Abstract
The paper investigates the effect of two different gas mixtures on chemical, physical and microbiological quality of veal meat packed in modified atmosphere during chill storage. Experimental gas atmospheres tested were O246 (46% O2, 31% N2 and 23% CO2) and O270 (70% O2, 8% N2 and 22% CO2). Samples were stored at 4°C for 14 days and tested at 0, 2, 4, 6, 8, 10, 12 and 14 days after packaging. The different O2 concentration influenced many parameters. Lower O2 concentration showed a greater increase of a* values (P<0.01) from the 2nd to the 8th packaging day, and a lower increase in drip loss values, thiobarbituric acid reactive substances and protein oxidation (P<0.001). Total aerobic mesophilic and psychrophilic count showed a gradual increase in bacterial load over storage time in both the experimental treatments. Results obtained showed that O246 is better than O270 in calf meat packaging because of a belated decline of meat quality, particularly about oxidative parameters.
Key Words:
Introduction
Modified atmosphere packaging (MAP) in food industry is recognised to maintain the desired properties of meat for the desired period of storage and display (McMillin, Citation2008). Furthermore, fresh meat packaging is carried out to protect products and avoid cross-contamination, to facilitate food handling and identification, to present product in an attractive way to consumers and especially to reduce gas and water vapour deteriorative effects, delaying biological and physical food spoilage (Yam et al., Citation2005).
Different meat organoleptic properties are usually considered in meat quality evaluation by consumers, such as colour, flavour, tenderness and texture (Bredahl et al., Citation1998). These meat characteristics not only are affected by the pre-slaughtering management, but also by biochemical and microbiological modifications during post-mortem storage (Sierra et al., Citation2006). The gases commonly used for MAP are oxygen, carbon dioxide and nitrogen. Oxygen function was to maintain myoglobin in its oxygenated form and stabilise meat redness; carbon dioxide inhibited the growth of aerobic spoilage bacteria and nitrogen had a filler function (Jakobsen and Bertelsen, Citation2000).
Several authors showed that high oxygen maintained oxymyoglobin pigment and cherry red colour (O’Grady et al., Citation2000), but may induce other oxidative reactions resulting in undesirable flavours (Rhee and Ziprin, Citation1987; Estevez and Cava, Citation2004). Fatty acids oxidation caused rancidity and changed chromatic, physical and nutritional meat properties (Kanner, Citation1994). High oxygen during fresh meat chill storage resulted in a low enzyme activity, col lagen solubility with worse tenderness (Rowe et al., Citation2004a) and caused the formation of protein complex and non-enzymatic browning products (Mercier et al., Citation2004).
The present study aims to investigate the relationship between two different gas mixtures in MAP and a number of indicators, such as colour stability, lipid and protein oxidation, tenderness and microbiological properties in veal calf mus cle. The choice of investigating veal meat was dictated by the poor literature about the effects of MAP on a bovine fresh meat fundamentally different from beef both from a physical and chemical perspective, such as low fat content, and a good smooth flavour product (Vieira et al., Citation2005), eating quality (Monteiro et al., Citation2013), fatty acids profile (Aldai et al., Citation2012), low myoglobin concentration (De Palo et al., Citation2013).
Materials and methods
Animals
A total of 8 Italian Holstein Friesian bull calves were used in the present trial. They were reared in the same farm. Calves were housed individually in pens (1.2 m2 per calf) during the first 8 wk and thereafter in groups of 4 calves (1.8 m2 per calf) per pen as described by De Palo et al. (Citation2013). After birth, animals received mother’s colostrum milked and supplied by artificial teats within 2 h of life. After the first colostrum supply, it was administered three times per day. Then, after 48 h and during the first 8 wk, they were fed commercial milk replacer (Alpuro B.V., Uddel, the Nederlands) according to a commercial feeding schedule to produce veal meat. After 8 wk, besides milk replacer, a corn silage was supplied to calves. The structures, housing, and feeding techniques were adopted according the EU directive 2008/119. Animals were slaughtered at the age of 218 d with an average body weight of 237 kg, after transportation to a slaughterhouse 32 km far from farm, with a travel made almost exclusively on an state expressway.
Meat sampling and packaging
The half carcasses were stored at 4±0.5°C for 24 h and then dissected in a temperature-controlled room (12±1°C). Samples were obtained from the right side of carcasses and in particular from the LM (between the 7th and the 12th rib).
Sixteen samples of longissimus dorsi muscle (LM) were collected from each of the 8 carcasses, for a total of 128 samples. Samples were placed in extruded polystyrene trays (AERpack PCM0330; Coopbox Italia, Reggio Emilia, Italy) packed in MAP with the same barrier film (Cryovac LID 2050; Cryovac, Passirana di Rho, Italy) () and two different gas mixtures: eight samples were packed with 46% O2, 31% N2, 23% CO2 (O246) and eight with 70% O2, 8% N2, 22% CO2 (O270). The MAP packed samples were processed using a heat sealer (CVS-PN 35; Mondini S.p.A., Cologne, Italy). Each sample (packed in each tray) consisted of 2 slices (thickness of 0.5 cm) and 3 pieces of meat. One slice was used to evaluate chromatic parameters and to perform microbiological analyses. The second slice was used to calculate water holding capacity (WHC), collagen and oxidative variables [protein oxidation, 2-thiobarbituric acid (TBA), i.e. thiobarbituric acid reactive substances (TBARS), and hydroperoxides]. One of the three pieces of meat (a parallelepiped with 3 by 6 by 6 cm sides) was used for the Warner Blatzer shear force (WBSF) evaluation on cooked meat and pH measurement. Another piece of meat (a cube with 1.5 cm per side) was contained in a small polyvinyl chloride (PVC) container and used to calculate drip loss. The PVC container was a cube of 3 cm per side open on 1 surface. The last piece of meat (a cube with 1.5 cm per side) was used to measure cooking loss.
Samples were transported under controlled temperature (+4±0.5°C) to the laboratories and they were stored at +4±0.5°C. All samples were stored in the same refrigerated cell, on a tabl e, in dark conditions. Laboratory analyses were performed at 2, 4, 6, 8, 10, 12, and 14 d of storage. The samples identified as T0 (24 h after slaughtering) were analysed on the same day of packaging within 2 h from collection time. Assignment of each sample to a treatment was made randomly.
Meat quality and microbiological analysis
Colour evaluation
Surface meat colour was measured at 1 min after opening the packaging during each experimental time: T0 (packaging day) and then 2, 4, 6, 8, 10, 12, 14 d of storage. The surface colour of calf slices was determined according to the CIE L*, a*, b* (CIE, 1976) colour system using Minolta CR-300 colorimeter (light source D65; Minolta Camera Co. Ltd., Osaka, Japan). Reflectance measurements were collected from a 0° viewing angle with an A pulsed xenon arc lamp with a reading surface of 8 mm diameter. These measurements were performed in three different points of each sample. Moreover, on each point, three measurements were performed by rotating the detector system of 90° from the previous one, for a total of nine measurements for sample. The 9 readings per sample were made at each time point and averaged for statistical analysis. The colorimeter was cali brated on the Hunterlab colour space system using a white title (L*=99.2, a*=1.0, b*=1.9). The a* and b* values were used to determine chroma=(a2 + b2)1/2 and hue (°)=tan–1 (b/a) according to Little (Citation1975) and Mancini and Hunt (Citation2005).
pH, water holding capacity, cooking loss and drip loss
Intramuscular pH was recorded using a portable pH meter with glass electrode shaped to easily penetrate meat (Carlo Erba pH 710; Carlo Erba Reagenti, Milano, Italy). Before each measurement, the pH meter was automatically calibrated for muscle temperature and using solutions with 4 and 7 pH values (Crison, Lainate, Italy).
The WHC was measured using the centrifugation method according to Bouton et al. (Citation1971). Samples weighing 3 g were collected from each slice and were then centrifuged at 60,000×g for 1 h at 10°C with an Allegra 64R centrifuge (Beckman Instruments Inc., Brea, CA, USA). After centrifugation, fluid was removed from the remaining samples and weighed again, and the centrifugation loss was calculated as the difference in weight before and after centrifugation. Water holding capacity was measured twice on each sample.
For the cooking loss determination, cubic meat pieces with 1.5 cm per side were weighed [initial weight (IW)] and then cooked in plastic bags in water bath at 80°C until they reached the internal temperature of 75°C, measured by a copper constantin fine-wire thermocouple fixed in the geometrical center of the sample (Model 5SC-TT-T-30-36; Omega Engineering Inc., Stamford, CT, USA). Cooked samples were cooled, fluids were removed using paper towel, and reweighed [final weight (FW)]. The cooking loss was calculated as a percentage of weight loss: [(IW-FW)/IW]×100 (Bertram et al., Citation2003).
Drip loss was evaluated on the second cubic piece with 1.5 cm per side at each experimental time (0, 2, 4, 6, 8, 10, 12, and 14 d of storage). They were placed in a PVC container with known weight (KW) during packaging in MAP. When trays were opened, each container with meat and liquid was weighed (IW). Then the meat sample was removed from the PVC container, fluids as well were removed, and weighed again (FW). The drip loss was calculated according this formula: {[(IW-KW)-FW]/(IW-KW)}×100.
Shear force and collagen
At each experimental time (0, 2, 4, 6, 8, 10, 12, and 14 d of storage), the WBSF was recorded on cooked meat. First, the parallelepiped samples with 3×6×6 cm were subdivided into 2 equal parts, cutti ng them perpendicularly to the fibres axis. One was cooked in a plastic bag in water bath at 85°C to an internal temperature of 75°C, measured with a copper constantin fine-wire thermocouple fixed in the geometrical center of the sample (Model 5SC-TT-T-30-36; Omega Engineering Inc.). Cores (1.27 cm diameter) were cut from each cooked sample parallel to the muscle fiber direction. The cores were sheare d perpendicular to the muscle fibres orientation using an Instron 1140 apparatus (Instron, High Wycombe, UK) provided with a computer, using a crosshead speed of 50 mm/min and a load cell of 50 N. Each core was sheared 3 times and these 3 values measured were used to obtain the mean value for each sample. Results were expressed in kg/cm2. Total collagen and collagen solubility were calculated as described by De Palo et al. (Citation2013).
Thiobarbituric acid reactive substances, protein oxidation and hydroperoxides
Lipid oxidation was assessed by the TBARS method (Buege and Aust, Citation1978) and expressed as milligrams of malondialdehyde (MDA) per kilogram meat. Meat samples (2 g) were homogenised in 20 mL of 100 mM phosphate buffer (pH 7.0) for 2 min using a homogeniser. An aliquot of homogenate (1 mL) was transferred to a glass tube and added with 50 L of butylated hydroxytoluene (7.2% in ethanol) and with 1950 L of TBA/trichloracetic acid (TCA)/HCl solution (0.375% TBA, 15% TCA, and 0.25 N HCl). Samples were shaken and incubated for 15 min at 90°C in a bath. Subsequently, they reached room temperature (15 to 30°C) and were centrifuged at 2000×g for 15 min at 4°C. Supernatant absorbance at 531 nm was measured against the blank containing 2 mL of TBA/TCA/HCl solution in 1 mL of distilled water. The TBARS were calculated compared with a standard curve constructed with 1,1,3,3-tetramethoxypropane.
Two aliquots of homogenate (50 L each) previously prepared for the TBARS determination were added with 1 mL 10% TCA and then centrifuged at 1200×g for 3 min at 4°C to measure protein oxidation. The first aliquot was used as a standard and added with 1 mL of 2 M HCl solution. The second aliquot was added with 1 mL of 2 M HCl containing 10 mM 2,4-dinitrophenyl hydrazine (DNPH). Samples were incubated for 1 h at room temperature (15 to 30°C) and shaken every 20 min, and then 1 mL of 10% TCA was added. The samples were vortexed for 30 s and centrifuged 3 times at 1200×g for 3 min at 4°C and the supernatant removed. The pellet was washed with 1 mL of ethanol:ethyl acetate (1:1), shaken, and centrifuged 3 times at 1200×g for 3 min at 4°C and the supernatant removed. The pellet was then dissolved in 1 mL 20 mM sodium phosphate 6 M guanidine hydrochloride buffer. Samples were then shaken and centrifuged at 1200×g f or 3 min at 4°C. Carbonyl concentration was measured on the DNPH treated sample at 360 nm with a Beckman Coulter DU800 (Beckman Instruments Inc.) and expressed as nanomoles carbonyl per milligrams protein. Protein concentration was measured according to Biuret assay (Tokur and Korkmaz, Citation2007). To determine hydroperoxides, 2 mL of homogenate (previously prepared for the TBARS determination) were added with 4 mL of CH3OH and 2 mL of CHCl3. The samples were vortexed for 30 s and were added with 2 mL of CHCl3 and 1.6 mL of 0.9% NaCl. The samples were shaken for 1 min and then centrifuged at 3500×g for 10 min at 4°C. Two milliliters of lipid extract were sampled from the lower chloroform phase and processed with 1 mL of CH3COOH/CHCl3 and 50 L of KI (1.2 g/1 mL distilled water). Samples were stored for 5 min in a dark room and added with 3 mL of 0.5% of CH3COOCd and then vortexed and centrifuged at 4500×g for 10 min at 40°C. The absorbance of the upper layer at 353 nm is determined against a reagent blank which is handled in the same manner except that the lipid sample is replaced by distilled water. Results were expressed in micromoles per gram according to Buege and Aust (Citation1978).
Total aerobic mesophilic and psichrofilic count
For each sample, these tests were performed: total aerobic mesophilic (TAMC) and psychrophilic count (TAPC), and Escherichia coli count (ECC). Each test was performed on d 0, 2, 8, 10, 12, and 14 of storage at 4°C. For each sample, 10 g of meat was removed aseptically, transferred to stomacher bags (bioMérieux, Marci l’Etoile, France) containing 90 mL of 0.85% sterile tryptone sal t solution, and homogenised using a stomacher (International Pbi S.p.a., Milano Marittima, Italy) for 60 s at room temperature. The homogenates were serially diluted 10-fold for microbial count. Total aerobic mesophilic count and ECC were performed using TEMPO System (bioMèrieux). Samples used for TAMC measurement were incubated for 40 h at 30°C and samples used for ECC measurement were incubated for 24 h at 37°C. Total aerobic psychrophilic count was performed with pour-plate method (Harrigan, Citation1998) using Plate Count Agar (Oxoid Basingstoke, Hampshire, England) incubated aerobically for 7 d at 4°C.
Evaluation of gas mixture changes during storage
Headspace gas concentrations were measured at each time point using a gas detector provided with a syringe (PBI-Dansensor CheckPoint O2/CO2; PBI-Dansensor’s, Ringsted, Denmark). Each measurement was taken three times per sample, before opening the MAP pack, holing across the extruded polystyrene tray, on a sample never holed before, with a disposable needle inserted on the syringe of the instrument, inserted through an adhesive backed rubber septum (PBI Dansensor’s), placed on extruded polystyrene to prevent pack leakage between measurements. The mean value between the three recordings was used for the further statistical evaluations.
Statistical analysis
A total of 16 samples from each of 8 carcasses were collected. The samples were randomly assigned to 2 treatments: 8 with O246 and 8 with O270. The 8 samples for each gas mixture were tested at 8 different storage days. A 2×8 (gas mixture×storage days) treatment was performed. The data set was submitted to 2-way ANOVA using the general linear model (SAS Inst. Inc., Cary, NC, USA). The gas mixture, the storage time, and the binary interaction between these 2 variables were included as fixed effects.
Gas mixtures were analysed separately, considering as fixed effect the storage time, and applying the post hoc Tukey’s test for repeated measures to evaluate the differences between the experimental times (SAS, Citation1999). All the data were expressed as least square mean and mean SE. Significance was set as P<0.05. Microbiological data were transformed into logarithms of the number of colony forming units (cfu/g). Statistics were performed using Statview (SAS, Citation1999) with statistical significance settled at P<0.05.
Results and discussion
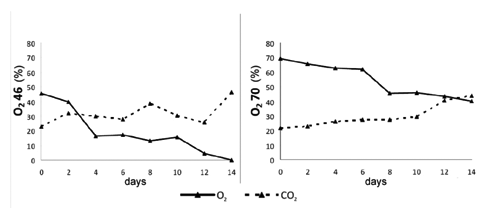
In both packagings, CO2 slowly increased during storage and O2 decreased, but in different ways (). In O246, oxygen decreased till 0% at the 14th storage day, with a greater drop between the 2nd and 4th day. In O270, oxygen dropped to 40.4% at the 14th storage day, with a greater drop between the 6th and 8th day.
Results showed an influence of gas mixture on the main quality parameters of fresh veal calf meat, such as colour, chemical, physical, oxidative and microbiological properties (). These data reflected the results obtained by Zakrys et al. (Citation2008, Citation2009) on beef, by Lund et al. (Citation2007a, Citation2007b) on pork, and by Berruga et al. (Citation2005) on lamb.
The present trial showed that the gas mixture used for MAP influenced oxidation is responsible for meat colour and its main sensory characteristics. Gas exchange between inside and outside the trays was determined by several factors: gases partial pressure and differences in the partial pressure on both sides of the film, storage temperature and relative humidity, but also top film type, thickness and its gas permeability (Lee et al., Citation2008; De Palo et al., Citation2013).
Different gas mixtures influenced colour pa rameters recorded (). The instrumental a* values displayed a negative correlation with days, indicating a decrease in red colour of samples packed in O246 and O270 over time, as described by Zakrys et al. (Citation2009) about beef steaks. The samples packed in O270 showed a* values constant until the 12th day and then significantly lower on the 14th (P<0.01), so the decrease in the redness was more delayed in the presence of a higher concentration of O2, as already widely described in literature. Previous finding by Jayasingh et al. (Citation2002) reported that ground beef packed in high oxygen MAP maintained a bright red colour until the 10th day of storage. Sørheim at al. (Citation1999) also reported a gradual discolouration in beef loin steaks packed under high oxygen between days 3 and 10 of display. According to John et al. (Citation2005) beef steaks packaged under high oxygen had a desirable red colour until the seventh day of storage, and then browned by day 14. In the current study it appears that the O270 atmosphere also provided lower values of redness and chroma compared to O246, especially at 48 h (P<0.05) and 96 h (P<0.01) after the packaging. Oxygen maintained the fresh meat red colour through the formation of oxymyoglobin (Mancini and Hunt, Citation2005) but, in this case, it probably had a reduced effect on the oxidative stability on veal calf meat, maybe due to a lower myoglobin content of this meat because of young slaughtering age and animal feeding (Gariépy et al., Citation1998). Important were also top film properties, particularly their permeability to water vapour and gas, and the residual concentration of O2, which was progressively reduced in the package over storage time.
The instrumental L* and b* values showed an opposite trend compared to a*, indicating an increase in lightness over time and a rise of yellowness up to 2 storage days. The oxidation does not involve only the myoglobin, but also lipids with a consequent increasing in meat yellowness (O’Grady et al., Citation2000; Tang et al., Citation2006). The lipid oxidatio n involved the formation of free radicals, which influenced myoglobin oxidative stability (Faustman et al., Citation1989): therefore lipid oxidation promoted myoglobin oxidation (Lin and Hultin, Citation1977).
Although high oxygen levels promoted oxidation (Estevez and Cava, Citation2004), instrumental b* values showed no significant differences between the two gas mixtures. These values were very low, and appeared accept able relative to oxidation of lipids. Moreover, the O270 samples maintained higher L* values, indicating that they became lighter than those packed with less oxygen during storage. The increased lightness over time was also linked to the cell membranes denaturation in muscle myofibrils, moving intracellular water towards the extracellular space (Yu et al., Citation2005; Mortensen et al., Citation2006). So, meat lightness was linked to WHC. In fact, the WHC was defined as the meat ability to retain water (Offer and Trinick, Citation1983), which is normally present into muscle cells and bound to protein in the intercellular spaces with a reduced mobility (Huff-Lonergan and Lonergan, Citation2005). The proteolysis occurring physiologically with cell death reduced WHC (Huff-Lonergan and Lonergan, Citation2005). In the current trial the WHC () progressively reduced over time showing no significant differences between the two atmosphere. Meat with low WHC had higher drip loss (den Hertog-Meischke et al., Citation1997). Both in O246 and O270 samples, drip loss increased over storage time, but slices packed under higher oxygen showed significantly greater drip loss, probably due to reduced proteolysis. A negative correlation between degradation of desmin by proteolysis and drip loss has been demonstrated by Melody et al. (Citation2004) and Morrison et al. (Citation1998). These proteins degrade from 45 min to 6 h post-mortem. Degradation of these proteins produces the expulsion of water from the intramyofibrillar spaces and the retention of water in the intracellular space. So, reduced degradation of proteins such as desmin causes an increased shrinking of the muscle cell and, then, of drip loss. Lund et al. (Citation2007a) also described an increase in drip loss over chill storage time in pork slices packed under high O2 concentrations. Moreover, desmin degradation, played an important role in meat WHC as well as contributed to meat aging and tenderising during post-mortem storage (Huff-Lonergan et al., Citation1996; Zhang et al., Citation2006). In beef meat the calpain system (µ-calpain and m-calpain) is believed to play a central role during post-mortem tenderisation (Huff-Lonergan et al., Citation1996). Because calpains contain histidine, protein oxidation influenced their activities (Lametsch et al., Citation2007), degrading histidine and inactivating or inhibiting their protease activity (Harris et al., Citation2001; Rowe et al., Citation2004a, Citation2004b). In fact, in the current study, instrumental WBSF of cook ed meat, although there were some differences between the two gas mixtures, declined early during storage and then remained constant over time (). According to Lund et al. (Citation2007a) high oxygen atmosphere storage promoted a significant decrease in tenderness for beef and porcine meat, due to protein cross-linking from oxidative processes. These results coincide with consumer assessment on beef steaks packed in MAP with oxygen levels higher than 40%, imparting more toughness and less juiciness potentially due to protein oxidation (Zakrys et al., Citation2009). So, protein oxidation is considered to play a role in meat tenderness, because of its demonstrated action in controlling proteases (Starke-Reed and Oliver, Citation1989).
Protein oxidation increased during storage time both in O246 than in O270 samples (), showing values significantly greater in slices packed with higher oxygen compared to others since from the second day. Previous studies by Lund et al. (Citation2007a), Zakrys et al. (Citation2008, Citation2009), showed increase in protein oxidation with the growth of O2 concentration, resulting in lower tenderness and juiciness, flavour deterioration and discoloration (Xiong, Citation2000). Physical and chemical changes in oxidised meat protein include hydroperoxides formation.
Lipid oxidation, measured by TBARS, also increased during shelf-life and in relation to oxygen concentration. In fact, O270 slices had TBARS values significant greater than O246 since 6th day of storage. These results are supported by previous studies (O’Grady et al., Citation2000; Zakrys et al. Citation2008, Citation2009), which reported that oxidative stability decreased with storage time and treatments aimed to modify TBARS concentration may be useful only in the presence of low O2.
The positive correlation between storage time and lipid oxidation are confirmed by the increased levels of hydroperoxides formed by unsaturated fatty acids oxidation (McMillin, Citation2008), and TBARS, used as lipid oxidation measurement (Lund et al., Citation2007a). Despite veal calf meat usually presents low levels of myoglobin (Gariépy et al., Citation1998), it showed the same discoloration of bee f meat.
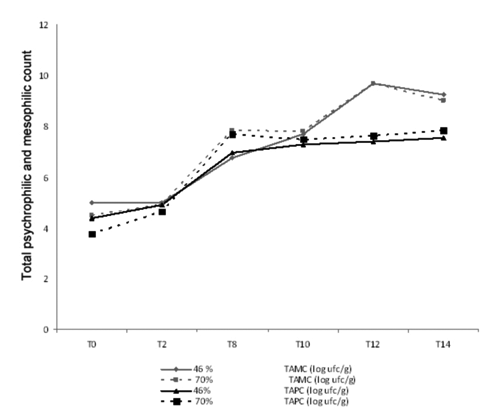
The two different atmosphere packaging did not affect total aerobic mesophilic and psychrophilic counts. In addition the results related to the TAMC and the TAPC () showed a significant increase in bacterial load over time with an increase of about 4 log cfu/g and 3 log cfu/g respectively in association with the appearance of unpleasant odors and slime surface. Moreover, at 8th day, the TAMC and the TAPC have reached values of about 7 log cfu/g, which is considered as the upper limit for acceptable microbiological quality meat as defined by ICMFS (Citation1986).
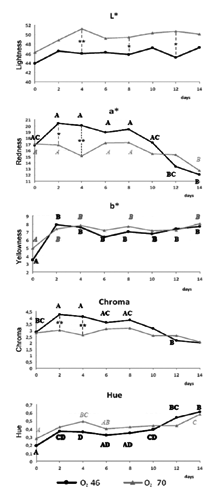
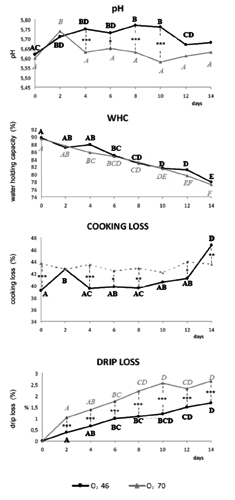
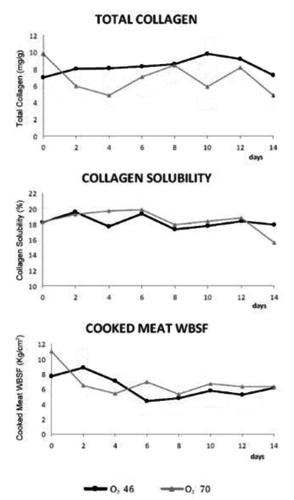
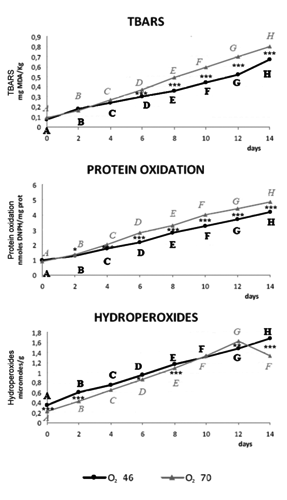
Table 1. Properties of film package.
Table 2. Influence of gas mixture on the main quality parameters of fresh veal calf meat through analyses of variance.
Conclusions
The results clearly showed that O270 atmosphere was responsible for an earlier decline in meat quality, evidenced by increased drip and cooking loss and higher protein and lipid oxidation. Particularly, protein oxidation, higher in O270 MAP, has proved critical in determining meat quality (colour, WHC, drip and cooking loss) and shelf-life. The mixture with the highest concentration of oxygen did not benefit colour, because of the particular chemical and colourimetric properties of veal calf meat. So, it seems more advantageous to use MAP gas mixtures with O2 concentration of 46 than 70%, in order to improve shelf-life and avoid an increase in oxidative reactions and physical changes related to them.
Acknowledgments
The research was granted by the University Aldo Moro, Bari, Italy. The Authors are grateful to Siciliani SpA and Giorgio Samoilis for their co-operation. The authors thank Dr. Giovanna Calzaretti for her support in laboratory work and Mr. Francesco D’Onghia for his support in field activities.
References
- AldaiN. LavínP. KramerJ.K.G. JarosoR. MantecónA.R., 2012. Breed effect on quality veal production in mountain areas: emphasis on meat fatty acid composition. Meat Sci. 92:687-696.
- BerrugaM.I. VergaraH. GallegoL., 2005. Influence of packaging conditions on microbial and lipid oxidation in lamb meat. Small Ruminant Res. 57:257-264.
- BertramH.C. AndersenH.J. KarlssonA.H. HornP. HedegaardJ. NørgaardL. EngelsenS.B., 2003. Prediction of technological quality (cooking loss and Napole Yield) of pork based on fresh meat characteristics. Meat Sci. 65:707-712.
- BoutonP.E. HarrisP.V. ShorthoseW.R., 1971. Effect of ultimate pH upon the water-holding capacity and tenderness of mutton. J. Food Sci. 36:435-439.
- BredahlL. GrunertK.G. FertinC., 1998. Relating consumer perceptions of pork quality to physical products characteristics. Food Qual. Prefer. 4:273-281.
- BuegeJ. AustS.D., 1978. Microsomial lipid peroxidation. Method Enzymol. 52:302-310.
- CIE, 1976. Colorimetry: official recommendations of the International Commission on Illumination. Comisión Internationale de l’Eclairage, Paris, France.
- De PaloP. MaggiolinoA. CentoducatiP. TateoA., 2013. Effects of two different packaging materials on veal calf meat quality and shelf life. J. Anim. Sci. 91:2920-2930.
- den Hertog-MeischkeM.J.A. Vada-KovácsM. SmuldersF.J.M., 1997. The effect of simulated transport of fresh meats on their water-holding capacity as assessed by various methods. Meat Sci. 46:1-8.
- EstevezM. CavaR., 2004. Lipid and protein oxidation, release of iron from heme molecule and colour deterioration during refrigerated storage of liver pate. Meat Sci. 68:551-558.
- FaustmanC. CassensR.G. SchaeferD.M. BuegeD.F. SchellerK.K., 1989. Vitamin E supplementation of Jolstein steer diets improves sirloin steak colour. J. Food Sci. 54:485-486.
- GariépyC. DelaquisP.J. PommierS. De PassilléA.M.B. FortinJ. LapierreH., 1998. Effect of calf feeding regimes and diet EDTA on physico-chemical characteristics of veal stored under modified atmospheres. Meat Sci. 48:101-115.
- HarriganW.F., 1998. Determination of the number, and detection, of viable microorganisms in a sample. In: HarriganW.F. ( ed.) Laboratory methods in food microbiology. Elsevier, Amsterdam, The Netherlands, pp 53-61.
- HarrisS.E. Huff-LonerganE. LonerganS.M. JonesW.R. RankinsD., 2001. Antioxidant status affects colour stability and tenderness of calcium-chloride injected beef. J. Anim. Sci. 79:666-677.
- Huff-LonerganE. LonerganS.M., 2005. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 71:194-204.
- Huff-LonerganE. MitsuhashiT. BeekmanD.D. ParrishF.C. OlsonD.G. RobsonR.M., 1996. Proteolysis of specific muscle structural proteins by µ-calpain at low pH and temperature is similar to degradation in post-mortem bovine muscle. J. Anim. Sci. 74:993-1008.
- ICMFS, 1986. Sampling for microbiological analysis: principles and scientific applications. Vol. 2. International Commission on Microbiological Specifications for Foods, University of Toronto Press, Toronto, Canada.
- JakobsenM. BertelsenG., 2000. Colour stability and lipid oxidation of fresh beef. Development of a response surface model for predicting the effects of temperature, storage time, and modified atmosphere composition. Meat Sci. 54:49-57.
- JayasinghP. CornforthD.P. BrennandC.P. CarpenterC.E. WhittierD.R., 2002. Sensory evaluation of ground beef stored in high-oxygen modified atmosphere packaging. J. Food Sci. 67:3493-3496.
- JohnL. CornforthD. CharlesE. SørheimO. PetteeB.C. WhittierD.R., 2005. Colour and thiobarbituric acid values of cooked top sirloin steaks packaged in modified atmospheres of 80% oxygen, or 0.4% carbon monoxide, or vacuum. Meat Sci. 69:441-449.
- KannerJ., 1994. Oxidative processes in meat and meat products: quality implications. Meat Sci. 36:169-189.
- LametschR. KnudsenJ.C. ErtbjergP. OksbjergN. TherkildsenM., 2007. Novel method for determination of myofibril fragmentation post mortem. Meat Sci. 75:719-724.
- LeeD.S. YamK.L. PiergiovanniL., 2008. Food packaging science and technology. CRC Press, Boca Raton, FL, USA, pp 79-108.
- LinT.S. HultinH.O., 1977. Oxydation of myoglobin in vitro mediated by lipid oxidation in microsomial fractions of muscle. J. Food Sci. 42:136-140.
- LittleA., 1975. Research note: off on a tangent. J. Food Sci. 40:410-411.
- LundM.N. HviidS.M. SkibstedH.L., 2007a. The combined effect of antioxidants and modified atmosphere packaging on protein and lipid oxidation in beef patties during chill storage. Meat Sci. 76:226-233.
- LundM.N. LametschR. HviidS.M. JensenO.N. SkibstedH.L., 2007b. High-oxygen packaging atmosphere influences protein oxidation and tenderness of porcine longissimus dorsi during chill storage. Meat Sci. 77:295-303.
- ManciniR.A. HuntM.C., 2005. Current research in meat colour. Meat Sci. 71:100-121.
- McMillinK.W., 2008. Where is MAP going? A review and future potential of modified atmosphere packaging for meat. Meat Sci. 80:43-65.
- MelodyJ.L. LonerganS.M. RoweL.J. HuiattT.W. MayesM.S. Huff-LonerganE., 2004. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 82:1195-1205.
- MercierY. GatellierP. RenerreM., 2004. Lipid and protein oxidation in vitro, and antioxidant potential in meat from Charolais cows finished on pasture or mixed diet. Meat Sci. 66:467-473.
- MonteiroA.C.G. GomesE. BarretoA.S. SilvaM.F. FontesM.A. BessaR.J.B. LemosJ.P.C., 2013. Eating quality of Vitela Tradicional do Montado-PGI veal and Mertolenga-PDO veal and beef. Meat Sci. 94:63-68.
- MorrisonE.H. MielcheM.M. PurslowP.P., 1998. Immunolocalisation of intermediate filament proteins in porcine meat. Fiber type and muscle-specific variations during conditioning. Meat Sci. 50:91-104.
- MortensenM. AndersenH.J. EngelsenS.B. BertramH.C., 2006. Effect of freezing temperature, thawing and cooking rate on water distribution in two pork qualities. Meat Sci. 72:34-42.
- O’GradyM.N. MonahanF.J. BurkeR.M. AllenP., 2000. The effect of oxygen level and exogenous α-tocopherol on the oxidative stability of minced beef in modified atmosphere packs. Meat Sci. 55:39-45.
- OfferG. TrinickJ., 1983. On the mechanism of water holding in meat: the swelling and shrinking of myofibrils. Meat Sci. 8:245-281.
- RheeK.I. ZiprinY.A., 1987. Lipid oxidation in retail beef, pork and chicken muscles as affected by concentrations of heme pigments and non-heme iron and microsomal enzymic lipid peroxidation activity. J. Food Biochem. 11:1-15.
- RoweL.J. MaddockK.R. LonerganS.M. Huff-LonerganE., 2004a. Influence of early post-mortem protein oxidation on beef quality. J. Anim. Sci. 82:785-793.
- RoweL.J. MaddockK.R. LonerganS.M. Huff-LonerganE., 2004b. Oxidative environments decrease tenderization of beef steaks through inactivation of µ-calpain. J. Anim. Sci. 82:3254-3266.
- SAS, 1999. The SAS system for Windows, velease 8.01. SAS Institute Inc., Cary, NC, USA.
- SierraV. CaballeroB. MochaM. MartinezM.J. OsorolK. TolivaD., 2006. Relationship between consumer scores and oxidation status of beef. pp 581-582 in Proc. 52nd Int. Congr. Meat Sci. Technol., Dublin, Ireland.
- SørheimO. NissenH. NesbakkenT., 1999. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Sci. 52:157-164.
- Starke-ReedP.E. OliverC.N., 1989. Protein oxidation and proteolysis during ageing and oxidative stress. Arch. Biochem. Biophys. 275:559-567.
- TangJ. Cameron FaustmanC. RichardA. ManciniR.A. SeyfertM. HuntM.C., 2006. The effects of freeze-thaw and sonication on mitochondrial oxygen consumption, electron transport chain-linked metmyoglobin reduction, lipid oxidation, and oxymyoglobin oxidation. Meat Sci. 74:510-515.
- TokurB. KorkmazK., 2007. The effects of an iron-catalyzed oxidation systems on lipids and proteins of dark muscle fish. Food Chem. 104:754-760.
- VieiraC. GarciaM.D. CardeñoA. ManteconA.R., 2005. Effect of diet composition and slaughter weight on animal performance, carcass and meat quality, and fatty acid composition in veal calves. Livest. Prod. Sci. 93:263-275.
- XiongY.L., 2000. Protein oxidation and implications for muscle food quality. In: DeckerE. FaustmanC. ( eds.) Antioxidants in muscle foods. John Wiley and Sons, Chichester, UK, pp 85-111.
- YamK.L. TakhistovP.T. MiltzJ., 2005. Intelligent packaging: concepts and applications. J. Food Sci. 70:R1-R10.
- YuL.H. LeeE.S. JeongJ.Y. PaikH.D. ChoiJ.H. KimC.J., 2005. Effects of thawing temperature on the physicochemical properties of pre-rigor frozen chicken breast and leg muscles. Meat Sci. 71:375-382.
- ZakrysP.I. HoganS.I. O’SullivanM.G. AllenandP. KerryJ.P., 2008. Effects of oxygen concentration on the sensory evaluation and quality indicators of beef muscle packed under modified atmosphere. Meat Sci. 19:648-655.
- ZakrysP.I. O’SullivanM.G. AllenP. KerryJ.P., 2009. Consumer acceptability and physiochemical characteristics of modified atmosphere packed beef steaks. Meat Sci. 81:720-725.
- ZhangW.G. LonerganS.M. GardnerM.A. Huff-LonerganE., 2006. Contribution of postmortem changes of integrin, desmin and μ-calpain to variation in water holding capacity of pork. Meat Sci. 74:578-585.