Abstract
The objective of this study was to evaluate the chemical composition, in vitro digestibility, gas production and fermentation kinetics of three Brachiaria cultivars: Brachiaria brizantha cv. Marandu, Brachiaria brizantha cv. Xaraes, and hybrid Brachiaria cv. Mulato, subjected to different levels of forage allowance (4, 7, 10 and 13% of the animal body weight), under rotational grazing cycles. Cultivar Xaraes presented higher contents of neutral detergent fibre, neutral detergent fibre corrected for ash and protein, acid detergent fibre and lignin, and lower production of gases in 96 h in the component leaf blade. There was a decrease of non-fibrous carbohydrates and fraction B2 and an increase in the fraction C of the carbohydrates in the components stem and leaf blade over the grazing cycles in all the cultivars. Cultivar Marandu presented higher digestibility values (in vitro organic matter digestibility and in vitro dry matter digestibility) in the components stem and leaf blade. Cultivar Mulato demanded a shorter time of colonisation, according to the calculations of fermentation kinetics. Regardless of the level of forage allowance and of the grazing cycles, the three Brachiaria cultivars were characterised by great fermentation quality due to the relations of the gas production potential and the gas production after 48 and 96 h.
Introduction
It is estimated that the total pasture area in Brazil is superior to 200 million ha, with more than its half comprised of cultivated grasses, and the genus Brachiaria occupies about 85% of this area (Vigna et al., Citation2011).
The genus Brachiaria spp. has around one hundred species, which have their distribution in the tropical regions of both hemispheres of the globe, mostly in Africa (De Souza-Kaneshima et al., Citation2010). The success of this grass genus is mainly due to the excellent adaptability to different production systems and climate and soil conditions; however, the inappropriate management of pastures has been the main limiting factor for raising animals to be a competitive activity facing the other agricultural activities. Therefore, the adjustment of forage allowance, which is a daily quantity of forage per 100 kg of animal body weight (BW), urges to optimise the use of pasture, enabling the maximum harvest green stuff or minimum losses by senescence. The great advantage of using the forage allowance would be to relate the plant to the animal, providing control of the supply of dry matter for each animal at the desired level, which is based on the consumption capacity as a function of BW.
The nutritional evaluation of feedstuffs for ruminants has had great importance to adequate the databases of the diet-formulation systems (Getachew et al., Citation1998). Therefore, getting to know the levels of nutrients, the rumen fermentation kinetics and the in vitro dry matter digestibility (IVDMD) is really important when evaluating a tropical forage, especially regarding to new genes of Brachiaria, such as Mulato and Xaraes.
The semiautomatic in vitro gas production technique (Maurício et al., Citation1999) is an alternative, which enables to describe the kinetics of fermentation in the rumen and to determine the rate and extent of the degradation of forages (Getachew et al., Citation1998), as well as measure products of fermentation of soluble and insoluble parts of substrates (Pell and Schofield, Citation1993).
The advantages of the utilisation of the in vitro technique in the evaluation of the nutritional value of the feedstuffs for ruminants are: fastness, physico-chemical uniformity of fermentation site and the convenience of keeping few animals fistulated, besides not being that much expensive.
According to the information presented so far, the objective of the present study was to evaluate the chemical composition, in vitro digestibility and gas production and fermentation kinetics of three Brachiaria cultivars: Brachiaria brizantha cv. Marandu, Brachiaria brizantha cv. Xaraes and hybrid Brachiaria cv. Mulato, subjected to levels of forage allowance (4, 7, 10 and 13% of the animal BW), under rotational grazing.
Materials and methods
The experiment was realised in the Forage and Pasture sector of the Department of Animal Science, Faculty of Agrarian and Veterinarian Sciences of Paulista State University (UNESP), Jaboticabal, SP, Brazil, located 21°15’22”S, 48°18’58”W and at a height of 595 m asl. According to Köppen classification, the predominant climate in Jaboticabal is Awa type, described as tropical of winter drought. The period of data collection and sampling in the field was from November 2008 to February 2009. The soil at the experimental area was characterised as an Oxisol (USDA, Citation1999).
The Brachiaria cultivars studied were Marandu [Brachiaria brizantha (Hochst. ex A. Rich.) Stapf.], Xaraes (Brachiaria brizantha) and Mulato (Brachiaria sp., hybrid of Brachiaria ruziziensis clone 44-6 and Brachiaria brizantha CIAT 6297) subjected to four forage allowances (4, 7, 10 and 13% of the animal BW).
The experiment comprised three areas, one for each Brachiaria cultivar, totalising 3397 m2. The size of the areas was defined in accordance with the forage allowances and the total area of each cultivar ( and ). The four forage allowances were allocated in the plots or experimental units with three repetitions, composed of 12 plots per cultivar, totalising 36 plots in the experiment.
Four grazing cycles (GC) were performed at fixed intervals of 21 days (1st GC: 12/11/2008; 2nd GC: 01/04/2009; 3rd GC: 01/25/2009; and 4th GC: 02/16/2009). For grazing, non-lactating cows of Holstein breed, with average BW of 450 kg, were used under rotational grazing (Allen et al., Citation2011). The distribution of animals was adjusted so as to allow the pre-determined forage allowance for each plot and cultivar.
Samples were collected in pre-grazing condition by adopting a 1 m2 at three points of the plot representing the average pasture height. The forage found within the area of the square was cut at the soil level and separated into morphological components: stem and leaf blade. The samples obtained were pre-dried in a forced circulation oven at 55°C during 72 h and then processed in a Willey-type mill with 1 mm mesh sieve.
Pre-dried samples were subjected to analyses of dry matter (DM), mineral matter (MM) and crude protein (CP) according to AOAC (Citation2005), neutral detergent fibre (NDF) and acid detergent fibre (ADF) according to Van Soest et al. (Citation1985). Total carbohydrates (TC) and non-fibrous carbohydrates (NFC) were calculated according to Sniffen et al. (Citation1992), through the formulas TC (%)=100 - [% CP +% ether extract (EE) +% ash] and non-fibrous carbohydrates NFC (%)=100 - [% CP +% EE +% neutral detergent fibre corrected for ash and protein (NDFap) +% ash]. The fraction C was obtained by multiplying the lignin (LIG) value by 2.4, and fraction B2, by the difference between NDFap and fraction C (Sniffen et al., Citation1992). In vitro dry matter digestibility was determined by the procedure of Tilley and Terry (Citation1963), with two incubation stages of 48 h each, and the in vitro digestibility of DM and NDF (IVDMD and IVNDFD) were determined by the treatment with neutral detergent solution. In the first stage, 0.2 g of dried and ground samples were weighed and placed in glass bottles of 100 mL capacity and 40 mL of buffer solution were added according to McDougal (Citation1949) and 10 mL rumen fluid obtained from three crossbred heifers with average live weight of 337 kg and approximately 24 months of age, cannulated in the rumen and duodenum, kept on Brachiaria brizantha cv. Marandu grass, were added. A day before the incubation, samples were weighed, placed in bottles and kept in oven at 39°C, and the growth medium and respective solution were also prepared, under continuous flow of CO2 and kept in bain-marie at 39°C, according to Theodorou et al. (Citation1994).
On the incubation day, the inoculation in the glass bottles was performed with constant bubbling of CO2, and bottles were conditioned in bain-marie at 39°C for 48 h. The bottles were agitated during the first stage at 2, 4, 6, 8, 12, 16, 24 and 36 h after incubation. After the first stage, 2 mL of HCl at 6 N [concentrated HCl 12 N, with equal amount of distilled water (1:1)], and 6 mL of pepsin at 5% (50 g/L) were added, which were incubated again for 48 more h at 39°C, repeating the process of agitation described in the first stage. After digestion with pepsin, the content of the tubes was transferred to crucibles previously weighed. The tubes were washed with warm distilled water for the total recovery of the remaining particles. After filtration, crucibles were taken to oven at 105°C for 12 h, cooled in a desiccator weighed, and finally taken to muffle furnace at 550°C for 3 h.
In vitro gas production was evaluated considering the analysis protocol described by Theodorou et al. (Citation1994) modified by Mauricio et al. (Citation1999), considering the volume of gases produced from the measurement of the pressure generated by the accumulation of gases in the fermentation process of the incubated samples, by utilising a pressure gauge (digital pressure gauge Pressure Meter Delta OHM-HD 2124.1; Delta Controls Ltd., Molesey, UK), for the adjustment of the equation of production of volume of gases.
Glass bottles with final volume of 50 mL (rumen inoculum + growth medium) were incubated in water bath at 39°C. The pressures generated by the gases produced were measured with a digital pressure gauge after 2, 4, 6, 8, 10, 12, 24, 26, 28, 30, 32, 36, 48, 52, 56, 60, 72, 76, 80, 84, 96, 100, 104, 108, 120, 124, 128, 132 and 144 h of fermentation. For the adjustments of variation, bottles considered blank were incubated, containing the solutions of incubation without substrate and an internal pattern (Tifton 85 grass hay) with known gas production profile. To predict in vitro organic matter digestibility (IVOMD) and IVDMD by the technique of in vitro gas production of the incubated samples, the equations recommended by Menke and Steingass (Citation1988) were utilised:
DMD=14.88 + (0.889*gases 24) + (0.045*CP) + (0.065*MM)
where, gases 24 is in vitro production of gases in 24 h of fermentation (mL/0.2 g DM), and CP and MM values are expressed in g/kg of DM and g/kg OM, respectively. The same equation described previously, but with in vitro production of gases taken in 48 h (gases 48 h) and 72 h (gases 72 h) of fermentation, was calculated.
The microbial fermentation patterns were estimated in the fermentation periods of 2, 4, 6, 8, 10, 12, 24, 26, 28, 30, 32, 36, 48, 52, 60, 72, 76, 80, 84 and 96 h, according to the model of France et al. (Citation1993), based on the accumulated average production of gases of each sample in a certain period of fermentation:
In which A is the accumulated volume of gases produced up to time t;Af is the asymptotic volume of the gases produced; b and c are parameters of the model; and t represents a discrete time of colonisation.
The model of France et al. (Citation1993) was adjusted to the data of gas production to estimate the duration of colonisation and the potential gas production [asymptote of gas production of the model (A)]. The non-linear procedure of SAS (Citation2002) was used to adjust the model to the data. The cumulative gas production at 48 and 96 h after incubation was used to calculate the ratios between the cumulative gas production 96 h after incubation and gas production potential (REL 1) and the ratios between the cumulative gas production after 48 and 96 h of incubation (REL 2).
The experimental design was subdivided into plots through the time (grazing cycles or hour after incubation), having a factorial of 3x4 (three Brachiaria cultivars and four forage allowances) and four grazing cycles as repeated measures in the plots.
Yijkl=value observed in the plot that received Brachiaria i, forage allowance j, repetition k and time l; αi=effect on Brachiaria i; βj=effect on forage allowance j; (αβ)ij=effect on the interaction between Brachiaria i and the forage allowance j; k(αβ)ij=effect on k repetition inside the interaction of Brachiaria i and forage allowance j; δl=effect of time l; (αδ)il=effect on the interaction between Brachiaria i and the time l; (βδ)jl=effect on the interaction between the forage allowance j and the time l; (αβδ)ijl=effect on the interaction among the Brachiaria i, forage allowance j and the length l; ɛijkl=randon error in the plot that received the the Brachiaria i, forage allowance j and the time l.
The data were analysed through Proc GLM and Proc Mixed of statistical package SAS (Citation2002). The results were subjected to variance analysis using the orthogonal contrast analysis for the forage allowances and grazing cycles, and Tukey test for the cultivars, at a significant level of 5%.
Results and discussion
There was no significant interaction between the cultivars and the forage allowances in the results of the bromatological analyses ().
Cultivars Marandu and Xaraes presented superior DM values in the component stem (P<0.05) to those obtained from cultivar Mulato ().
The cultivars differed from each other statistically (P<0.05) in the variables ADF in the fraction stem and NDF, NDFap and ADF in the fraction leaf blade, in which cultivar Xaraes presented higher values, cultivar Marandu showed intermediate values and cultivar Mulato lower ones, except for ADF of the component stem, in which cultivar Marandu had lower content than cultivar Mulato.
Higher NDF, NDFap and ADF values in the component leaf blade of cultivar Xaraes were already expected, since this cultivar had presented elevated elongation and final length over the grazing cycles, according to Magalhães (Citation2010).
Nave et al. (Citation2010) worked with cultivar Xaraes at the same period of the year, in which the current experiment was realised, and found cell wall contents similar to those of this study (68.8; 35.5; and 41.9) for NDF of the component leaf blade and ADF for components leaf blade and stem, respectively.
Hare et al. (Citation2009) in a trial with Brachiaria Brizantha got the same amount of ADF (37.5) for the component stem and lower for leaf (31.3) for the cultivar Marandu in this study (). Note that the above study showed lower values for CP (6.8, 10.3, 7.0, 9.8) and NDF (68.1, 59.0, 65.2, 62.4) for the components of stem and leaf blade of Marandu and Mulato compared to this one.
A cubic effect was observed in the variables DM, CP, NDF, NDFap, ADF and LIG in the component stem at the grazing cycle. In the component leaf blade, there was a linear effect on LIG, quadratic effect on DM and cubic effect on CP, NDF, NDFap and ADF.
Van Soest (Citation1994) reported that the NDF content is the most limiting factor for roughage intake; the contents of the constituents of the cell wall superior to 55 to 60% in the DM are correlated negatively with forage intake. Overall, the fibrous constituents (NDF, ADF and LIG) are negatively correlated to the digestibility (Wilson et al., Citation1983; Weiss, Citation1994).
In this study there was a decrease in protein content and an increase in LIG content in different Brachiaria cultivars with the advance of the grazing cycles. According to Jung and Allen (Citation1995), physiological age of the plant advances, consequently, an increase in the percentages of cellulose, hemicellulose and LIG, reducing the proportion of the potentially digestible nutrients (soluble carbohydrates, proteins, minerals and vitamins), which represent reduction in digestibility.
Buxton and Redfearn (Citation1997) claims that the susceptibility to the rumen degradation of the fibrous portion varies either because of the age or the level of maturation of the forage. Thus, as the plant develops, there is decrease in the protein content and increase in the fibrous content, associated with the elevation in the LIG content. Due to the covalent bonds with hemicellulose, LIG forms a barrier that prevents the enzymatic hydrolysis from the structural carbohydrates, limiting the digestion of the cell wall of the forage.
According to Magalhães (Citation2010), the decrease in the concentration of CP in the component stem over the grazing cycles is a result from the increase in the pasture height affected by the physiological stage of the plants and by the degree of maturity from the accumulation of forage, which caused competition for light and increase in the elongation of the stem.
The average values for NDFap of cultivar Xaraes were significantly higher to those obtained from Xaraes an Mulato cultivars in grazing cycles 1 and 2, and did not differ from cultivar Marandu in cycles 3 and 4 ().
The greatest NDFap contents of cultivar Xaraes can be related to the highest height of this cultivar over all the grazing cycles (Magalhães, Citation2010), demonstrating the effect of aging for non-grazed leaf blades and the highest elongation and lignification of component stem, which is necessary to support the weight of the plant, increasing the mass of this component, once it was possibly at an advanced stage of maturation and lignification, increasing the fibrous components of the cell wall, and consequently, the NDFap contents.
The average values of total carbohydrates, non-fibrous carbohydrates, fraction B2 and C of the fraction stem were not affected by cultivars, forage allowances and grazing cycles ().
Also in the leaf blade fraction, cultivar Mulato stood out for presenting the greatest values of NFC in relation to the other cultivars analysed, which can be explained by the fact that cultivar Mulato presented lower NDF content, as shown in and 3.
In components stem and leaf blade, the TC presented cubic effect on the grazing cycles (P<0.05 and P<0.01, respectively). Velásquez et al. (Citation2010), observed a linear increase in the values of TC in the cutting ages, these results are different from those obtained in the current study, which had a tendency towards the maintenance of the total carbohydrate contents in the grazing cycles. With the exception of the third grazing cycle, which had a tendency towards the reduction of TC values in the components stem and leaf blade.
Non-fibrous carbohydrates contents had cubic and quadratic effects (P<0.05) over the grazing cycles in the components stem and leaf blade, respectively. In a study with three forage species, Marandu, Tifton 85 and Tanzania under different cutting ages, Velásquez et al. (Citation2010) observed a decrease in the fraction of non-fibrous carbohydrates and an increase in the contents of LIG in the cutting ages; these results were similar to those obtained in this study, except for the fourth grazing cycle of the component stem, in which, in this period, there was a discrete increase in the NFC content in relation to the second and third grazing cycles; however, it remained with contents below the first grazing cycle.
The tendency towards the reduction of the NFC contents over the grazing cycles was due to the increase in the contents of the cell wall () throughout them, once, according to Jung and Allen (Citation1995), the variation in the amount of this fraction interferes directly in the availability of energy to the ruminant, i.e. the advance in plant age causes increase in the constituents of the cell wall, thus reducing the NFC contents, and consequently, the supply of energy of quick degradation to the rumen microorganisms.
Fraction B2 presented a linear decrease (P<0.01) over the grazing cycles in the component stem and quadratic effect (P<0.05) in the component leaf blade. According to Russel et al. (Citation1992), the fraction B2 of forages is the main source of energy for the microbial growth. Nevertheless, elevated values of this fraction, which presents a slow degradation rate, along with fraction C (indigestible), tend to affect intake negatively by the rumen fill, thus affecting the animal performance (Mertens, Citation1987).
Fraction C increased linearly in the components stem and leaf blade over the grazing cycles (P<0.01). This occurred due to the increase of LIG content with advancing age of the plant.
There was no significant difference between cultivars Marandu and Mulato in the variables IVOMD and IVDMD. Cultivar Mulato had a much better result for NDFD than cultivars Marandu and Xaraes ().
Cultivars Marandu and Mulato presented the lowest contents of NDF, ADF and LIG, which are directly correlated to digestibility.
According to the data from Velásquez et al. (Citation2010), who analysed the chemical composition of different species of tropical forages twice through the year (January to March and April to June), IVDMD of the tropical forages is at around 60%, which is similar to the average of the results obtained in the present study ().
Nave et al. (Citation2009) found IVDMD values of cultivar Xaraes in the components leaf blade (68.50%) and stem (65.2%) respectively superior and inferior to the present study (56.91% for leaf blade and 50.95 to 58.23% for stem between grazing cycles). Velásquez et al. (Citation2010), thus, in a study with marandu cultivar, from January to March, at three regrowth ages (28, 35 and 42 days), obtained superior results of IVDMD for the fraction stem (70.65; 64.39; 61.60) in the present study ( and 6).
The gas production is a result of the total fermentation of the substrate and, consequently, the disappearance of the DM. The gases arise directly from the microbial degradation of the feedstuffs, and indirectly from the buffer reaction with the acids generated as a result of the fermentation (Velásquez et al., Citation2010). It can be observed that the gas production increased throughout the fermentation period, tending to stabilise at around 144 h ().
Dry matter digestibility and OMD decreased linearly with the advance of the grazing cycles (), which can be explained by the elevation in the fibrous components (NDF, ADF and LIG), which are negatively correlated with the digestibility (Wilson et al., Citation1983).
In this study, the biggest changes that occurred in the chemical composition of the cultivars studied were a result from the maturation of the plants. The proportion of potentially digestible components tended to decrease, and the proportion of fibre increased due to the accumulation of non-grazed forage over the grazing cycles. The digestibility of the cell wall is one of the main factors to limit the performance of ruminants in tropical countries (Wattiaux et al., Citation1991) adjusted according to cultivar, forage allowance and grazing cycle, for the fractions stem and leaf blade, respectively. In the parameters of France et al. (Citation1993), which numerically describe the rumen fermentation kinetics (), there was no significant difference (P>0.05) in the maximum potential of gas production (A) and in parameter L, which estimates the time of colonisation (h) between the cultivars and the grazing cycles in the component stem. There was no statistical difference in gas production after 48 and 96 h of incubation between the cultivars. The parameter that estimates the time of colonisation (L) represents the period between the beginning of incubation and the microbial action on the sample tested. The reduction in the time of colonisation are fostered by the presence of readily fermentable substrates and by physic and chemical characteristics of the cell wall of the sample, capable of facilitating the microbial colonisation.
Castro et al. (Citation2007), in a study with cultivar Marandu at four cutting ages (28, 56, 84 and 112 days), obtained L values in the range from 1.24 up to 1.38, which is below the values obtained in here.
According to Getachew et al. (Citation2004), based on the fact that the gases produced reflect the degradation of the sample tested, the rate and the maximum potential of gas production are probably the main parameters for evaluating the quality of forages tested by the gas production technique. Thus, the most fermentable or digestible forages would be those, which present the greatest values of maximum potential associated with high gas production rate, likewise, resulting in more fermentation of the material in a shorter period of incubation.
Castro et al. (Citation2007), in a study with Brachiaria brizantha cv. Marandu at three cutting ages found A values between 230 and 232 mL/g DM, which are values below the ones found in the present study, which has a more elevated LIG content than the above study. Lignin markedly affects the extent of rumen degradation, and consequently, the fermentation of polysaccharides (Van Soest, Citation1994).
The value of REL 1 was between 0.83 and 0.98, i.e. 83 to 98% of the potential was obtained during the gas production assay, thus proving that incubation for 96 h was enough to achieve the goals proposed by in vitro gas production technique.
The fermentation quality of the feedstuff can be analysed by the relation of REL 2, for the mean retention time in the rumen, which is of 48 h, and it is expected that the greatest fermentation happens in this period and that the relation be close to 1. The lowest values of this relation were found in the third grazing cycle, and the highest ones were found in cultivars Marandu and Mulato in the fraction stem and in the first grazing cycle in the fraction leaf blade. No significant effect was observed between cultivars, forage allowance and grazing cycles in the two fractions for this relation.
Conclusions
The cultivars studied of different forage allowance presented similar chemical composition and in vitro digestibility and gas production, which indicates their use can provide the same result in the animal performance.
Acknowledgments
The authors are grateful to Sao Paulo State Research Foundation (FAPESP; São Paulo, Brazil) for funding this research.

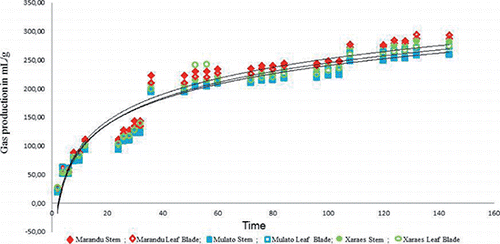
Table 1. Area per plot of each cultivar according to levels of forage allowance.
Table 2. Mean values of dry matter, crude protein, neutral detergent fibre, neutral detergent fibre corrected for ash and protein, acid detergent fibre, and lignin of the components stem and leaf blade of Brachiaria cultivars managed under grazing in rotational stocking at different forage allowances.
Table 3. Unfolding of the interaction between cultivar and grazing cycles in the neutral detergent fibre corrected for ash and protein of the component leaf blade from Brachiaria cultivars managed under grazing in rotational stocking at different forage allowances.
Table 4. Fractionation of carbohydrates of the components stem and leaf blade from Brachiaria cultivars managed under grazing in rotational stocking at different forage allowances.
Table 5. Observed means of in vitro organic matter, dry matter digestibility and neutral detergent fibre digestibility of the components stem and leaf blade of Brachiaria cultivars managed under grazing in rotational stocking at different allowances.
Table 6. Unfolding of the interaction between cultivar and grazing cycles of the in vitro dry matter and neutral detergent fibre digestibility in the component stem of Brachiaria cultivars managed under grazing in rotational stocking at different allowances.
Table 7. Means of in vitro dry and organic matter digestibility values of the components stem and leaf blade obtained through the technique of in vitro gas production of Brachiaria cultivars managed under grazing in rotational stocking at different forage allowances.
Table 8. Estimated parameters associated with the ruminal kinetics of Brachiaria cultivars managed under grazing in rotational stocking at different forage allowances.
References
- Allen V.G. BatelloC. Berretta E.J. HodgsonJ. KothmannM. LiX. MclvorJ. MilneJ. MorrisC. PeetersA. SandersonM.,2011. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 66: 2-28.
- AOAC, 2005. Official methods of analysis. 18th ed., Association of Official Analytical Chemists, Gaithersburg, MD, USA.
- Buxton D.R. Redfearn D.D.,1997. Plant limitations to fiber digestion and utilization. J. Nutr. 127: 814-818.
- Castro G.H.F. Graça D.S. Gonçalves L.C. Mauricio R.M. Rodriguez N.M. BorgesI. Tomich T.R.,2007. Cinética de degradação e fermentação ruminal da Brachiaria brizantha cv. marandu colhida em diferentes idades ao corte. Arq. Bras. Med. Vet. Zoo. 59: 1538-1544.
- De Souza-Kaneshima A.M. SimioniC. Felismino M.F. Mendes-Bonato A.B. Risso-PascottoC. PessimC. Pagliarini M.S. Do Valle C.B.,2010. Meiotic behaviour in the first interspecific hybrids between Brachiaria brizantha and Brachiaria decumbens. Plant Breeding 129: 186-191.
- FranceJ. Dhanoa M.S. Theodorou M.K. Lister S.J. Davies D.R. IsacD.,1993. Model to interpret gas accumulation profiles associated with in vitro degradation of ruminant feeds. J. Theor. Biol. 163: 99-111.
- GetachewG. BlummelM. Makkar H.P.S. BeckerK.,1998. In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim. Feed Sci. Tech. 72: 261-281.
- GetachewG. Robinson P.H. De Peters E.J. Taylor S.J.,2004. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Tech. 111: 57-71.
- Hare D.M. TatsapongP. PhengphatS.,2009. Herbage yield and quality of Brachiaria cultivars, Paspalum atrotum and Panicum maximum in north-east Thailand. Trop. Grasslands 43: 65-72.
- Jung H.G. Allen M.S.,1995. Characteristics of plant cell walls affecting intake and digestibility of forages by ruminants. J. Anim. Sci. 73: 2774-2790.
- Magalhães M.A.,2010. Características morfogênicas, estruturais e composição química de cultivares de Brachiaria sub-metidas a níveis de oferta de forragem sob pastejo rotativo. Degree Diss., Universidade Estadual Paulista, Jaboticabal, SP, Brazil.
- Mauricio R.M. Mould F.L. Dhanoa M.S. OwenE. Channa K.S. Theodorou M.K.,1999. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Tech. 79: 321-330.
- Mc Dougal E.I.,1949. Studies on ruminal saliva. The composition and output of sheep’s saliva. Biochem. J. 43: 99-109.
- Menke K.H. SteingassH.,1988. Estimation of the energetic feed value obtained from chemical analysis and gas production using rumen fluid. Anim. Res. Dev. 28: 7-55.
- Mertens D.R.,1987. Predicting intake and digestibility using mathematical models of ruminal function. J. Anim. Sci. 64: 1548-1558.
- Nave R.L.G. Pedreira C.G.S. Lima C.G.D.,2009. Canonical correlations amonschemical, physical and morphological characteristics of xaraes palisadegrass under rotational grazing. Sci. Agric. 66: 270-275.
- Nave R.L.G. Pedreira C.G.S. Pedreira B.C.,2010. Nutritive value and physical characteristics of Xaraes Palisadegrass as affected by grazing strategy. S. Afr. J. Anim. Sci. 40: 285-293.
- Pell A.N. SchofieldP.,1993. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 76: 1063-1073.
- Russel B.J. O’Connor J.D. Fox D.J. Van Soest P.J. Sniffen C.J.,1992. A net carbohydrate and protein system for evaluation cattle diets: ruminal fermentation. J. Dairy Sci. 70: 3551-3581.
- SAS,2002. SAS user’s guide: statistics. SAS Inst. Inc., Cary NC, USA.
- Sniffen C.J., O’ Connor D.J. Van Soest P.J.,1992. A net carbohydrate and protein system for evaluating cattle diets: carbohydrate and protein availability. J. Anim. Sci. 70: 3562-3577.
- Theodorou M.K. Williams B.A. Dhanoa M.S.,1994. A simple gas production method using a pressure transducer to determine the fermentation kinetic of ruminant feeds. Anim. Feed Sci. Tech. 48: 185-197.
- Tilley J.M.A. Terry R.A.,1963. A two stage technique for in vitro digestion of forages crops. Grass Forage Sci. 18: 104-111.
- USDA, 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. 2nd ed. United States Department of Agriculture, Natural Resources Conservation Service ed., Washington, DC, USA.
- VanSoest P.J.,1994. Nutritional ecology of the ruminant. 2nd ed. Cornell University Press, Ithaca, NY, USA.
- VanSoest P.J. Robertson J.B. Lewis B.A.,1985. Analysis of forage and fibrous foods. Cornell University Press, Ithaca, NY, USA.
- Velásquez P.A.T. Berchielli T.T. Reis R.A. Rivera A.R. Dian P.H.M. Teixeira I.A.M.A.,2010. Composição química, fracionamento de carboidratos e proteínas e digestibilidade in vitro de forrageiras tropicais em diferentes idades de corte. Rev. Bras. Zootecn. 39: 1206-1213.
- Vigna B.B.Z. JungmannL. Francisco P.M. Zucchi M.I. Valle C.B. Souza A.P.,2011. Genetic diversity and population structure of the Brachiaria brizantha germplasm. Trop. Plant Biol. 4: 157-169.
- Wattiaux M.A. Mertens D.R. Satter L.D.,1991. Effect of source and amount of fiber on kinetics of digestion and specific gravity of forage particles in the rumen. J. Dairy Sci. 74: 3872-3883.
- Weiss W.P.,1994. Estimation of digestibility of forages by laboratory methods. In:Fahey G.C.Jr. Collins M. Mertens D.R. (eds.) Forage quality evaluation and utilization. American Society of Agronomy Publ., Madison, WI, USA, pp 644-651.
- Wilson J.R. Brown R.H. Windham W.R.,1983. Influence of leaf anatomy on dry matter digestibility of C3, C4, and C3/C4 intermediate types of Panicum species. J. Agron. Crop Sci. 23: 141-146.
