Abstract
The present study was conducted to investigate the effects of the stems and leaves in ultrafined powder of Astragalus (SLASUP) on immune functions of chickens, and to provide a basis for clinical application of the stems and leaves of Astragalus. Two hundred and fifty Hyaline brown chicks were assigned randomly into five groups at 1 day of age, fifty chickens each. Group I was the control group, receiving no herbs; Group II was the positive herbal Group, birds in this group being given 0.5% powder of Astragalus root in feedstuff (0.5% ARP Group); Group III was the 0.5% SLASUP Group; Group IV was the 1% SLASUP group; and Group V was the 1.5% SLASUP Group. Chickens in the three SLASUP Groups were given SLASUP powder in the feedstuff. All birds in the five groups were immunised on day 7 and 21 with Newcastle disease (ND) vaccine. The blood samples on days 21, 28, 35, 42 and 49 were collected to determinethe ND virus (NDV) antibody titers, the contents of interleukin-2 (IL-2) and the immunoreactive fibronectin (IFN-ᵧ) in the sera of 21, 35, 49-day-old chicken. The results indicate that both 1 and 1.5% SLASUP can improve the immune functions of chickens significantly by increasing the antibody titers, IL-2 and IFN-ᵧ contents. Stems and leaves in ultrafined powder of Astragalus may be clinically useful to boost the immune response in preventing infections such as ND in poultry farms.
Introduction
With the fast growing development of poultry farming industry, epidemic diseases of chickens have become more and more complex. It should be a very effective alternative to prevent and treat various kinds of diseases in chickens with herbal medicines (Wei, Citation2009). Chinese herbal medicine has been used for thousands of years in China. It is derived from natural sources such as plants, minerals and animals. The herbals contain polysaccharides, alkaloids, glycosides, etc., and have both nutritional and medicinal properties. Fructus Ligustri Lucidi, when supplemented to the ration, could increase heifers average daily gain (ADG), final body weight and feed efficiency, increase concentration of superoxide dismutase (SOD) and reduce concentration of malondialdehyde (MDA) to improve the blood antioxidant function, and reduce concentrations of prostaglandin E2 (PGE2) and immunoreactive fibronectin (IFN-ᵧ) to improve immune function (Qiao et al., Citation2013). Mannan oligosaccharide and an essential oil blend could enhance performance and also increase eggshell weight as free radical scavengers (Bozkurt et al., Citation2012). The ecofriendly and non-hazardous nature of Chinese herbal medicines for animals, absence of residual effects, minimum problems of drug resistance and absence of side effects further instill the interest in herbal medications. However, as the current price of Chinese herbal medicines increasing from time to time and the limited natural sources of available herbs, the competition for humans and animals to consume the same kind of herb and the increasing cost of herbs have generated the need to explore new herbal sources or unused parts of the traditionally used herbs. The herbal Astragalus (Astragalus membranaceus) is the dried root of leguminous plants [Astragalus membranaceus (Fisch.) Bge or Astragalus membranaceus (Fisch.) Bge.Var. mongholicus (Bge.) Hsiao]. Astragalus has several functions such as replenishing Qi and raising Yang (both terms are somewhat equivalent in meaning to immune function) to strengthen spleen, tonifying defensive Qi (defensive function), stabilising the superficial (skin defensive function), and stopping sweating, clearing toxins and abscess pus, promoting wounds healing, and circulating body fluids to reduce edema (Liu and Xu, Citation2002). Astragalus, when used with the other herbs in the formula Huangqi Maxingshigan decoction, could up-regulate IFN-ᵧ and down-regulate IL-4 levels, enhance cell mediated immunity and the production of sIgA (Cheng et al., Citation2011). In some researches, chicks treated with different levels of Astragalus membranaceus root powder in diet showed significant increases in absolute immune organ weights, serum total protein, albumin, A/G ratio and immunoglobulin type G compared to untreated one (Abdelrafea et al., 2013). Zhang et al. (Citation2013) reported that dietary supplementation of Astragalus root powder (ARP) at the concentration of 5 g/kg of diet enhanced serum antioxidant status in broilers. Astragalus tinctures could stimulate immune cells as quantified by CD69 expression on CD4 and CD8 T cells (Brush et al., Citation2006). Astragalus membranaceus extract may increase the release of immune response mediator and cell migration via hypothalamus-pituitary-adrenal (HPA) axis to activate immune response in macrophages (Qin et al., Citation2012), stimulate the number of the potentially beneficial bacteria (bifidobacteria and lactobacilli), while reducing the number of the potentially harmful bacteria (Bacteroides spp. and Escherichia coli) (Guo et al., Citation2004), and enhance the antibody response to a T-dependent antigen injected into normal mice or mice immunodepressed by cyclophosphamide or radiation treatment or by aging (Zhao et al., Citation1990). Lots of studies have shown that Astragalus polysaccharides (APS), a kind of natural effective component of Astragalus, can enhance the immunological functions, increase the number of leukocytes and lymphocytes, improve the transformation rate of lymphocytes and the activity of macrophages, induce the interferon production, strengthen the immunity effect of many kinds of vaccines (Jiang et al., Citation2011; Yuan and Chen., Citation2000; Wang et al., Citation2006; Zhao et al., Citation1993; Wang et al., Citation2011); and some herbal compound polysaccharides including APS have the best effects in lymphocyte proliferation and raising the antibody titers (Guo et al., Citation2012; Kong et al., Citation2004). According to some researches, Astragaloside IV and flavonoids in Astragalus can also improve immunity (Lin et al., Citation2011; Jiao et al., Citation1999). Recent studies have indicated that these important effective components still exist in the stems and leaves of Astragalus (Zhang, Citation2010; Zhu and Jiang, Citation2004). All these reports indicate that there is really a need to explore the medicinal values of the stems and leaves of Astragalus in addition to the traditional use of the root part.
Several reports claim that the stems and leaves of Astragalus have been proved to possess the same properties as the root part in mice (Jiao et al., Citation1999; Zhang, Citation2010). The stems and leaves of Astragalus, if pulverised to superfine powder, are easier to be extracted, releasing higher concentrations of APS (Liu et al., Citation2006; Dong et al., Citation2008; Li et al., Citation2008) and exert the immunoenhancing functions in chickens (Chen et al., Citation2009). In the present study, the immunoenhancing effects of the stems and leaves of Astragalus in ultrafine powder (SLASUP) were investigated in the chickens by measuring the antibody titers, the contents of IFN-ᵧ and interleukin-2 (IL-2) after immunised with Newcastle disease (ND) vaccine, with positive results. This study can be used as a reference for future studies to develop effective Chinese herbal medicine immune-enhancers. Stems and leaves of Astragalus in ultrafine powder may be clinically useful to boost the immune response in preventing infections such as ND in poultry farms.
Materials and methods
Preparation of Chinese herbal medicine
Astragalus (root) was purchased from the herbal market of Anguo in Baoding and authenticated. The root was processed to common powder as usual (ARP). The stems and leaves of Astragalus were processed to ultrafine powder (SLASUP).
Animals and treatments
Two hundred and fifty one-day-old Hyaline brown chickens were purchased from a chicken farm in Xushui county, China. All chickens were fed with normal feedstuff in the first week for acclimatisation, and then divided randomly into five groups, with fifty chickens in each group. Group I was the blank control Group. Birds in Group I were fed with the common basal diet containing no herbals. Group I was the positive herbal control, with 0.5% ARP added to the diet. Birds in Group III were given 0.5% SLASUP to their diet, Group IV received 1% SLASUP in their diet, and, Group V received 1.5% SLASUP. All the birds of the five groups were immunised with ND and infectious broncheitis divalent vaccine (LaSota +H120 strain) at 7 days of age by nasal and eye drops. Then, at day 21, the chickens were given the second immunisation of ND and infectious broncheitis divalent vaccine (LaSota +H52 strain) by nasal and eye drops. At the same day, they were injected with ND oil vaccine intramuscularly. All the chickens were kept in the same comfortable environment with proper temperature, humidity and light cycles. The animal care protocol in this experiment was approved by the Animal Welfare Committee of Agricultural University of Hebei, Baoding, China.
Reagents
Newcastle disease virus (NDV) hemagglutination test antigen was obtained from China Institute of Veterinary Drugs Control, Beijing, China. Chicken IL-2 and IFN-ᵧ ELISA Kits were obtained from Research and Diagnostic Systems Inc. (Minneapolis, MN, USA).
Assay of Newcastle disease virus antibody titers in serum
The blood samples of eight chickens at 21, 28, 35, 42, 49 days of age were collected randomly from each group, and allowed to clot at 37°C for 30 min. The samples were seperated by centrifugation at 3000×g for 10 min, and the sera stored at 4°C. The NDV antibody titers were measured using the red blood cell agglutination test and the red blood cell agglutination inhibition (HI) test.
Measurements of the interleukin-2 and immunoreactive fibronectin contents
The blood samples of eight chickens at the ages of 21, 35, 49 days were collected randomly from each group, and allowed to clot at 37°C for 30 min. The sera were separated by centrifugation at 3000×g for 10 min, and stored at 4°C. The contents of IL-2 and IFN-ᵧ were measured with the Chicken IL-2 and Chicken IFN-ᵧ ELISA Kits, respectively. Measurements were conducted according to the manufacturer’s instructions.
Read the optical density (OD) at 450 nm using a standard microplate reader. The standard curve was generated by pooling the average OD (450 nm) obtained from each of the six standard concentrations on the vertical (Y) axis vs the corresponding concentration on the horizontal (X) axis. The amount in an unknown sample was calculated according to the standard curve.
Statistical analysis
Data were expressed as mean±standard deviation. Data were analysed with software of SPSS 17.0, and by one-way variance (ANOVA) test and log rank test. P values below 0.05 or 0.01 were considered significant, or very significant.
Results and discussion
The dynamic changes of the Newcastle disease virus antibody titers
There were significant enhancements in NDV antibody titers of chickens in the four herbal groups (II-V) compared with that of the none-herbal group (Group I) at different time points post-vaccination. The NDV antibody titers of chickens in Group II (0.5% ARP Group) on day 35 were significantly higher than that in the none herb control Group (Group I) (P<.05). The average antibody titers of the chickens in Group III (0.5% SLASUP Group) on day 28 were markedly higher than that in Group I (P<0.01), while the NDV antibody titers of chickens in Group IV (1% SLASUP Group) on days 35 and 42 were significantly higher than Group I (P<.05). However the NDV antibody titers of the chickens in Group V (1.5% SLASUP Group) were higher than the control Group (Group I) on days 28, 35, 42 and 49 (P<0.01). When compared with the 0.5% ARP Group, there was significant elevation in Group V (1.5% SLASUP Group) at day 28 (P<0.01) and day 49 (P<.05). Dynamic changes of NDV antibody titers were listed in .
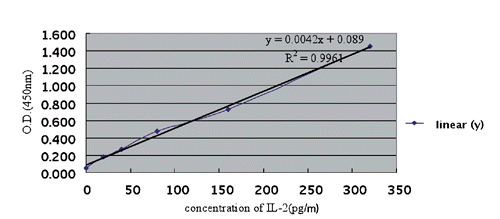
The standard curve of interleukin-2
The standard curve of IL-2 was generated by pooling the average OD (450nm) obtained from each of the six standard concentrations on the vertical (Y) axis vs the corresponding concentration on the horizontal (X) axis (). Regressive equation: y=0.0042x+0.089; R=0.9980
The contents of the interleukin-2 in serum
The IL-2 contents of chickens in five different groups were evaluated on days 21, 35, 49. It showed that the IL-2 contents in serum of chickens decreased as the chicken’s age grew. The IL-2 contents of chickens in Group II (the 5% ARP Group) on day 21 were signicantly higher than that in Group I (the none herbal control) and the 0.5% SLASUP Group (P<.05). The IL-2 contents of the chickens in Group V (1.5% SLASUP Group) and II (the 5% ARP Group) on day 35 were significantly higher than that in Group I (P<.05), while the IL-2 contents of the chickens in Group V and III on day 49 were significantly higher than that in Group I, P<.01 ().
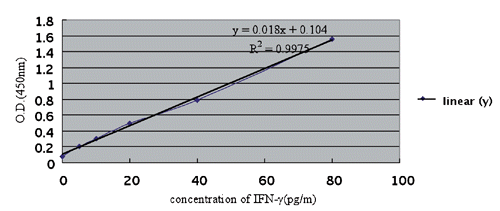
The standard curve of immunoreactive fibronectin
The standard curve of IFN-ᵧ is generated by pooling the average OD (450nm) obtained from each of the six standard concentrations on the vertical (Y) axis vs the corresponding concentration on the horizontal (X) axis (). Regressive equation: y=0.018x+0.104; R=0.9987.
The contents of immunoreactive fibronectin
The IFN-ᵧ contents of the chickens decreased as the chicken’s age grew in all the five groups. The values changed from 9.63±0.61 at day 21 to 6.36±0.33 at day 35, to 3.66±0.34 at day 49 in Group I. There were the same trends in the other four groups. There were no significant differences between the 0.5% ARP Group (Group II) and the control Group. For details please see . When comparison was made between the SLASUP Groups and the control Group I, we found that only Group IV (1.0% SLASUP Group) showed higher IFN-ᵧ contents on day 35 and day 49 in comparison to the none herbal control Group (I) or the positive herbal control (Group II, 0.5% ARP Group), P<.01 ().
General remarks
Immunopotentiators are a kind of substances enhancing the body’s immune responses in a nonspecific way used separately or with other antigens. They can improve the body’s immune functions, attenuate the immunologic disorders caused by environmental stresses, and help to prevent and treat infectious and conditional diseases (Cui et al., Citation2001). Humoral immunity is such a type of immune mechanism that protects itself by means of producing antibodies by B cells. Therefore, the antibody level in serum is one of the main indicators to assess the immunologic status. APS is mainly a kind of natural and effective components of the herb Astralagus. In recent years, several researchers have done extensive studies on enhancing the body’s immune functions with herbals and the effects of APS on the body’s active immunisation as an immunopotentiator. The results showed that APS increased the NDV antibody titers (Chen et al., Citation2009; Wang et al., Citation2007; Zhao et al., Citation2004; Li et al., Citation2004; Si and Min, Citation2008). In our present study, SLASUP enhanced the NDV antibody levels of chickens. The chickens in 1.5% SLASUP Group showed the highest antibody titers than the other SLASUP groups, and especially than the 0.5% ARP Group (). Our results are somewhat in agreement with Chen and his colleague’s whose research indicates the ultrafine powder shows better immunoenhancing effect than ordinary powder of Astragalus in chickens (Chen et al., Citation2009). These results indicate the SLASUP has the potential for clinical use as an immunopotentiator.
Interleukin is a lymphokine that controls some aspects of the immune response by conveying signals between white blood cells and lymphocytes. Both lymphokine and haemocyte growth factors are cytokines, and they work together on hematopoiesis and immunomodulation through intercoordination and interaction. Interleukins play a very important role in information transmission, activation and regulation of immunocytes, mediating the activation of T and B cells, proliferation and differentiation of T cells and B cells and Inflammatory responses. Different interleukins are designated by number. For example, interleukin-2 (IL-2) stimulates the proliferation of T cells and the synthesis of other T cell-derived cytokines, induces the growth and cytolytic functions of NK cells to produce lymphokine-activated killer cells, activates phagocytes. It is a growth factor and stimulates antibody synthesis in B cells, and may promote apoptosis in antigen-activated T cells. It is used pharmaceutically as an anti-tumor drug. Shao et al. (Citation1994) reported that 5% Astragalus injection could enhance proliferation of T cells and production of IL-2 stimulated by Con A in mice. Other studies showed that the IL-2 contents in mice treated with Astragalus decoction were significantly higher than that in the control Group (Huang and Liu, Citation2008; Zhang et al., Citation2000). Xu et al. (Citation1997) also found that Astragalus was able to improve the IL-2 level significantly in mice. Our results are in accordance with all of the research results mentioned above in that there was a significant difference between the SLASUP and the control Group ().
Interferons are a family of naturally-occurring proteins which are a kind of antiviral cytokines having pleiotropic regulatory effects on immune system, and secreted by cells of the immune system (for example, white blood cells, natural killer cells, fibroblasts, and epithelial cells). Interferons play an important role in the first line of defensing against viral infections. They are parts of the non-specific immune system and will induce at an early stage in viral infection-before the specific immune system responds. Three classes of interferons have been identified: α, β, and ᵧ. IFN-ᵧ (immune interferon) belongs to Type II interferon, it is the major interferon produced by lymphocyte cultures that is immunologically stimulated by mitogens or antigens. The major producer cells are T lymphocytes, and its major activity is immunoregulation. The biological effects of IFN-ᵧ are widespread, as almost every cell type is altered upon interaction with this protein. Thus it has become very apparent that IFN-ᵧ is a multipotent cytokine whose regulation and effects are complex and essential to host survival (Young and Bream, Citation2007). The development of new generation vaccines has focused on the use of natural immunologic adjuvants that are capable of enhancing a protective immune response (Lowenthal et al., Citation1998). One study showed that the dosage of 200 mg/kg APS could significantly enhance the IFN-ᵧ level of the immunosuppressive dogs (Qiu et al., Citation2010). Two saponins of Astragalus had positive effect on Th1 cytokine release (IL-2 and IFN-ᵧ), and created powerful immunoregulatory effects without the stimulation of inflammatory cytokines in mice (Ay e et al., Citation2011). Our present research indicates the IFN-ᵧ contents of chickens in the 1% SLASUP Group is significantly higher than the non-herbal control or the ARP Group.
Our present study has demonstrated that SLASUP can improve the immune function of chickens, and a certain dosage of them has even stronger effect than 0.5% ARP. The results of our study are similar to those of several researches on SLASUP. Wang (Citation2008) compared the different doses of ultrafine powder and the ordinary powder of the same herbal formula named Huang Huo San (Astragalus and Epimedium) on the influence of the Avian Influenza AIV (H) antibody titers. He found that the 0.5% ultrafine powder Group showed stronger elevating effect than 1.0 and 1.5% ordinary powder Groups. According to the report by Cheng (Citation2011), the ultrafine powder of Astragalus could significantly increase the weights of immune organs of immunosuppressed mice, prolong the swimming time of mice, and the lower-dose could achieve the same effect as the decoction of Astragalus. Chen et al. (Citation2009) studied the effects of superfine Astragalus particles and crude Astragalus particles on the chicken’s immune functions by measuring the indexes of immune organs and the chicken’s immunity to ND. The results indicated that compared to the crude Astragalus particles, the superfine Astragalus particles have advantages in increasing the indexes of bursa of Fabricius and thymus gland and the average NDV antibody titers in serum. In our present study, the stem and leaf of Astragalus in superfine powder have the same effect as or even better effect than the powder of radix astragali. This might be due to that after the Chinese herbal medicines were superfinely pulverised, the particle surface area of the herbals increased, as a result, the releasing quantity and rate of effective components increased too (Hou, Citation2001).
Conclusions
The SLASUP enhanced the immunological functions of chickens including the NDV antibody titers, the contents of IFN-ᵧ and IL-2. This study can be used as a reference for future studies to develop effective Chinese herbal medicine immune-enhancers. The SLASUP may be clinically used as an adjuvant in boosting immune responses in poultry farms.
Acknowledgments
This study was financially supported by the Ministry of Science and Technology of China, Beijing, China (No. 2011BAD34B02).
Table 1. The Newcastle disease virus antibody titers in different experiment groups (log2).
Table 2. Effect of Astragalus on interleukin-2 (pg/mL).
Table 3. Changes of immunoreactive fibronectin in different groups (pg/mL).
References
- AyşeN. TunaN. ÖzlemY. GüzideA. ShabanaK. ErdalB., 2011. Evaluation of the immunomodulatory properties in mice and in vitro anti-IFNlammatory activity of cycloartane type saponins from Astragalus species. J. Ethnopharmacol. 139 :574-581.
- BozkurtM. TokuşoğluO. Küçükyılmaz K. AkşitH. Çabuk M. Uğur Çatlı A. SeyrekK. Çınar, M., 2012. Effects of dietary mannan oligosaccharide and herbal essential oil blend supplementation on performance and oxidative stability of eggs and liver in laying hens. Ital. J. Anim. Sci. 11 :223-229.
- BrushJ. MendenhallE. GuggenheimA. ChanT. ConnellyE. SoumyanathA. BureshR. BarrettR. ZwickeyH., 2006. The effect of Echinacea purpurea, Astragalus membranaceus and Glycyrrhiza glabra on CD69 expression and immune cell activation in humans. Phytother. Res. 20 :687-695.
- Chen H.J. Tang Z.W. Chen X.B., 2009. Effects of super fine Astragalus and crude Astragalus particles on the immune function of chickens. J. Anhui Agric. Sci. 4 :1549-1550.
- ChengH., 2011. A study of Astragalus ultrafine on enhancing immune function and anti-fatigue. Clinical Journal of Chinese Medicine 24 :30-31.
- Cheng J.J. Li Q.Y. Shi W.Y. Zhong X.H., 2011. Effects of Huangqi Maxingshigan decoction on infectious laryngotracheitis in chickens. Ital. J. Anim. Sci. 10 :124-130.
- Cui B.A. Zhang S.M. Yang M.F. Wang X.B. QiuY. WangL., 2001. Comparing Astragalus and levamisole on immune functions of chickens. Veterinary Pharmaceuticals and Feed Additives 6 :1-2.
- Dong Y.L. HaoL. Zhou P.P., 2008. The effect of ultramicro-pulverization on the Astraglus polysaccharides dissolubility. J. Anhui Agric. Sci. 9 :3742-3744.
- El-Shafei A.A. Al-Gamal M.A. Abdelrahman A.S. Arafa M.M., 2013. Influence of different levels of astragalus root powder in broiler chick diets on the physiological and biochemical changes. J. Appl. Sci. Res. 9 :2104-2118.
- Guo F.C. Williams B.A. Kwakkel R.P. Li H.S. Li X.P. Luo J.Y. Li W.K. Verstegen M.W.A., 2004. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poultry Sci. 83 :175-182.
- GuoL. WangD. HuY. ZhaoX. WangY. YangS. WangJ. FanY. HanG. GaoH., 2012. Adjuvanticity of compound polysaccharides on chickens against Newcastle disease and avian influenza vaccine. Int. J. Biol. Macromol. 50 :512-517.
- Hou L.B., 2001. The application of cellular level ultramicro-pulverization in Chinese drugs pharmaceutics. Journal of Chinese Herbal Medicine 10 :765-766.
- Huang H.P. Liu C.P., 2008. Experimental study of Astragalus membranaceus on immunological function of mice. Nei Mongol Journal of Traditional Chinese Medicine 3 :53-54.
- Jiang R.X. ZhaoX. SunY. SongB. Xia M.L., 2011. Effect of Astragalus polysaccharides on immunological function in mice. Journal of Qiqihar University of Medicine 4 :510-511.
- JiaoY. WenJ. Yu X.H. Zhang D.S., 1999. Influence of flavonoid of Astragalus embranaceus’s stems and leaves on the function of cell mediated immunity in mice. Chinese Journal of Integrated Traditional and Western Medicine 6 :356-358.
- KongX. HuY. RuiR. WangD. LiX., 2004. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int. Immunopharmacol. 4 :975982.
- LiN. She R.P. Han L.J. Wang K.Z., 2004. Effect of Astragalus root extractions on chicken growth and immunological function. Chinese Journal of Veterinary Science and Technology 5 :61-64.
- LiY. Yang Y.H. Cai G.X., 2008. The effect of ultramicro-pulverization on the extraction efficiency of main chemical compositions in Astragalus membranaceus. Chinese Traditional Patent Medicine 2 :229-231.
- LinL. ShenH. Wang L.X. Zhou X.B. WuJ. XuY. ZhaoS. Liu Z.W. GeC. Liu Y.J., 2011. Effects of Astragaloside IV and β-elemene on immunological function of dentritic cells in rats. J. Southeast Univ. 2 :294-297.
- LiuY. Shi W.Y. YangM. Che S.F. Zhong X.H., 2006. Influence of ultrafine comminution technology on dissolving of polysaccharide from both Astragalus membranaceus (Fisch.) Bunge and Epimedium brevicornum Maxim. Journal of Traditional Chinese Veterinary Medicine 1 :14-16.
- Liu Z.J. Xu J.Q., 2002. Traditional Chinese veterinary medicine. Chinese Agricultural Press, Beijing, China.
- Lowenthal J.W. O’Neil T.E. BroadwayM. Strom A.D. Digby M.R. AndrewM. York J.J., 1998. Coadministration of IFN-gamma enhances antibody responses in chickens. J. Interferon. Res. 8 :617-622.
- Qiao G.H. Shao C.M. ShaoT. YangX. Zhu X.Q. Li J.H. LuY., 2013. Effects of supplemental Chinese herbs on growth performance, blood antioxidant function and immunity status in Holstein dairy heifers fed high fibre diet. Ital. J. Anim. Sci. 12 :121-126.
- Qin Q.J. Niu J.Y. Wang Z.X. Xu W.J. Qiao Z.D. GuY., 2012. Astragalus membranaceus extract activates immune response in macrophages via heparanase. Molecules 17 :7232-7240.
- Qiu H.H. Cheng G.L. Xu J.Q. Zhang N.W. Liu F.H. Zhu X.Y. ZhaoJ. Zhang Y.J., 2010. Effects of Astragalus polysaccharides on associated immune cells and cytokines in immunosuppressive dogs. Procedia Vaccinol. 1 :26-33.
- Shao Q.X. Xu F.H. XiaS. Liu G.Z., 1994. The regulating effect of Astragalus membranaceus on immunological function of mice. Journal of Zhenjiang Traditional Chinese Medical College 4 :261-264.
- Si C.D. Min Y.H., 2008. Effect of Astragalus polysaccharide on immunity of broilers. Chin. J. Prev. Vet. Med. 12 :978-980.
- Wang D.Y. HuY. L. Sun J.L. Zhang B.K. Liu J.G., 2006. Effect of Chinese herbal medicinal ingredient prescription on peripheral T lymphocyte transformation and serum antibody titers of vhickens inoculated with ND vaccine. Chin. J. Vet. Sci. 2 :194-196.
- Wang F.H. Zhang J.L. Liu G.X., 2011. Immune enhancement of Astragalus and angelica polysaccharide on ND vaccine immunity. Journal of Animal Science and Veterinary Medicine 4 :1-6.
- Wang X.B. Chen G.Y. Wei Z.Y. Ren X.Y. Cui B.A., 2007. Comparison on effect of Astragalus polysacharide powders and injects on immune function and growth of chickens. China Poultry 3 :21-23.
- WangZ., 2008. The effect of Huanghuosan ultrafine powder on avian influenza (H9) of chickens. Poultry Husbandry and Disease Control 2 :5-6.
- Wei X.B., 2009. The application of Chinese herbal medicines in poultry farming. China Poultry 18 :32-33.
- Xu Z.G. Zhang A.H. Guo H.L. Liu X.L., 1997. Effect of Astragalus on NK cell activity and level of TNF-α and IL-2 in mice. Journal of Yunyang Medical College 3 :124-126.
- Young H.A. Bream J.H., 2007. IFN-gamma: recent advances in understanding regulation of expression, biological functions and clinical applications. Curr. Top. Microbiol. Immunol. 316 :97-117.
- Yuan H.X. Chen Y.C., 2000. The modern pharmacological research and clinical application of Astragalus. Journal of Shandong University of Traditional Chinese Medicine 5 :396-400.
- Zhang G.G. Yang Z.B. WangY. Yang W.R., 2013. Effects of Astragalus membranaceus root processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poultry Sci. 92 :178-183.
- Zhang G.Q., 2010. Investigation of active ingredients from roots, stem and leaves of Astragalus mongpholicus Bunge. Tianjin Pharmacy 5 :5-7.
- Zhang J.X. Qiu S.C. Li B.Q. Liu J.R. Peng Q.H., 2000. Effect of Astragalus membranaceus (AM) on immune function of mice. Li Shi Zhen Medicine and Materia Medica Research 6 :488-489.
- Zhao K.S. Ding L.X. Kong H.Y. Tian S.Y. Jin B.Q. Liu X.S., 1993. Effect of Astragalus on secretion of tumor necrosis factors in human peripheral blood mononuclear cells. Chinese Journal of Integrated Traditional and Western Medicine 13 :263-265.
- Zhao K.S. ManciniC. DoriaG., 1990. Enhancement of the immune response in mice by Astragalus membranaceusextracts. Immunopharmacology 20 :225-233.
- Zhao Y.L. Li G.X. Li H.Q., 2004. Influence of Astragalus on immune performance of Gushi chicken. Journal of Henan Agricultural Sciences 11 :90-91.
- Zhu Z.L. Jiang W.X., 2004. Study on the effective components in the overground organ of Astragalus membranaceus. Heilongjiang Medical Journal 6 :417-419.