Abstract
This study was performed to investigate changes in growth performance, immune organs weight and microstructure of broilers fed different levels of boron (0, 100, 200 and 400 mg/L) in drinking water for 6 weeks. A total of 240 newly-hatched Gushi chickens [body weight (BW)=34±2 kg; male: female=1:1] were equally divided into 4 treatments with 5 pens each (n=12 per pen). The birds were weighed after fasting for 12 h every two weeks until the end of experiment. The thymus, bursa and spleen samples were collected every two weeks and fixed in 4% paraformaldehyde phosphate buffer at room temperature (25°C). Results showed that BW and average daily gain significantly reduced by addition of 200 and 400 mg/L boron to drinking water compared to control group (P<0.05 or <0.01). Compared to controls, the thymus index of broilers fed 100 mg/L boron increased significantly from 4th to 6th week of age (P<0.05), whereas the weight and immune organs index of broilers fed 400 mg/L boron decreased significantly every week (P<0.05 or <0.01). Microscopic observation showed that the structure of immune organs of broilers fed 100 mg/L boron was well developed from 4th to 6th week of age, and that of broilers fed 200 and 400 mg/L boron was damaged every week. The present study suggests that appropriate supplements of boron (<100 mg/L) could improve the growth of immune organs in broilers after 4 weeks. However, high doses of boron (>200 mg/L) could significantly inhibit the growth of immune organs and even exhibit toxic effects.
Introduction
Boron, the fifth element in the periodic table, is the only nonmetal in the group IIIA elements, but contains characteristics between those of metals and nonmetals (Devirian and Volpe, Citation2003). Boron is ubiquitous in nature, and it is commonly found in soils, rocks, and surface and ocean waters in the form of boric acid and inorganic borates (Vaziri et al., Citation2001). It was accepted in 1923 as an essential nutrient for all vascular plants (Warrington, Citation1923). Since then, the role of boron has been extensively studied in plants. Some data showed that the predominant place of boron function in plants is in the primary cell walls where it cross-links the pectic polysaccharide rhamnogalacturonan-II (RG-II). The RG-IIs are small, structurally complex polysaccharides that represent an extreme example of the evolutionary conservation of wall polysaccharide structure (O’Neill et al., Citation2004). A borate transporter has been described to be essential for cellular boron uptake and cell growth in mammalian cells (Park et al., Citation2004). Evidence has been accumulating that boron may be required by humans and animals (Devirian and Volpe, Citation2003). For example, it has a significant effect on certain metabolic processes, such as coronary heart disease, arthritis and osteoporosis (Naghii and Samman, Citation1997), and affect metabolic parameters of animals such as chicken, swine and rat (Armstrong et al., Citation2000, Citation2001; Fort, Citation2002; Wallace et al., Citation2002; Bakken and Hunt, Citation2003; Devirian and Volpe, Citation2003; Hunt, Citation2003; Huel et al., Citation2004). Boron is also known to interact with calcium, vitamin D and magnesium, which are all important in bone metabolism. However, boron can be toxic in high doses. Nielsen (Citation1991) speculated that boron has such a role in cell membrane function or stability that it influences the response to hormone action, transmembrane signalling or transmembrane movement of regulatory cations or anions (Nielsen, Citation1991). Testicular atrophy and inhibited spermiation has been observed in rats exposed to high doses of boron (Chapin and Ku, Citation1994). Addition of 400 mg/L boron in drinking water has been shown to inhibit the development of intestinal villi in Gushi chickens (Wang et al., Citation2005). Although the above studies showed that high doses of boron can cause the damage of reproductive and digestive organs, appropriate supplementation of boron in the diet would produce positive effects on the physiological functions of animal. Current research confirmed that appropriate addition of boron enhances Fc-receptor expression and interleukin-6 production in cultured mammalian macrophages. It has been indicated that addition of boron can affect the production of cytokines by increasing the production of tumor necrosis factor-a and interferon-γ after a stress or disease challenge in pig (Armstrong and Spears, Citation2003). In addition, appropriate addition of boron can also improve performance and immune function of broilers, and promote bone development of ostrich chick (Hunt, Citation2003; Cheng et al., Citation2011). Gushi breed of chicken is one of the major chicken breeds found in Henan province, China. It has characteristics of roughage resistance, high level of egg production, tender meat, and genetic stability. Given the positive effect of boron, we expect important effects in Gushi chickens as well.
The object of this trial was to study the effects of different doses of boron in drinking water on the growth performance, weight and microstructure of immune organs in Gushi chickens.
Materials and methods
Animals and experimental design
The research were conducted at the Anhui Science and Technology University, Anhui, China (longitude 117°21, latitude 32°56, and altitude 17 m) during the spring (April and May) of 2013 and lasted for 42 d.
A total of 240 newly hatched Gushi chickens were obtained from the Laboratory Animal Breeding Center of Anhui Science and Technology University, and raised in the Center for Laboratory Animal of the same university. All procedures were carried out in accordance with the guidelines of Animal Care and Use Committee of Anhui province. The birds were raised and fed a basal ration () according to the National Research Council recommendations of the United States. The temperature was maintained at 20 to 28°C controlled by air-conditioning with 20:4 (light:dark) cycle, and the relative humidity was maintained at 60 to 70 pa vapour pressure/100 pa saturation vapour pressure. Food (basal ration) and drinking water were made available ad libitum. Vaccination against Newcastle disease and infectious bursal disease was performed at days 7 and 14, respectively. Birds were individually identified and weighted using numbered leg bands and divided into control (C) group and three treatment groups (T1, T2, T3) with five replicates (12 birds/replicate) per group (n=60). The chickens in C were given water with no boron addition for 6 weeks. The chickens in T1, T2, T3 respectively drank water supplemented with 100, 200 and 400 boron mg/L for 6 weeks, in the form of boron acid (Sinopharm Chemical Reagent Co. Ltd., Shanghai, China; purity>99.5 g boron acid/g, boron content>17.4 g boron/100 g boron acid).
Sample collection and preparation
Twenty chickens from each group (4 birds each replicate) were weighed and sacrificed by bloodletting through the carotid artery after injecting 100 g urethane/L at 2nd, 4th and 6th weeks, respectively. The thymus, bursa and spleen samples were dissected immediately. Then the samples were weighed on an electronic analytical balance and fixed in 40 g paraformaldehyde/L at room temperature for 24 h.
Observation of microstructure
After fixation, the samples were dehydrated by graded ethanol treatment, cleared in xylene, paraffin embedded, and serially sectioned (6 µm) using rotary microtome. Every 30th section was stained with hematoxylin and eosin and sealed with neutral gum. At least 10 sections per immune organ and a total of 100 sections per treatment groups were used to microscopic observation and measurement by light microscope and Olympus DP73 microscopic image analysis system (including DP controller and Image-Pro Express; Olympus, Tokyo, Japan).
Statistical analysis
Indexes of immune organs were counted by the formula: [organ weight/body weight (BW)]×100%, according to Liu et al. (Citation2006). At least 50 fields in ten sections of each immune organ were photographed using a ×20 objective with microscope to analyse the change of microstructure following the methods of Liu et al. (Citation2010). All experimental data were analysed using SPSS version 17 software (SPSS Inc., Chicago, IL, USA). The homogeneity of data variances was analysed by ANOVA using general linear model (GLM) procedure and normality of data distribution was tested by the Kolmogorov-Smirnov test. Descriptive statistical indicators were determined (mean and standard error) for assessed the measure of the BW and average daily gain (ADG). The statistical significance of means in this study was compared by the Dennett’s test. The difference between control and treatment groups was confirmed with the level of significance set at P<0.05 and the level of extreme significance set at P<0.01.
Results and discussion
Effects of boron supplemented in drinking water on animal growth performance
The effects of boron supplemented in drinking water on the BW and ADG of broilers are shown in . The BW of broilers fed 100 mg boron/L in drinking water was lower than that of chickens in C at week 2 (P<0.05), but the ADG did not differ significantly (P>0.05). However, the BW and the ADG of broilers fed 100 mg boron/L in drinking water increased obviously compared to C at week 6, and the difference in the ADG was significant (P<0.05). During the experimental phase, high doses of boron (200 and 400 mg boron/L in drinking water) caused significant reduction (P<0.05 or P <0.01) in the BW and ADG values, compared to C. These results showed that low-dose boron could improve the growth in the later stage, but high-dose boron could inhibit the growth in broilers.
Effects of boron on the weight and index of immune organs
The effects of boron supplemented in drinking water on the immune organs weight and index are illustrated in and . The weight () and index () of bursa and spleen in the broilers fed 100 mg boron/L in drinking water from 2nd to 6th week of age were not different from C (P>0.05), but the weight and index of thymus from 4th to 6th week of age increased significantly (P<0.05) ().The spleen weight from week 2 to 6 and the spleen index from week 4 to 6 in broilers fed 200 mg boron/L in drinking water was significant lower than that of the broilers in C (P<0.05) ( and ). In addition, the immune organs weight and index of the broilers consuming 400 mg boron/L in drinking water throughout the experiment decreased significantly (P<0.05) ( and ), indicating that exposure of high-dose boron could inhibit the immune function in broilers.
Effects of boron on microstructure of the thymus
As shown in , the thymic cortex areas and the thymus cortex-medulla ratio of broilers fed 100 mg boron/L in drinking water from 2nd to 4th week of age were not different from C (P>0.05), but the thymic cortex areas increased significantly at week 6 (P<0.05). The thymic cortex areas at week 2 and the thymus cortex-medulla ratio from week 2 to 4 in broilers fed 200 mg boron/L in drinking water were significantly lower than that of the broilers in C (P<0.05), but the thymic cortex areas of the 4-to-6-week-old broilers and the thymus cortex-medulla ratio of the 6-week-old broilers were not significantly different from C (P>0.05). The thymic cortex areas and the thymus cortex-medulla ratio of the broilers consuming 400 mg boron/L in drinking water throughout the experiment decreased significantly (P<0.05 or P<0.01) ( and ), indicating that exposure of high-dose boron could inhibit the thymus development in broilers. Compared to the thymus microstructure () in broilers of C of the same age, the thymus of the birds fed 100 mg boron/L in drinking water showed a slight damage at 2nd week of age (), but the thymus started to rapidly develop at week 4 (), the number of lymphocytes within the thymic parenchyma increased obviously, and the thymic lobules became enlarged gradually. The structure of the thymus developed well by 6th week of age (). The damage of thymus in the broilers fed 200 mg boron/L in drinking water from 2nd to 4th week were more obvious compared to the broilers fed 100 mg boron/L (,).
The thymus in the broilers fed 400 mg boron/L in drinking water revealed severe degenerative appearance, compared to C and the other treatment groups, especially at week 2. The thymic interlobular septum became obviously thick, the thymus parenchyma revealed obvious atrophy and rapid degeneration due to the reduction and exhaustion of lymphocytes in the thymic lobules (). In the medulla of the thymus, the epithelial-reticular cells and the Hassall’s corpuscles increased, and the centre of some degenerated corpuscles presented the keratinous fragments or the homogenous and transparent changes at week 2 (). The skeletal muscle which is observed in the edge of thymic lobul (), presented obvious cross-striations with some unknown cells (). The thymus in the birds consuming 400 mg boron/L in drinking water still showed degeneration at week 4 (), but it showed slight recovery () up to week 6.
Effects of boron on microstructure of the bursa
As is shown in , the bursal nodule areas and the number of bursal nodule of broilers fed with 100 mg boron/L in drinking water from 2nd to 6th week of age were not different from the control group (P>0.05). The bursal nodule areas from 2nd to 4th week of age and the number of bursal nodule in 2-week of age broilers fed with 200 mg boron/L in drinking water were significantly lower than that of the broilers in the control group (P<0.05), but that of the 6-week -old broilers were not different from the control group (P>0.05). The bursal nodule areas from 2nd to 6th week of age and the number of bursal nodule from 4th to 6th week of age broilers consuming 400 mg boron/L in drinking water were decreased significantly throughout the experiment (P<0.05 or <0.01). However, the number of bursal nodule in 2-week of age broilers fed with 200 and 400 mg boron/L in drinking water were significantly higher than that of the broilers in the control group (P<0.05).
The structure of the bursa in broilers from weeks 2 to 6 were normal and did not show pathological changes in the control group (,,). Compared to the control group of the same week of age, the bursa of the broilers fed with 100 mg boron/L in drinking water from 2nd to 6th week of age were not different. The damage of bursa in the broiler fed with 200 mg boron/L in drinking water was obvious (), the areas of bursal nodules obviously decreased, and the number of bursal nodule was increased at first and then decreased from 2nd to 6th week of age, and the number of lymphocytes was decreased in the bursal nodules. But the structure of the bursa in the birds slowly recovered from weeks 4 to 6 ( and ). The damage of the bursa in the broiler fed with 400 mg boron/L in drinking water was more severe, and the plicae of bursa in birds were very narrow (). The bursal nodule areas was obviously decreased (), but the number of bursal nodule was obviously increased in the 2-week of age broilers. The cortical region of bursal nodules became very thin and the ratio of the medulla mainly consisting of epithelial-reticular cells increased in the 4-week of age broilers (). The number of lymphocytes in the bursal nodules, especially in the medulla, decreased rapidly (). In addition, the degenerated bursal nodules vestiges consisting of columnar epithelial cells were observed in the bursa of fabricius during the later experimental phase (), and a vacuolar structure was seen in the medulla of some bursal nodules in the 6-week of age broilers ().
Effects of boron on microstructure of the spleen
As shown in , the areas of splenic nodule and periarterial lymphatic sheath in the broilers fed 100 mg boron/L in drinking water were not different from C from week 2 to 6 (P>0.05). The areas of splenic nodule and periarterial lymphatic sheath in the broilers fed 200 mg boron/L in drinking water from 2nd to 6th week of age were significantly lower than that of the broilers in C, except the areas of periarterial lymphatic sheath in the 6-week-old broilers (P<0.05). The areas of splenic nodule and periarterial lymphatic sheath in the broilers consuming 400 mg boron/L in drinking water throughout the experiment decreased significantly compared to C (P<0.05 or <0.01), indicating that exposure of high-dose boron also could inhibit the spleen development in broilers The spleen of broilers in C from week 1 to 6 showed well development (,,,,). The changes in the histological structure of spleen in experimental broilers exhibited obvious correlation with the supplemental doses of boron, compared to the broilers in C. The microstructure of the spleen in the broilers fed 200 mg boron/L in drinking water showed a slight inhibition at week 2 (), but the spleen development was seen from week 4 to 6. The development of the spleen in the broilers fed 100 mg boron/L in drinking water showed an obvious inhibition from week 2 to 4 (,,). The areas of splenic nodules were obviously small and the germinal centres of splenic nodules were difficult to observe (). The periarterial lymphatic sheaths became thinner and their lymphocytes were loosened (). However, at 6th week of age, the microstructure of the spleen in the broilers fed 200 mg boron/L was obviously improved (,). The spleen of broilers fed 400 mg boron/L in drinking water showed severe hypoplasia from week 2 to 6. The splenic nodules which had no germinal centres obviously decreased in the number and the areas from week 2 to 4 (,). The periarterial lymphatic sheaths became very thin (). Up to 6th week of age, the development of the spleen was still inferior to that of the broilers in C and other treatment groups to some degree (,).
Effects of boron supplemented in drinking water on the development of immune organs in broiler
The immune organs in birds include the thymus, spleen and bursa. The thymus mainly cultivates T lymphocytes (T cells) and mediates cellular immunity. The bursa mainly cultivates B lymphocytes (B cells) and mediates humoral immunity. The spleen is a peripheral immune organ, which mainly filters blood and generates an immune response. Boron plays a role in regulating enzymatic activity in pathways involved in energy substrate metabolism, insulin release and the immune system. It modifies insulin release by altering the metabolism of nicotinamide adenine dinucleotide phosphate, and may affect glucose, lipid and protein metabolism (Armstrong et al., Citation2001; Devirian and Volpe, Citation2003; Hunt, Citation2003). Boron could also decrease plasma aspartate transaminase activity. At physiological amounts, boron has been shown to increase serum antibody in broilers and rats (Hunt, Citation1994; Bai and Hunt, Citation1996). Our results showed that the supplementation of 100 mg/L boron in drinking water slightly inhibited the growth of broilers and the development of immune organs in 2-week-old broilers. Supplementation of 100 mg/L boron in drinking water also slightly inhibited the proliferation and differentiation of T and B cells in immune organs in the early growth, but produced the positive effect on the broilers from week 4 to 6 compared with C, which might be due to the effect of boron on the metabolism of protein, glucose and lipids by regulating enzymatic activity (Li et al., Citation2013), further influence on T and B cells proliferation and the development of immune organs. Previous studies had found that boron at the physiological dosage was a regulator of energy metabolism, and may have a role as a cofactor for some enzymatic reactions (Hunt et al., Citation1994; Devirian and Volpe, Citation2003; Hunt, Citation2003).
Toxic effects of high-dose boron on the development of immune organs in animal experiments had showed that boric acid did not cause any gene mutations, but had obvious toxicity (Fail et al., Citation1998; Armstrong et al., Citation2001). For example, the T cell activity has been shown to be inhibited in rats fed boron-rich diet (Hunt, Citation2003). A significant decline in the reproductive performance and feed intake have been detected in boron toxicity (Kloppmann et al., Citation2005; Geyikoglu and Turkez, Citation2008). Testicular atrophy and inhibition of spermiation in rats has been reported with boron poisoning (Scialli et al., Citation2010; Duydu et al., Citation2011). Shortening of the villi and reduced depth of the cryptae in the jejunum of broilers have also been reported with boron poisoning (Wang et al., Citation2005). In the present study, the supplementation of 200 mg/L boron in drinking water caused obvious inhibition of growth performance and development of immune organs in the broiler with decrease of BW, ADG, weight and organs index of immune organs from week 2 to 4. The supplementation of 400 mg/L boron in drinking water produced obvious toxic effects, and showed more significant inhibition of growth performance and development of immune organs. The depletion of the lymphocytes caused atrophy and degeneration of the immune organs in 2-to-6-week-old broilers fed 400 mg boron/L. The above results indicate that high-dose boron significantly inhibited the development of immune organs and the proliferation of lymphocytes in the broilers. Possible reasons include: i) the toxic effects of boron on the central nervous system directly or indirectly impacted the immune organs and lymphocytes (Ku and Chapin, Citation1994); ii) the regulatory role of boron in the metabolism and respiratory burst mechanism impacted the immune organs and lymphocytes (Devirian and Volpe, Citation2003).
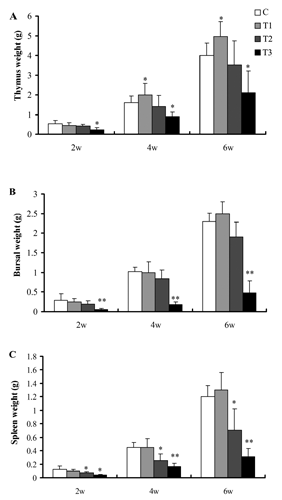
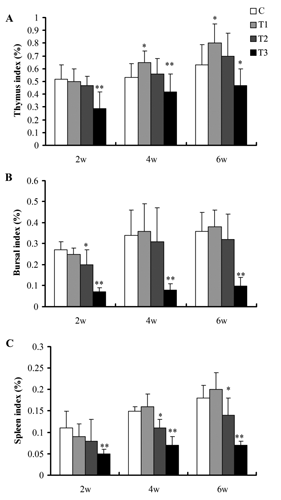
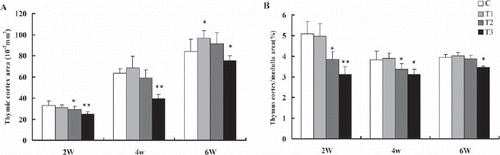
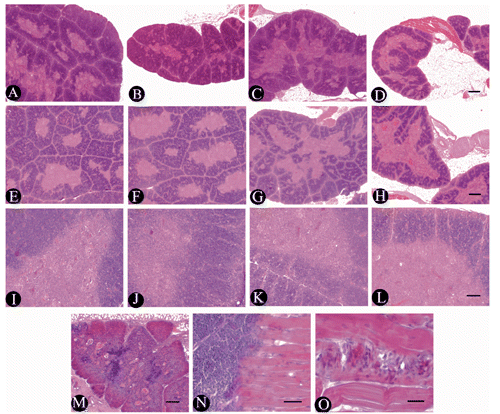
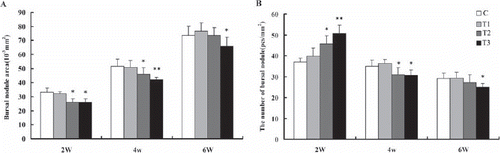
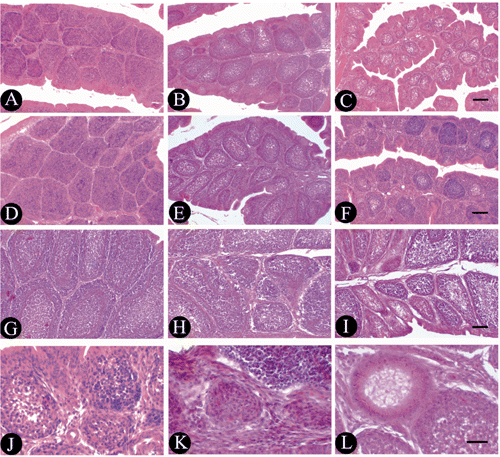
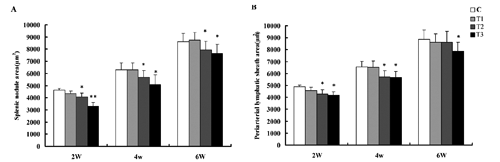
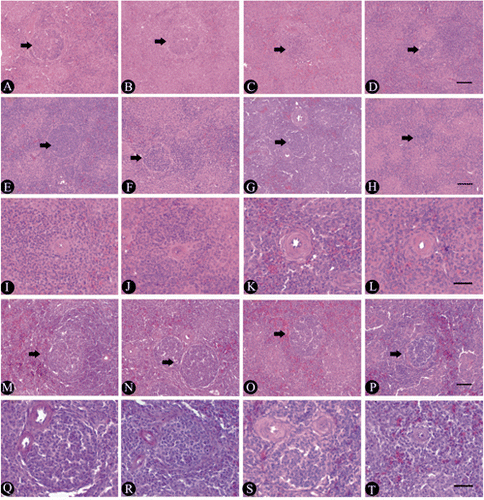
Table 1. Ingredients of the basal diet and its nutrition levels.
Table 2. Effects of boron supplemented in drinking water on body weight and average daily gain of broilers.
Effects of boron supplemented in drinking water on immune function of broilers
After removing the thymus in rats, a significant decrease of lymphocytes in the blood has been demonstrated causing the lack of the lymphocytes in thymus-dependent regions of lymph nodes and spleen, and reduced antibody-producing capability. Removal of the thymus in broilers has been shown to be associated with decreased number of lymphocytes in the blood and inhibition of the immune response. Moreover, removal of the bursa in 17-day-old chick embryo has been shown to be associated with loss of lymphocytes and reduced antibody-producing capability after antigenic stimulation in hatched chicks. These studies showed that the early development of central immune organs is very important to the peripheral lymphoid organs and immune function. In the present study, adding 100 mg boron/L in drinking water did not impact the structure of immune organs, and produced positive effects on the development of immune organs in 4-to-6-week-old broilers, indicating that appropriate addition of boron could obviously improve the cellular and humoral immune response and immune function in late developmental stage. However, adding 200 mg boron/L in drinking water caused definitive inhibition in the development of central and peripheral immune organs, and the inhibition gradually became significant with increasing boron dose. When 400 mg boron/L were added in drinking water, the thymus and bursa in the broiler from 2nd to 6th week of age degenerated sharply, and the areas of thymus-dependent region of the spleen and splenic nodules were decreased. Although restoration appeared in the structure of central immune organs in the 6-week-old broilers, the development of the immune organs were not still imperfect compared with C, indicating that toxic dose of boron could significantly inhibit the development of the central and peripheral immune organs for a long time.
Conclusions
The present study showed the role of boron on growth performance and development of immune organs in broilers at different dose levels. The supplementation of 100 mg boron/L in drinking water had positive effect on the growth performance and the development and microstructure of the immune organs in 4-to-6-week-old broilers. The supplementation of high-dose boron (>200 mg/L) obviously inhibited the growth of broilers and damaged the microstructure of immune organs. The toxic effects of boron on the immune organs obviously correlated with addition of boron. The results indicated that appropriate addition of boron (<100 mg/L) in drinking water could improve the immune function in the broiler in the later growth, while the high-dose boron could inhibited the immune function.
Acknowledgments
This work was supported by Key Projects of Scientific Research of Education Ministry, China (210095); Natural Science fund of Anhui, China (1308085MC50,1208085QC68); Key Discipline Construction Program of Anhui Science and Technology University, China (AKXK20101-2); Provincial Natural Science Research Projects of Colleges and Universities, Anhui, China (KJ2012B057); Talent Fund of Anhui Science and Technology University, China (ZRC2013354).
References
- ArmstrongT.A. SpearsJ.W., 2003. Effects of boron supplementation of pig diets on the production of tumor necrosis factor-a and interferon-γ. J. Anim. Sci. 81:2552-2561.
- ArmstrongT.A. SpearsJ.W. CrenshawT.D. NielsenF.H., 2000. Boron supplementation of a semipurified diet for weanling pigs improve feed efficiency and bone strength characteristics and alters plasma lipid metabolites. J. Nutr. 139:2575-2581.
- ArmstrongT.A. SpearsJ.W. LloydK.E., 2001. Inflammatory response, growth, and thyroid hormone concentrations are affected by long-term boron supplementation in gilts. J. Anim. Sci. 79:1549-1556.
- BaiY. HuntC.D., 1996. Dietary boron (B) increases serum antibody concentrations in rats immunized with heat-killed mycobacterium tuberculosis (MT). FASEB J. 10:A819 (abstr.).
- BakkenN.A. HuntC.D., 2003. Dietary boron decreases peak pancreatic in situ insulin release in chicks and plasma insulin concentrations in rats regardless of vitamin D or magnesium status. J. Nutr. 133:3577-3583.
- ChapinR.E. KuW.W., 1994. The reproductive toxicity of boric acid. Environ. Health Persp. 102:87-91.
- ChengJ.Y. PengK.M. JinE.H. ZhangY. LiuY. ZhangN.B. SongH. LiuH.Z. TangZ.L., 2011. Effect of additional boron on tibias of African ostrich chicks. Biol. Trace Elem. Res. 144:538-549.
- DevirianT.A. VolpeS.L., 2003. The physiological effects of dietary boron. Crit. Rev. Food Sci. 43:219-231.
- DuyduY. BasaranN. UstundağA. AydinS. UndeğerU. AtamanO.Y. AydosK. DukerY. IckstadtK. WaltrupB.S. GolkaK. BoltH.M., 2011. Reproductive toxicity parameters and biological monitoring in occupationally and environmentally boron-exposed persons in Bandirma, Turkey. Arch. Toxicol. 85:589-600.
- FailP.A. ChapinR.E. PriceC.J. HeindelJ.J., 1998. General, reproductive, developmental, and endocrine toxicity of Boronated compounds. Reprod. Toxicol. 12:1-18.
- FortD.J., 2002. Boron deficiency disables Xenopus laevis oocyte maturation events. Biol. Trace Elem. Res. 85:157-169.
- GeyikogluF. TurkezH., 2008. Boron compounds reduce vanadium tetraoxide genotoxicity in human lymphocytes. Environ. Toxicol. Phar. 26:342-347.
- HuelG. YazbeckC. BurnelD. MissyP. KloppmannW., 2004. Environmental boron exposure and activity of delta-aminolevulinic acid dehydratase (ALA-D) in a newborn population. Toxicol. Sci. 80:304-309.
- HuntC.D., 1994. The biochemical of physiologic amounts of dietary boron in animal nutrition models. Environ. Health Persp. 102:35-43.
- HuntC.D., 2003. Dietary boron: an overview of the evidence for its role in immune function. J. Trace Elem. Exp. Med. 16:291-306.
- HuntC.D. HerbelJ.L. IdsoJ.P., 1994. Dietary boron modifies the effects of vitamin D3 nutrition on indices of energy substrate utilization and mineral metabolism in the chick. J. Bone Miner. Res. 9:171-182.
- KloppmannW. BianchiniG. CharalambidesA. DotsikaE. GuerrotC. KloseP. MareiA. PennisiM. VengoshA. VoutsaD., 2005. Boron contamination of Mediterranean groundwater resources: extent, sources and pathways elucidated by environmental isotopes. Geophys. Res. Abstr. 7:10162 (abstr.).
- KuW.W. ChapinR.E., 1994. Mechanism of the testicular toxicity of boric acid in rats: in vivo and in vitro studies. Environ. Health Persp. 102:99-105.
- LiS.H. LiW.C. ZhouJ.X. JinG.M. GuY.F. ChenH.L. ZhaoJ. HuangJ., 2013. Effects of drinking boron on the growth and the biochemical indexes and antioxidant ability in serum of rats. Chin. J. Vet. Sci. 3:486-492.
- LiuS.J. LuoX. LiD.X. ZhangJ. QiuD.L. LiuW. LiangS. YangZ.R., 2006. Tumor inhibition and improved immunity in mice treated with flavone from Cirsium japonicum DC. Int. Immunopharmacol. 6:1387-1393.
- LiuW.J. WangZ.X. ChenY.X., 2010. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period. Anat. Rec. 293:1315-1324.
- NaghiiM.R. SammanS., 1997. The effect of boron supplementation on its urinary excretion and selected cardiovascular risk factors in healthy male subjects. Biol. Trace Elem. Res. 56:273-286.
- NielsenF.H., 1991. Nutritional requirements for boron, silicon, vanadium, nickel, and arsenic: current knowledge and speculation. FASEB J. 5:2661-2667.
- O’NeillM.A. IshiiT. AlbersheimP. DarvillA.G., 2004. Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide. Annu. Rev. Plant. Biol. 55:109-139.
- ParkM. LiQ. ShcheynikovN. ZengW. MuallemS., 2004. NaBC1 is a ubiquitous electrogenic Na+ -coupled borate transporter essential for cellular boron home-ostasis and cell growth and proliferation. Mol. Cell. 16:331-341.
- ScialliA.R. BondeJ.P. Bruske-HohlfeldI. CulverB.D. LiY. SullivanF.M., 2010. An overview of male reproductive studies of boron with an emphasis on studies of highly exposed Chinese workers. Reprod. Toxicol. 29:10-24.
- VaziriN.D. OveisiF. CulverB.D. PahlM.V. AndersenM.E. StrongP.L. MurrayF.J., 2001. The effect of pregnancy on renal clearance of boron in rats given boric acid orally. Toxicol. Sci. 60:257-263.
- WallaceJ.M. Hannon-FletcherM.P. RobsonP.J. GilmoreW.S. HubbardS.A. StrainJ.J., 2002. Boron supplementation and activated factor in healthy men. Eur. J. Clin. Nutr. 56:1102-1107.
- WangJ. GuY.F. LiS.H. ShangC.F. ChenH.L., 2005. Effect of boron toxicosis on jejunum development in broilers. Chin. J. Vet. Sci. 25:514-517.
- WarringtonK., 1923. The effect of boric acid and borax on the broad bean and certain other plants. Ann. Bot.-London 37:629-667.
