Abstract
The objective of this study was to investigate the serum protein electrophoretic pattern of growing calves with changing nutrition, in order to evaluate the concentrations of serum protein fractions for different age groups. One hundred and eleven clinically healthy calves of a Slovak spotted breed, low-land black spotted breed, or their crossbreeds were included into this study. They were divided into three groups according to their age and nutrition: Group I – calves at the age of 1-2 weeks (milk period, n=30); Group II – calves at the age of 1-2 months (transitional feeding period, n=32); Group III – calves at the age of 3-5 months (solid feeding period, n=49). Blood samples were taken by direct puncture of v. jugularis. Blood serum was analysed for total serum protein concentrations, and for the relative values of protein fractions – albumin, α1-, α2-, β1-, β2-and γ-globulins. The results showed a significant effect of age on the serum total protein concentrations and the most of protein fractions. In younger calves significantly higher relative concentrations of α1-, α2- and β1-globulins compared with older animals were recorded (P<0.05). On the other hand, the relative values of β2-, γ-globulins, and total proteins in younger calves were significantly lower than those measured in older ones (P<0.05). It is concluded that there are marked age-dependent differences in serum protein fractions in growing calves, and this should be taken into consideration when interpreting serum protein profile.
Introduction
The blood serum is composed of hundreds of different proteins and concentrations of total proteins and several specific proteins are of clinical value (Joliff, Citation1992). Protein electrophoresis is a standard technique to separate and determine the protein components in plasma or serum in clinical biochemistry. Serum proteins are by electrophoresis resolved into 5-6 bands, comprising albumin, α-, β- and γ-globulins (Vaden et al., Citation2009). Routine serum protein electrophoresis is recognised as the most reliable assessment of protein profiles and has replaced the biochemical determination of albumin and albumin/globulin ratio in the ability to predict abnormalities of clinical significance (Werner and Reavill, Citation1999). The importance of considerable species differences to the overall interpretation of serum protein electrophoretic pattern in the veterinary medicine was established and constitutes a continued challenge to the clinicians and to the laboratories to continue the pursuit of species-specific, even age-, gender- and nutrition-specific values and ranges for several serum protein fractions.
Separation of serum protein fractions is very important for the diagnosis of many different diseases, including paraproteinaemias, immune deficiency, various protein abnormalities and for the determination of the underlying nature of hyperproteinemia or hyperglobulinemia (Whicher et al., Citation1987). Identifying and quantifying protein fractions enable the identification of animals with altered serum protein pattern, which may reflect responses to changes in homeostasis or disease (Alberghina et al., Citation2011). Many times, abnormalities found in proteinogram are not related to illness conditions, but with physiological and individuals conditions (França et al., Citation2011). The interpretation of biochemical constituents depends on the knowledge of the variation that exists not only among different species of animals, but also among different groups of animals. Factors like age, stage of development and growth, breed, pregnancy, nutrition are directly related to changes in various biochemical parameters (Egli and Blum, Citation1998; Mohri et al., Citation2007). These studies confirmed that changes in nutrition in young animals, body growth and development are accompanied by dynamic changes in many indices of haematological, protein, mineral, enzymatic, energy, and other profiles. Moreover, the age and nutrition of animals are important factors that may affect also the electrophoretic pattern of serum proteins. Therefore, to maximise the diagnostic value of serum protein electrophoresis, it is essential for clinicians to have access to well-established age-and nutrition-specific serum protein electrophoretic patterns.
The aim of the current study was to quantify the serum protein fractions using agarose gel electrophoresis in growing calves (at the age ranged from 1-2 weeks till 5 months) in order to evaluate the effect of age and nutrition on serum protein electrophoretic pattern.
Materials and methods
Animals and blood sample collection
This study was carried out on one hundred and eleven clinically healthy calves from large-scale dairy farms with a similar conventional calf rearing program. The calves were of a Slovak spotted breed, low-land black spotted breed, or their crossbreeds at the age from 1 week till 5 months. The animals were divided into the following age, physiological and nutritional groups: Group I – calves at the age of 1-2 weeks [milk feeding period, n=30, average body weight (BW)=41 kg]; Group II – calves at the age of 1-2 months (transitional feeding period, n=32, average BW=63 kg); Group III – calves at the age of 3-5 months (solid feeding period, n=49, average BW=119 kg). The calves from the Group I were kept loosely in individual pens. These calves till the age of 2 days were fed 2 L of colostrum 3 times a day. The calves from the age of 3 days received 3 L of whole milk 2 times a day. During the transitional pre-ruminant feeding period (Group II), the calves were housed loosely in individual pens and fed 2.5 L of milk replacer administered twice a day, meadow hay and concentrates with free access to water. The calves from the age of 3 months (Group III) were housed loosely in larger groups of animals, and received hay and concentrated mixed feed (in the amount gradually increasing from 0.1 up to 1.5 kg per calf and day) with free access to water. Before blood sample collection, all animals were clinically examined using standard physical examination procedures (Jackson and Cockcroft, Citation2002). The evaluated calves were in good general health without any abnormalities or obvious clinical signs of diseases. Blood samples for the analyses were collected from calves by direct puncture of v. jugularis into serum gel separator tubes without anticoagulant. The samples from the calves were collected at the same time 2 h after the morning feeding. Blood samples were allowed to clot at room temperature, and then centrifuged at 3000 g for 30 min to separate serum. The harvested blood serum was dispensed into plastic tubes, and stored at -20°C until analysed for total serum protein concentrations and for the identification of serum protein fractions.
Laboratory analyses
Blood serum was analysed for the concentrations of total proteins (TP, g/L), and serum protein fractions. Total protein concentrations were assessed on an automated biochemical analyser Alizé (Lisabio, Pouilly-en-Auxois, France) by the biuret method using commercial diagnostic kits (Randox Laboratories Ltd., Crumlin, UK). Serum protein fractions were separated by zone electrophoresis on a buffered agarose gel at pH 8.8 on an automated electrophoresis system Hydrasys (Sebia Corporate, Evry, France) using commercial diagnostic kits Hydragel 7 Proteine (Sebia Corporate) according to the procedure described by the manufacturer. The electrophoretic gels were scanned, and the serum protein fractions were visualised and displayed on densitometry system Epson Perfection V700 (Epson America Inc., Long Beach, CA, USA) by light transmission and automatic convertion into an optical density curve presentation. Protein fractions were identified and quantified by computer software Phoresis version 5.50 (Sebia Corporate), and if necessary, corrected by visual inspection of the electrophoretogram.
Serum proteins were separated into the following fractions in order of fastest to slowest mobilities: albumin, α1-, α2-, β1-, β2-, and γ-globulins. The relative concentrations (%) of the protein fractions were determined as the percentage of the optical absorbance. Albumin:globulin ratios (A/G) were computed from the electrophoretic scan.
Statistical analyses
Arithmetic means (x) and standard deviation (SD) for each evaluated variable and age group of animals were calculated using descriptive statistical procedures. One-way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were used to compare the differences between the results and to evaluate the significances of means between the different age groups of calves. All statistical analyses were carried out using the programme GraphPad Prism V5.02 (GraphPad Software Inc., La Jolla, CA, USA).
Results and discussion
The data referring to the concentrations of total proteins, serum protein fractions and A/G ratios in three different age and nutritional groups of calves expressed as average values and SDs of means, including the significance of differences in means between the groups of animals are presented in . The distribution of individual values of the concentrations of serum total proteins and relative values of albumin, α-, β- and γ-globulin fractions in the evaluated groups of calves are shown in .
The concentrations of total proteins in the blood serum of calves showed a trend of significantly increasing values with the advanced age and changes in feeding (P<0.001, , ). The values ranged from 61.4 g/L in the youngest calves to 68.8 g/L in the oldest animals. Serum protein electrophoresis identified in calves six distinct bands, comprising albumin, two α-globulin (α1 and α2), two β-globulin (β1 and β2), and γ-globulin fractions. Albumin was the most prominent fraction in young, as well as older calves, with non-significantly higher relative concentrations in calves in the milk and transitional feeding period compared to those in the solid feeding period (, ). Significantly highest mean relative concentration of α1-globulins was observed in the youngest calves (), the differences found in the evaluated age groups were significant (P<0.001). A significant effect of the age was found in the relative concentrations of α2-globulins (P<0.001), with the significantly lowest mean value in the oldest calves (). Significant age- and feeding-related differences between the evaluated groups of calves were found also for β1- and β2-globulins. The relative concentrations of β1-globulins significantly decreased with increasing age of the calves (; P<0.001). An opposite trend was observed in the relative concentrations of β2-globulins. The lowest mean value was found in the youngest calves (). Similarly, the relative concentrations of γ-globulins showed a trend of significantly higher values with increasing age (; P<0.001). Significantly highest mean value was recorded in the oldest calves. The A/G ratios showed no significant differences between the evaluated groups of calves. The lowest A/G mean value was recorded in the youngest calves.
Various studies on age-dependent dynamics of many hematological and biochemical variables in calves were carried out. These studies stated that because of the factors influencing the concentrations of biochemical variables, it is extremely important to know the physiological values and, thus, be able to distinguish them to the pathological conditions (Egli and Blum, Citation1998; Mohri et al., Citation2007). Seeing that serum protein electrophoresis is an important auxiliary diagnostic method for the identification of blood proteins, helpfull to clinical biochemistry, it is necessary to know the protein profile of healthy calves of different ages, for posterior comparison with pathological processes. The adaptation of calves to various environmental factors, including nutrition, is accompanied by intense morphological, functional, as well as biochemical changes (Blum, Citation2006; Piccione et al., Citation2009). In the present study, we evaluated the changes in the serum protein electrophoretic pattern in calves at different ages (from 1-2 weeks till 5 months), which included three different types of feeding regimes of calves: milk, transient (milk and solid), and solid feed. Significant age-related variations in total protein concentrations were observed in this study, with a trend of gradually increasing values with advancing age. Kaneko (Citation1997) stated that with age, the total serum protein concentrations tend to increase. Similar increase in blood protein concentrations in growing calves was reported by Mohri et al. (Citation2007) and Ježek et al. (Citation2006). Undoubtedly, the reason for the demonstrated effect was the adequate nutrition (Blum and Hammon, Citation2000; Hammon et al., Citation2002). Knowles et al. (Citation2000) found the lowest concentrations of albumin after birth, and then it increased till the age of 80 days. Similar variations in the concentrations of albumin in calves during a shorter period after the birth, were reported by Bertoni et al. (Citation2009) and Osorio et al. (Citation2013). The results of an experiment conducted on neonatal piglets demonstrated that an increased dietary amino acids supply enhances hepatic albumin synthesis (Davis et al., Citation1998). These authors stated that the increase of albumin together with the age is a physiological phenomenon and higher concentrations of albumin in young and growing animals help to maintain the metabolic balance. According to Piccione et al. (Citation2011), the albumin levels in goat kids showed a significant decrease in the first 14 days after birth with a subsequent increase. This trend reflects the albumin’s medium half-life that ranges from 14 to 16 days in ruminants, after which period the liver is responsible for albumin synthesis (Lassen, Citation2004). For the relative concentrations of albumin, a non-significant age-related variation between calves at different ages was observed in this study, with values being higher in younger calves compared to older ones. It has been shown also in horses, goats, and camels that the age of investigated animals may markedly influence the electrophoretic pattern of serum proteins with decreasing relative concentrations of albumin and increasing globulins with advancing age (Chaudhary et al., Citation2003; Alberghina et al., Citation2010).
The results of the presented study indicated marked variations between the groups of calves in the relative concentrations of α-, β-, as well as γ-globulin fractions. Previously, Keay and Doxey (Citation1982) found differences in α- and β-globulins between calves at the age of 3-4 weeks and adult cattle, with higher values in calves. In the current study, the relative concentrations of α1- and α2-globulins recorded in young calves were higher than the values obtained in older ones. Similarly, Szewczuk et al.
Our study indicated in young calves significantly higher relative concentrations also for β1-globulins compared to older animals. Similarly, higher concentrations of β1-globulins were found by Szewczuk et al. (Citation2011) in calves on day 5 of life compared to older ones, as well as by Piccione et al. (Citation2011) in goat kids after birth. This is because the most important proteins included in β1-globulin fraction are the complement, which like the acute phase proteins are involved in the environmental stress response (Bernabucci et al., Citation2009). An opposite trend was observed in the concentrations of β2-globulins. Small amounts of immunoglobulins can also be present in this fraction. Therefore, higher concentrations of β2-globulins in older calves may be attributed to an increase in antibody production because of the active imunity, that begins after the age of 1 month, and an increase of exposure to ambiental agents in older animals (Tizard, Citation2009; França et al., (Citation2011). showed in the blood serum of calves higher concentrations of α-globulins on day 5 of life compared with values found on day 30 of life. The α-fraction includes the majority of acute phase proteins (haptoglobin, ceruloplasmin, α1-acid glycoprotein, α1-antitrypsin), which increase rapidly with inflammatory status and stressors (Kaneko, Citation1997). On the other hand, the environmental conditions represent an important trigger for the shift in acute phase proteins, and some increases in the concentrations of α-globulins may be present in conditions not related to inflammatory diseases (Lomborg et al., Citation2008). Therefore, higher relative concentrations of α-globulins in calves are not necessarily a sign of the activation of inflammatory processes, or a sign of a disease. Higher values of α-globulins in young calves obtained in our study may be related to the exposure of animals to changing nutritional and rearing factors, and may be associated with the normal process of growth. Kaneko et al. (Citation2008) reported that blood sera of newborn and young calves contain large amounts of α-globulins due to higher concentrations of some of the proteins from this fraction, which have to protect young animals from immunologic attacks. According to Bishop et al. (Citation2010), α1-fetoprotein represents one of these proteins, which is synthesised in the developing fetus and then by the parenchymal cells of the liver. Therefore, higher relative concentrations of α1-globulins in calves recorded in our study may reflect the accumulation of α1-fetoprotein in the blood serum of young animals. However, additional studies are needed to identify the individual proteins associated with higher relative concentrations of α1-globulins in calves compared with adult cattle.
The proteins that compose the γ-globulin fraction are principally the immunoglobulins (IgG, IgA, IgE, IgM). In the present study, higher concentrations of γ-globulins in older calves compared to the group of the youngest animals were recorded, which may be connected to good alimentary canal absorption and the normal process of growth. Similar changes were observed in calves by Mohri et al. (Citation2007), with an increase of γ-globulins and their stabilisation in older animals. Paltrinieri et al. (Citation2008) reported also that in foals from birth until twelve months of age there is a gradual increase in immunoglobulins. Chaudhary et al. (Citation2003) suggested that lower concentrations of γ-globulins in young calves are caused by the immaturity of the lymphoid system, and that these lower values remain until the production of globulins by the maturing immune system. According to Kaneko et al. (Citation2008), due to the age, there is an increase in γ-globulin concentrations because of a higher exposure of an organism to changing environmental factors and pathogens, including bacteria, viruses, fungi and parasites. Presented results suggest a marked shift in the concentrations of albumin and globulins in young calves compared with older ones (higher relative concentrations of albumin and lower percentages of γ-globulins in younger animals), presumably caused by changing globulin patterns during development. Thomas (Citation2000) reported that over the life span of animals, there are changes in the relative concentrations of protein fractions with advancing age (decrease in albumin, and increase in globulins). Age is thus an important consideration in the interpretation of results of serum protein electrophoresis.
The albumin/globulin ratio is of special interest for clinicians because it allows systematic classification of the electrophoretic profile and identification of dysproteinemias (Kaneko, Citation1997). In our study, presented data showed non-significant variations in A/G ratios reflecting the changes found in the relative concentrations of albumin and globulin fractions. According to Alberghina et al. (Citation2010), the A/G ratio must be interpreted cautiously with attention paid to which part of the ratio has changed.
Conclusions
In conclusion, the results of the current study showed that the serum protein electrophoretic patterns are significantly influenced by the age and changing nutrition of the animals and this must be taken as a very important factor in determinig the concentrations of serum protein fractions in calves. The most significant differences between calves at different ages were observed in the relative concentrations of α1-, α2-, β1- and γ-globulins. While the relative concentrations of α- and β1-globulins were significantly higher in young calves, the values of γ-globulins were higher in older animals. The shift in the concentrations of albumin and globulin fractions suggests that the age of evaluated animals should be taken into consideration when interpreting serum protein electrophoretic profiles. For the most of serum protein fractions, age specific values should be available for precise interpretation of laboratory results in animals. Seeing that serum protein electrophoresis may be a useful diagnostic tool also in bovine medicine, the obtained data would be useful for clinicians in the determination and differentiation of dysproteinemias and the evaluation of various pathological conditions in calves, providing a basis for further specific laboratory investigations. Moreover, the proper interpretation of serum protein electrophoretic pattern requires the analysis of some specific serum proteins.
Acknowledgments
This work was supported by the Slovak Research and Development Agency under contract No. APVV-0475-10 and by VEGA Scientific Grants No. 1/0592/12 and 1/0812/12 from the Ministry of Education.
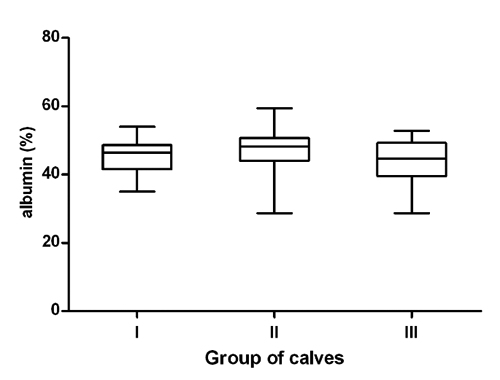
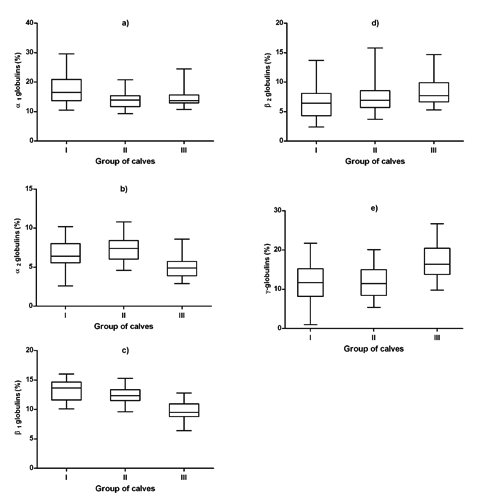
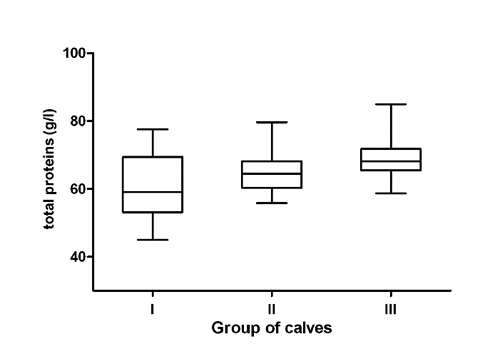
Table 1. Concentrations of total proteins, serum protein fractions and albumin/globulin ratios in the evaluated age and nutritional groups of calves (mean±SD).
References
- AlberghinaD. CasellaS. VazzanaI. FerrantelliV. GiannettoC. PiccioneG. 2010. Analysis of serum proteins in clinically healthy goats (Capra hircus) using agarose gel electrophoresis. Vet. Clin. Path. 39:317-321.
- AlberghinaD. GiannettoC. VazzanaI. FerrantelliV. PiccioneG. 2011. Reference intervals for total protein concentration, serum protein fractions, and albumin/globulin ratios in clinically healthy dairy cows. J. Vet. Diagn. Invest. 23:111-114.
- BernabucciU. LaceteraN. DanieliP.P. BaniP. NardoneA. RonchiB. 2009. Influence of different periods of exposure to hot environment on rumen function and diet digestibility in sheep. Int. J. Biometeorol. 53:387-395.
- BertoniG. FerrariA. GubbiottiA. TrevisiE. 2009. Blood indices calves: relationship with mother values and changes in the first days of life. pp 595-597 in Proc. 16th Nat. Congr. ASPA, Torino, Italy. Ital. J. Anim. Sci. 8(Suppl.2):595-597.
- BishopM.L. FodyE.P. SchoeffL.E. 2010. Clinical chemistry: techniques, principles, correlations. Lippincott Williams & Wilkins, Philadelphia, PA, USA.
- BlumJ.W. 2006. Nutritional physiology of neonatal calves. J. Anim. Physiol. An. N. 90:1-11.
- BlumJ.W. HammonH.M. 2000. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves. Livest. Prod. Sci. 66:151-159.
- ChaudharyZ.I. IqbalJ. RashidJ. 2003. Serum protein electrophoretic pattern in young and adult camels. Aust. Vet. J. 81:625-626.
- DavisT.A. BurrinD.G. FiorottoM.L. ReedsP.J. JahoorF. 1998. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate.J. Nutr. 128:347-350.
- EgliC.P. BlumJ.W. 1998. Clinical, haematological, metabolic and endocrine traits during the first three months of life of suckling Simentaler calves held in a cow-calf operation. J. Vet. Med. A 45:99-118.
- FrançaR.T. CostaM.M. MartinsD.B. PagnoncelliM. LealM.L. MazzantiC.M. PalmaH.E. KunertC.P. PaimF.C. dos Anjos LopesS.T. 2011. Protein profile of buffaloes of different ages. Acta Sci. Vet. 39:995-999.
- HammonH.M. SchiesslerG. NussbaumA. BlumJ.W. 2002. Feed intake patterns, growth performance and metabolic and endocrine traits in calves fed unlimited amounts of colostrums and milk by automate strating in the neonatal period. J. Dairy Sci. 85:3352-3362.
- JacksonP.G.G. CockcroftP.D. 2002. Clinical examination of farm animals. Blackwell Publ., Oxford, UK.
- JežekJ. KlopčičM. KlinkonM. 2006. Influence of age on biochemical parameters in calves. Bull. Vet. Inst. Pulawy 50:211-214.
- JoliffC.R. 1992. Analysis of the plasma proteins. J. Clin. Immunoassay 15:151-161.
- KanekoJ.J. 1997. Serum proteins and the dysproteinemias. In: KanekoJ.J. (ed.) Clinical biochemistry of domestic animals. Academic Presss, London, UK, pp 117-138.
- KanekoJ.J. HarveyJ.W. BrussM.L. 2008. Clinical biochemistry of domestic animals. 6th ed. Elsevier Academic Press, London, UK.
- KeayG. DoxeyD.L. 1982. A comparison of the serum protein electrophoretic patterns of young and adult animals. Vet. Res. Commun. 5:271-276.
- KnowlesT.G. EdwardsJ.E. BazeleyK.J. BrownS.N. ButterworthA. WarrissP.D. 2000. Changes in the blood biochemical and haematological profile in neonatal calves with age. Vet. Rec. 18:593-598.
- LassenE.D. 2004. Laboratory evaluation of plasma and serum proteins. In: ThrallM.A. (ed.) Veterinary hematology and clinical chemistry. Lippincott Williams & Wilkins, Philadelphia, PA, USA, pp 401-415.
- LomborgS.R. NielsenL.R. HeegaardP.M.H. JacobsenS. 2008. Acute phase proteins in cattle after exposure to complex stress. Vet. Res. Commun. 32:575-582.
- MohriM. SharifiK. EidiS. 2007. Hematology and serum biochemistry of Holstein dairy calves: age related changes and comparison with blood composition in adults. Res. Vet. Sci. 83:30-39.
- OsorioJ.S. TrevisiE. BallouM.A. BertoniG. DrackleyJ.K. LoorJ.J. 2013. Effect of the level of maternal energy intake prepartum on immunometabolic markers, polymorphonuclear leukocyte function, and neutrophil gene network expression in neonatal Holstein heifer calves. J. Dairy Sci. 96:3573-3587.
- PaltrinieriS. GiordanoA. VillaniM. ManfrinM. PanzaniS. VeronesiM.C. 2008. Influence of age and foaling on plasma protein electrophoresis and serum amyloid A and their possible role as markers of equine neonatal septicaemia. Vet. J. 176:393-396.
- PiccioneG. CasellaS. GiannettoC. VazzanaI. NiuttaP.P. GiudiceE. 2009. Influence of age on profile of serum proteins in the calf. Acta Vet.-Beograd 85:413-422.
- PiccioneG. ScianóS. MessinaV. CasellaS. ZumboA. 2011. Changes in serum total proteins, protein fractions and albumin-globulin ratio during neonatal period in goat kids and their mothers after parturition. Ann. Anim. Sci. 11:251-260.
- SzewczukM. Czerniawska-PiątkowskaE. PalewskiS. 2011. The effect of colostral supplement on the serum protein fractions, health status and growth of calves. Arch. Tierzucht 54:115-126.
- ThomasJ.S. 2000. Overview of plasma proteins and protein electrophoresis. In:FeldmanB.F. ZinkiJ.G. JainN.C. (eds.) Schalm’s veterinary haematology. 5th ed. Lippincott Williams & Wilkins, New York, NY, USA, pp 891-904.
- TizardI.R. 2009. Veterinary immunology: an introduction. 8th ed. Saunders Elsevier, St. Louis, MO, USA.
- VadenS.L. KnollJ.S. SmithS.W.K., TilleyL.P. 2009. Protein electrophoresis. In: S.L. VadenJ.S. KnollS.W. SmithK. TilleyL.P. ( eds.) Blackwell’s five minute veterinary consult: laboratory tests and diagnostic procedures. Wiley-Blackwell Ames, IA, USA, pp 501-503.
- WernerL.L. ReavillD.R. 1999. The diagnostic utility of serum protein electrophoresis. Vet. Clin. N. Am.-Exot. Anim. Pract. 2:651-662.
- WhicherJ.T. CalvinJ. RichesP. WarrenC. 1987. The laboratory investigation of paraproteinaemia. Ann. Clin. Biochem. 24:119-132.
