Abstract
An experiment was conducted to evaluate the effects of two lyophilised aqueous extracts of Lysiloma acapulcensis (LAE) and Phitecellobium dulce (PDE) tree leaves on in vitro assessment of hatching of eggs, larval development and migration of gastrointestinal nematodes of sheep using a general linear model. Treatments contained extracts from both species at concentrations of 0, 125, 250 and 500 µg/mL. Both albendazole and levamisole were used at a level of 1% as positive control. The extract of LAE, compared to PDE, showed better inhibition (P<0.05) of egg hatching. Different doses of both the LAE and PDE extracts showed a larvicidal effect (P<0.05) on all larvae exposed to different doses of the extracts. In the larval migration assay, a similar effect with levamisole at doses of 250 and 500 µg/mL occurred with the LAE extract. The extract of P. dulce had a lower larvicidal effect (P<0.05) than levamisole and L. acapulcensis extracts. Using aqueous extracts of both species of L. acapulcensis and P. dulce could be a promising alternative to synthetic anthelmintics as treatments of gastrointestinal nematodes of sheep in organic and conventional production systems under subtropical conditions.
Key Words:
Introduction
Gastrointestinal parasitism in small ruminants in tropical regions has been classified as a major health and welfare problem. Helminthic infections are a major cause for reduced productivity in livestock, particularly those owned by poor farmers (Hounzangbe-Adote et al., Citation2005). Development of resistant strains of nematodes to synthetic anthelmintics (Jackson and Coop, Citation2000) and the move to organic farming systems over the past few years have increased the demand for alternatives to chemoprophylaxis as a means of reducing the use of anthelmintic drugs to control parasites (Athanasiadou et al., Citation2000; Waller and Thamsborg, Citation2004).
Use of plants containing high levels of condensed tannins (CT) or CT extracts (CTE) are potential alternative methods to reduce the worm burden, nematode female fecundity and egg hatchability (Athanasiadou et al., Citation2001; Nguyen et al., Citation2005; Githiori et al., Citation2006; Meja-Hernandez et al. Citation2014). These plants, with anthelmintic properties, are promising due to their beneficial effects on health, rather than for their direct nutritional value. Both L. acapulcensis and P. dulce are native tree species widely distributed in the semi-arid regions of Mexico, which have high condensed tannins concentrations (Camacho et al., Citation2010), and constitute a substantial part of the diet consumed by grazing goats and sheep.
The objective of the present study was to evaluate the anthelmintic effects of Lysiloma acapulcensis (LAE) and Phitecellobium dulce (PDE) on egg hatching, P. dulce larval development and migration of gastrointestinal nematodes of sheep.
Materials and methods
Plant materials
Fresh leaves of L. acapulcensis and P. dulce were harvested in July 2011 from an area located at the Centro Universitario Universidad Autónoma del Estado de México, Temascaltepec, Mexico. Geographically, this is located at 19°02’04” west longitude at an elevation of 1720 m asl. The climate is moderately humid with an average temperature of 15 to 18°C an annual rainfall of 950 to 1000 mm (García, Citation1987).
Preparation of plant extracts
Plant extracts were prepared according to Salem et al. (Citation2006). Briefly, 100 g of each plant species leaves were chopped and extracted with distilled water (100 mL of water: 10 g of leaves). This solution was incubated for 48 h at room temperature, and then filtered through three layers of cheesecloth. Finally, the remaining fractions were lyophilised and kept refrigerated at 4°C in air-tight containers until use for biological assays.
Chemical composition and condensed tannins determination
Lyophilised samples of LAE and PDE were analysed for dry matter (DM) (method 934.01), organic matter (OM) (method 942.05), crude protein (CP) (method 954.01) according at AOAC (Citation1990). Neutral (NDF) and acid detergent fiber (ADF) were determined by the method of Van Soest et al. (Citation1991). Total condensed tannins (TCT) were analysed using the butanol-HCL method (Terrill et al., Citation1992), as modified by López et al. (Citation2004), as internal standards using L. acapulcensis. Analyses of the free (FCT1), protein- (PCT) and fibre-(FCT2) bound CT were conducted following Porter et al. (Citation1986). Purification was with Sephadex LH-20 as described by Asquith and Butler (Citation1985), with the modifications of Hedqvist et al. (Citation2000).
Gastrointestinal nematodes eggs recovery
A mixture of gastrointestinal nematodes (95% Haemonchus contortus, 2% Trichostrogylus colubriformis and 3% Oesophagostomum columbianum) was recovered as described by Hubert and Kerboeuf (Citation1992). Faeces (~1000 g) were obtained from two sheep, maintained as experimental donors, by hanging a faecal collection bag on the sheep overnight. The fresh faeces were suspended in water and cleared of organic debris by filtration through 250, 150, 100 and 60 µm sieves. Eggs were collected on a 32 µm sieve and the organic debris was further cleared by centrifugation (3500 rpm for 5 min) in saline solution. The concentration in the suspension was estimated by counting the number of eggs in 20 µL aliquots, where the eggs suspension was adjusted to 400 eggs/mL.
Treatments
Nine treatments were evaluated, being: P. dulce (PD-125, PD-250 and PD-500 µg/mL), L. acapulcensis (LA-125, LA-250 and LA-500 µg/mL), phosphate buffer solution (PBS, was used as negative control and solvent of extracts), albendazole and levamisole both at 1% (ABZ and LV, were used as positive control) and dimethyl sulphoxide (DMSO, was used as negative control and solvent of ABZ).
Eggs hatch assay
The in vitro egg hatch assay was based on the method of Marie-Magdeleine et al. (Citation2010). One mL of egg suspension was distributed in each well of 48 well plates containing ~400 eggs/well, and mixed with the same volume of plant extracts (i.e., 125, 250, 500 µg/mL). Albendazole at a 1% concentration was used as the positive control. The plates were incubated at 25°C for 48 h, when one drop of Lugol’s iodine solution was added to stop egg hatching. All the eggs and first stage larvae (L1), in each plate were counted with three replicates for each concentration and control.
Larval development assay
The procedure used was described by Hubert and Kerboeuf (Citation1992) with modification of Assis et al. (Citation2003). One thousand larvae (L1 and L2) were distributed into the wells of the 24 weel plates. Larvae were recovered by the procedures described for eggs hatch assay and feed for 48 h in a nutritive solution of fecal juice and Amphotericin B to avoid fungal development. At this time, the same volume of plants extracts (i.e., 125, 250, 500 µg/mL) were added and albendazole was used as the positive control as previously. There were three replicates for each treatment. Third- stage larvae (L3) were obtained 7 d later. At this time, the parasites were counted under an inverted microscope by separation of the larvae into third stage larvae (L3), and larvae of other development stages (L1 and L2).
Larval migration assay
The procedure used was that of Marie-Magdeleine et al (Citation2010). One thousand live L3 were added to centrifuge tubes (total of 30 tubes) containing negative control (PBS), a positive control (Levamisole at 1% concentration) and each extract to be tested (i.e., 125, 250, 500 µg/mL). Use of PBS aimed to avoid interference with any non-specific effect due to pH change. All incubations were for 3 h at 25°C. Thereafter, the L3 from each tube was washed with PBS and centrifuged (5000 rpm for 5 min) three times. Larvae were then transferred to sieves (inserts equipped with a 20 µm mesh positioned a conical tube). After 3 h at room temperature, the numbers of L3 larvae which migrated through the mesh were counted in an optical microscope at 40 x in a 10% aliquot.
Statistical analyses
Data were transformed into Arcoseno √x and analysed with a completely randomised design using SAS (Citation2006), where mean comparisons used Tukey’s test at a confidence level of 95% (Steel and Torrie, Citation1980).
Results and discussion
Chemical composition and condensed tannins
The leaves of Lysiloma acapulsencis had higher concentrations of OM, NDF, ADF, FCT1, PCT and TCT than leaves of P. dulce which had a higher content of CP and FCT2 ().
Eggs hatch assay
Both aqueous extracts of LAE and PDE, in addition to the negative and positive controls (i.e., PBS, ABZ, and DMSO), affected gastrointestinal nematodes egg hatching. Phosphate buffer solution and DMSO had the highest (P<0.05) egg hatching (96.7 and 68.1%, respectively) followed by PDE and LAE (P<0.05), with ABZ having the lowest (P<0.05) hatching percentage, i.e. 30.5% (). Within PDE extract doses, there was increased hatching as the dose of extract increased (62.7, 59.6, and 56.3% for PD-125, PD-250, and PD-500, respectively) while, in LAE, the highest hatching (47.4%) was with the highest extract dose (i.e., 500 µg/mL) compared to the intermediate dose of 250 µg/mL which had the lowest value (32.6%; ).
Larval development and migration assay
Both the LAE and PDE extracts at different doses, and ABZ, resulted in almost no larval development (P<0.05) compared to 100 and 86.4% larval development for DMSO and PBS, respectively (). The highest (P<0.05) larval migration was with PBS (79.3%) compared to other treatments which had values (P<0.05) of 2.9% for LV-500 and 16.1% with PD-500. The lowest (P<0.05) larval migration was with LV at all doses (4.6, 6.7, and 2.9% for LV-125, LV-250, and LV-500, respectively; ).
Eggs hatch and larval development assay
This study examined the in vitro bioactive properties of aqueous extracts from L. acapulcensis and P. dulce on common gastrointestinal nematodes of sheep. Results showed that LAE has an ovicidal effect with doses of 250 and 500 µg/mL, which may be due to the condensed tannins in L. acapulcensis (116.3 g/kg DM; ). Ademola and Eloff (Citation2011), in their study with Vernonia amygadalina acetone extracts, inhibited egg hatching and larval development of H. contortus larvae at a concentration of 957 µg/mL. It is well known that CT of tanniniferous plants varies in their molecular weight and composition (Mueller-Harvey, Citation2006; Gea et al., Citation2011). Thus possible anthelmintic effects of tanniniferous plants may depend on their CT contents as well as on other CT parameters such as the monomeric composition and degree of polymerisation (i.e., average size of tannin molecules). Molan et al. (Citation2003) and Brunet and Hoste (Citation2006) suggested that prodelphidin-rich tannins and their monomers have a stronger anthelmintic activity against sheep nematodes than do procyanidin-rich tannins and their monomers.
In our study, LAE showed anthelmintic properties with reduced egg hatching (32.6%), although the dose of 250 µg/mL was higher than that of PDE at 59.6%. Alonso-Díaz et al. (Citation2008) showed that extracts of some tropical tanniniferous plants (i.e., Acacia pennulata, Lysiloma latisiliqum, Leucanena leucocephala), had in vitro anthelmintic effects against gastrointestinal nematodes using egg hatching and larval development assays. The hatching of nematode eggs is initiated by environmental stimuli which cause release of enzymes, such as proteases, lipases and chitinases, by the larvae which function to degrade the egg membrane (Mansfield et al., Citation1992). The tanniniferous compounds which are in extracts of L. acapusencis may act to inhibit activity of these enzymes. Both LAE and PDE are more potent inhibitors of larval development than of egg hatching. Indeed results were similar to those of Molan et al. (Citation2002), who found that extracts of Lotus pendunculatus, Lotus corniculatus, Hedychium coronarium and Onobrychis viciifolia, were more potent in inhibiting larval development (91%) than of egg hatching (34%). Unfortunately the design of this study did not include confirmation of the role of tannins in the anthelmintic effect observed using polivynil-pirrodilone or polyethylene glycol as tannin blocking agents. However, it is possible to speculate that the more specific and strong actions of the tanninrich extracts on larval development is related to the presence of proline and hydroxiproline rich proteins in the nematode larval sheath and cuticle, and the high affinity of tannins to those proteins (Page, Citation2001).
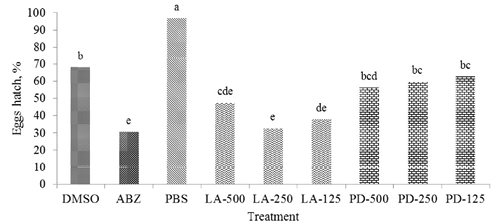
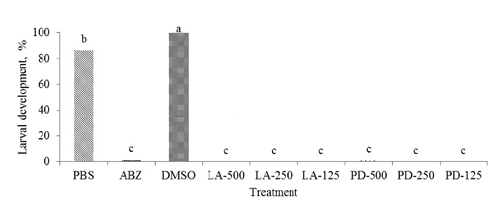
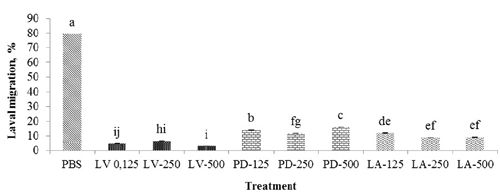
Table 1. Chemical composition and concentration of condensed tannins (g/kg DM) in leaves of L. acapulcensis and P. dulce.
Larval migration assay
The LAE (250 and 500 µg/mL) was more consistent on larval migration. Purified condensed tannins from several plant species were used in vitro against T. colubriformis and T. circumcincta (Molan et al., Citation2000). The viability, motility and migration ability of the L3 larvae of these nematodes were severely affected by the presence of CT in their environment. Lorimer et al. (Citation1996) found that CT extracts reduced migration of L3 larvae of T. colubriformis. Other in vitro studies have shown that both purified condensed tannins and terpenoids from several legumes reduced the mobility, and consequent migration ability, of ovine nematode larvae (Molan et al., Citation2000, Citation2003). In our study L. acapulsencis, a legumes with a high CT content, was probably responsible for its anthelmintic properties.
Tannins are biochemical structures with a nature consistent with its constitutive monomer (flavan-3-ol). Condensed tannins can be distinguished into four classes: i) prodelphinidins, ii) procyanidins, iii) prorobetinidins, and iv) profisetinidins. In legume forages, both prodelphinidins and procyanidins are commonly present. However their ratios strongly differ among plant species and/or varieties (Mueller Harvey et al., Citation2006). By experimentally measuring effects of the different flavan-3-ols (monomers) which constitutute either prodelphinidins or procyanidins, some dta suggests that prodelphinidins have more potent anthelmintic activity than do procyanidins (Molan et al., Citation2003; Brunet et al., Citation2006, Citation2008). This hypothesis is also supported by the reality that legume fodders with a high content of prodelphinidin and/or procyanidins (e.g. sainfoin, sericea lespedeza, Lotus pedunculatus) have shown more consistent anthelmintic activity than species with a low prodelphinidin and/or procyanidins ratio (Lotus corniculatus; Molan et al., Citation2003).
In vitro and in vivo assays indicate that tannins disturb the two early steps of nematode establishment, being larval exsheathment (Brunet and Hoste, Citation2006; Brunet et al., Citation2007) and penetration of the exsheated larvae within the digestive mucosae (Brunet et al., Citation2008). These functional modifications have been associated with major changes in larval ultrastructure. Similarly, observations of transmission and scanning electron microscopy on adult H. contortus, after in vitro and/or in vivo contact with tree rich plants. have shown modifications to the cuticle, the digestive tract and the female reproductive tract, which may explain their negative consequences on adult worm populations, particularly those affecting egg excretion (Brunet et al., Citation2011).
Ovicidal and larvicidal activity against gastrointestinal strongyles in our study showed evidence that extracts of the two trees have anthelmintic activity. This is of vital importance in southern Mexico State, and other areas of the world, where the production systems of sheep and goats during the dry period depend mainly on feeding animals trees and shrubs, among which the two species which we studied dominate. That is why use of extracts of both species tested may represent an alternative to control of gastrointestinal nematodes in sheep, reducing indiscriminate use of synthetic wormers. However in vivo studies are required to support the promising in vitro assay data of our study.
Conclusions
Aqueous extracts of Lysiloma acapulcensis and Phitecellobium dulce species could be beneficial as phytogenic anthelmintics in sheep, and could be used to control gastrointestinal nematodes in sheep under subtropical conditions.
References
- Ademola I.O. Eloff J.N., 2011. Anthelmintic activity of acetone extracts and fractions of Veronia amigdalina against Heamonchus contortus eggs and larvae. Trop. Anim. Health Pro. 43:521-527.
- Alonso-Díaz M.A. Torres-Acosta J.F.J. Sandoval-Castro C.A. Aguilar-Caballero A.J. Hoste H., 2008. In vitro larval migration and kinetics of exsheathment of Haemonchus contortus larvae exposed to four tropical tanniniferous plant extracts. Vet. Parasitol. 153:313-319.
- AOAC, 1990. Official methods of analysis. 15th ed., Association of Official Analytical Chemists, Washington, DC, USA.
- Asquith T.N. Butler L.G., 1985. Use of dyelabeled protein as spectrophotometric assay for protein precipitans such as tannin. J. Chem. Ecol. 11:1535-1544.
- Assis L.M. Bevilaqua C.M.L. Morais S.M. Vieira L.S. Costa C.T.C. Souza J.A.L., 2003. Ovicidal and larvicidal activity in vitro of Spigelia anthelmia Linn. extracts on Haemonchus contortus. Vet. Parasitol. 117:43-49.
- Athanasiadou S. Kyriazakis I. Jackson F. Coop R.L., 2000. Consequences of long term feeding with condensed tannins on sheep parasitized with Trichostrogulus colubriforimis. J. Parasitol. 30:1025-1033.
- Athanasiadou S. Kyriazakis I. Jackson F. Coop R.L., 2001. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: in vitro and in vivo studies. Vet. Parasitol. 99:205-219.
- Brunet S. Aufrere J. El BabiliF. Fouraste I. Hoste H., 2007. The kinetics of exsheatment of infective nematode larvae is disturbed in the presence of a tannin-rich plant extract. Parasitology 134:1253-1262.
- Brunet S. Hoste H., 2006. Monomers of condensed tannins affect the larval exsheathment of parasitic nematodes of ruminants. J. Agr. Food Chem. 54:7481-7487.
- Brunet S. Hoste H. Arroyo LópezC. Manolaraki F. Martínez-Ortiz de MontellanoC. Sotiraki S. Torres-Acosta F., 2011. The anthelmintic properties of tannin-rich legume forages: from knowledge to exploitation in farm conditions. In: RanillaJ.M. ( ed.) Challenging strategies to promote the sheep and goat sector in the current global context. CIHEAM/CSIC/Universidad de León/FAO, Zaragoza, Spain, pp 295-304.
- Brunet S. Jackson F. Hoste H., 2008. Effects of sainfoin (Onobrychis viciifolia) extract and monomers of condensed tannins on the association of abomasal nematode larvae with fundic explants. Int. J. Parasitol. 38:783-790.
- Camacho L.M. Rojo R. Salem A.Z.M. Provenza F.D. Mendoza G.D. Avilés F. Montañez-Valdez O.D., 2010. Effect of season on chemical composition and in situ degradability in cows and in adapted and unadapted goats of three Mexican browse species. Anim. Feed Sci. Tech. 155:206-212.
- García E., 1987. Modificaciones al sistema de clasificación climática de Koeppen. Universidad Nacional Autónoma de México, Mexico City, Mexico.
- Gea A. Stringano E. Brown R.H. Mueller-Harvey I., 2011. In situ analysis and structural elucidation of sainfoin (Onobrychis viccifolia) tannins for high throughput germplasm screening. J. Agr. Food Chem. 59:405-503.
- Githiori J.B. Athanasiadou S. Thamsborg S.M., 2006. Use of plants in novel approaches for control of gastrointestinal helminths in livestock with emphasis on small ruminants. Vet. Parasitol. 139:308-320.
- Hedqvist H. Mueller-Harvey I. Reed J.D. Krueger C.G. Murphy M., 2000. Characterization of tannins and in vitro protein digestibility of several Lotus corniculatus varieties. Anim. Feed Sci. Tech. 87:41-56.
- Hounzangbe-Adote M.S. Paolini V. Fouraste I. Moutairou K. Hoste H., 2005. In vitro effects of four tropical plants on three life-cycle stages of the parasitic nematode, Haemonchus contortus. Res. Vet. Sci. 78:155-160.
- Hubert J. Kerboeuf D., 1992. A micro larval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 130:442-446.
- Jackson F. Coop R.L., 2000. The development of anthelmintic resistance in sheep nematodes. Parasitology 120:95-107.
- López J. Tejada I. Vázquez C. De DiosG. Shimada A., 2004. Condensed tannins in humid tropical fodder crops and their in vitro biological activity. J. Sci. Food Agric. 84:295-299.
- Lorimer S.D. Perry N.B. Foster L.M. Burgess E.J., 1996. A nematode larval motility inhibition assay for screening plant extracts and natural products. J. Agr. Food Chem. 44:2842-2845.
- Mansfield L.S. Gamble H.R. Fetterer R.H. 1992. Characterization of the eggshell of Haemonchus contortus–I. Structural components. Comp. Biochem. Physiol. 103b:681-686.
- Marie-Magdeleine C. Udino L. Philibert L. Bocage B. Archimede H., 2010. In vitro effects of Cassava (Manihot esculenta) leaf extracts on four development stages of Haemonchus contortus. Vet. Parasitol. 173:85-92.
- Mejia-Hernandez P. Salem A.Z.M. Elghandour M.M.Y. Cipriano-Salazar M. Cruz-Lagunas B. Camacho L., 2014. Anthelmintic effects of Salix babylonica L. and Leucaena leucocephala Lam. extracts in growing lambs. Trop. Anim. Health Pro. 46:173-178.
- Molan A.L. Meagher L.P. Spencer P.A. Sivakumaran S., 2003. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylu colubriformis. Int. J. Parasitol. 33:1691-1698.
- Molan A.L. Waghorn G.C. Min B.R. McNabb W.C., 2000. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro. Folia Parasit. 47:39-44.
- Molan A.L. Waghorn G.C. Min B.R. McNabb W.C., 2002. Effect of condensed tannins on egg hatching and larval development of Trichstrongylus colubriformis in vitro. Vet. Rec. 150:65-69.
- Mueller-Harvey I., 2006. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agr. 86:2010-2037.
- Nguyen T.M. Van BinhD. Ørskov E.R., 2005. Effect of foliages containing condensed tannins on gastrointestinal parasites. Anim. Feed Sci. Tech. 121:77-87.
- Page A.P., 2001. The nematode cuticle: synthesis, modification and mutants. In: KennedyM.W. HarnettW. ( eds.) Parasitic nematodes: molecular biology, biochemistry and immunology. CAB, London, UK, pp 167-193.
- Porter L.J. Hirstich L.N. Chan B.G., 1986. The conversion of procyanidins and prodelphinidins to cyanidin and delhpinidin. Phytochemistry 25:223-230.
- Salem A.Z.M. Salem M.Z.M. El-Adawy M.M. Robinson P.H., 2006. Nutritive evaluations of some browse tree foliages during the dry season: secondary compounds, feed intake and in vivo digestibility in sheep and goats. Anim. Feed Sci. Tech. 127:251-267.
- SAS, 2006. SAS user’s guide: statistics. Version 9.0. SAS Inst., Cary NC, USA.
- Steel R.G.D. Torrie J.H., 1980. Bioestadística: principios y procedimientos. McGraw-Hill Interamericana, Santa Fé, Mexico.
- Terrill T.H. Rowan A.M. Douglas G.B. Barry T.N., 1992. Determination of extractable and bound condensed tannin concentrations in forage plants, protein meals and cereal grains. J. Sci. Food Agric. 58:321-329.
- Van SoestP.J. Robertson J.B. Lewis B.A., 1991. Methods for dietary fibre, neutral detergent fibre, and non-starch carbohydrates in relation to animal nutrition. J. Dairy Sci. 74:583-597.
- Waller P.J. Thamsborg S.M., 2004. Nematode control in green ruminant production systems. Trends Parasitol. 20:493-497.
