseph S.B. Mak P.A. Wilpitz D.C. Edwards P.A. TontonozP.,2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRalpha. P. Natl. Acad. Sci. USA 97 :12097-12102.
- Wang B.T. Ducker G.S. Barczak A.J. BarbeanR. Erle D.J. Shokan K.M.,2011. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. P. Natl. Acad. Sci. USA 108 :15201-15206.
- WangN. RanallettaM. MatsuuraF.,2006. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arterioscl. Throm. Vas. 26 :1310-1316.
- WangY. Rogers P.M. SuC. VargaG. Stayrook K.R. Burris T.P.,2008. Regulation of cholesterologenesis by the oxysterol receptor, LXRalpha. J. Biol. Chem. 283 :26332-26339.
- WongJ. Quinn C.M. Brown A.J.,2006. SREBP-2 positively regulates transcription of the cholesterol efflux gene, ABCA1, by generating oxysterol ligands for LXR. Biochem. J. 400 :485-491.
- XuJ. DangY. Ren Y.R. Liu J.O.,2010. Cholesterol trafficking is required for mTOR activation in endothelial cells. P. Natl. Acad. Sci. USA 107 :4764-4769.
- ZanottiI. PotiF. PedrelliM. FavariE. MoleriE. FranceschiniG. CalabresiL. BerniniF.,2008. The LXR agonist T0901317 promotes the reverse cholesterol transport from macrophages by increasing plasma efflux potential. J. Lipid Res. 49 :954-960.
- ZhaoC. Dahlman-WrightK.,2009. Liver X receptor in cholesterol metabolism. J. Endocrinol. 204 :233-240.
