Abstract
The present study aims to investigate the effects of dietary Pantoea-6® (extract of fermented wheat flour with Pantoea agglomerans) and plant extracts (red clover and garlic) on eggshell quality and structure and intestinal histology. Sixty-six Boris Brown laying hens (30 weeks old) were allotted to 3 groups, each with eleven replicates of two chickens. The control group was fed a basal diet (18% crude protein, 2850 kcal/kg ME) and the other groups were fed the basal diet supplemented with 0.1% Pantoea-6® (including 0.06 g/kg lipopolysaccharide) and 0.1% plant extracts, respectively. There were no significant differences in laying performance and egg quality. However, these adverse effects occurred in the egg and albumen weight and eggshell breaking strength of the Pantoea-6® and plant extracts groups (P<0.05). Shell weight of the Pantoea-6® group was significantly higher than the other groups (P<0.05). Compared with the control, eggshell structure tended to have greater thickness in both dietary Pantoea-6® and plant extracts groups. The duodenum and jejunum of both Pantoea-6® and plant extracts groups showed higher values for cell area than those of the control (P<0.05). Moreover, cells on the villus tip surface were protuberated in both dietary Pantoea-6® and plant extracts groups, resulting in a rough surface. This study shows that Pantoea-6® and plant extracts at a 0.1% level might have a beneficial effect on egg and albumen weight, eggshell quality and structure parameters, as well as on small intestine histological parameters.
Introduction
In the egg industry, it is estimated that 8 to 11% of the eggs produced are lost due to damaged eggshell quality problems (Dunn et al., Citation2005). Thus, eggshell provides protection for the contents, influences the economic profitability of egg production, impacts on the containers used for marketing the eggs and provides a unique package for a valuable food (Hunton, Citation2005). Hen eggshell consists of ceramic materials constituted by a three-layered structure, namely, cuticle on the outer surface, a spongy (calcareous) layer and an inner lamellar (or mammillary) layer (Stadelman, Citation2000). The outer surface of the eggshell is covered with a mucin protein that acts as a soluble plug for the pores in the shell. The cuticle is also permeable to gas transmission (Tsai et al., Citation2006). The spongy and the inner lamellar layers form a matrix composed of protein fibres bonded to calcite (calcium carbonate) crystals. The two layers are also constructed in such a manner that there are numerous circular openings (pores). This structure permits gaseous exchange throughout the shell. The chemical composition of eggshell has been reported as: calcium carbonate (94%), magnesium carbonate (1%), calcium phosphate (1%) and organic matter (4%) (Stadelman, Citation2000; Hunton, Citation2005). These chemical elements from the hen’s diet must be broken down in the digestive system and then re-synthesised in the shell gland to form the eggshell. Dietary supplementation with natural substances, e.g. garlic, has been demonstrated to improve eggshell and egg quality (Qatramiz, Citation2006). Supplementation with black cumin (Nigella sativa L.) can increase the shell strength of eggs (Aydin et al., Citation2008). Radwan et al. (Citation2008) indicated that feeding dietary Curcuma Longa to laying hens could increase shell weight and thickness because it could improve the micro environment in the uterus which was the site of calcium deposition. Furthermore, the active substance was lipopolysaccharide (LPS), which is derived from the cell walls of Pantoea agglomerans, gramnegative bacteria that grow symbiotically with wheat (Kohchi et al., Citation2006). Suzuki et al. (Citation1992) reported that feeding dietary LPS enhanced eggshell strength. Gastrointestinal tract development and health are the key to productivity in all farm animals and poultry (Abdullah et al., Citation2010). The digestive functions could be considered the most limiting factors in production. Meimandipour et al. (Citation2010) and Yamauchi et al. (Citation2010) reported that small intestinal histology is markedly affected by dietary components and the histological changes in the small intestine correlate with small intestinal function. Thus, it is also possible that the presence of extraction of fermented wheat flour with Pantoea agglomerans and herb mixture could alter the intestinal histology and quality of eggshell. The purpose of this study was to evaluate the effect of adding Pantoea-6® (extract of fermented wheat flour with Pantoea agglomerans) and plant extracts (red clover and garlic) supplements on the laying performance, egg quality, eggshell structure and small intestinal histology of laying hens.
Materials and methods
Birds and management
The experiment was conducted according to the guidelines for the care and use of laboratory animals established by Kagawa University, Japan. In total, 66 Boris Brown laying hens (30 weeks old) were randomly divided into 3 experimental groups of 22 birds each as follows: the control group was fed a basal diet () and the other groups were fed the basal diet supplemented with 0.1% Pantoea-6® and 0.1% plant extracts (red clover and garlic). During the experiment, all birds were maintained in individual laying cages in an environmentally controlled room (at a temperature of around 27°C with a photoperiod of 16L:8D). Each group was fed ad libitum with its own diet for a period of 8 weeks. Water was continuously available from nipple drinkers. Feed intake and refusal were recored daily and body weight was measured weekly.
Egg quality examination
Eleven eggs from each group were collected weekly to measure egg quality. At first, the egg weight was recorded using an electronic digital balance. The shell-breaking strength was measured using an eggshell strength meter (accuracy: 0.1 kg/cm2; Fujihira Industry Co. Ltd., Tokyo, Japan). The eggs were broken onto a metal plate and the height of the albumen was measured as the distance between the metal plate and the electrode placed on top of the thick part of the egg. Then, the weights of the albumen, egg yolk and eggshell were measured using an electronic digital balance. Eggshell thickness was recorded as the mean value of measurements at three locations on the egg (air cell, equator and sharp end), measured by a dial thickness gauge (Peacock Ozaki, Tokyo, Japan). Yolk colour was measured by a subjective method using the Roche yolk colour fan (Roche Ltd., Basel, Switzerland). Haugh units were calculated for each individual egg using the following formula:
Haugh unit=100 log (H-1.7 W0.37+7.6) where H is the observed height of the albumen (mm) and W is the weight of eggs (g).
Examination of phosphorus, calcium and magnesium in eggshell
The phosphorus, calcium and magnesium contents of eggshell were measured at the end of the experiment by the Japan Food Research Laboratories (December 6, 2011, No. 11112776001-01-11112776006-01; Tokyo, Japan).
Blood component examination
At 38th week of age, 15 birds (5 birds/treatment) were randomly selected. Blood was drawn from the wing vein for determining total protein, albumin, albumin to globulin ratio (A/G), total amount of bilirubin (T-BIL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LD), glyceride, total cholesterol (T-cho), blood urea nitrogen (BUN), creatinin, calcium, inorganic phosphorus and glucose. Chemical elements were measured at a commercial laboratory (Wakamatsu Medical Research Laboratory, Kitakyushu, Japan).
Eggshell examination
At 38th week of age, three eggs were randomly chosen from each treatment for eggshell sampling. The samples of the air cell, equator and sharp end of the eggshell were cut transversely approximately 0.5×0.5 cm in size. The pieces were mounted on aluminium stubs with electrically conductive carbon paste, coated (E-1030 Ion Sputter; Hitachi Ltd., Tokyo, Japan) and viewed under a scanning electron microscope (SEM) (Hitachi S-4300SE/N; Hitachi Ltd.) at 8 kV. Images were used to observe the microstructure of the eggshells.
Tissue sampling
At 38th week of age, four chickens with similar mean body weight and egg production level were chosen from each treatment. They were euthanised under anesthesia with diethyl ether to obtain tissues for microscopic assessment. The whole small intestine was removed immediately and placed into a mixture of 3% glutaraldehyde and 4% paraformaldehyde fixative solution in 0.1 M cacodylate buffer (pH 7.4). The intestinal segments were cut from the duodenum, jejunum and ileum as follows: i) the duodenum segment was cut from the gizzard to the pancreatic and bile ducts; ii) the jejunum segment was cut from the duct to the Meckel’s diverticulum; and iii) the ileum segment was cut from the diverticulum to the ileocaecal-colonic junction. Tissue samples were taken from each part.
Light microscopy examination
A 2-cm long section of each intestine was fixed in Bouin’s solution for 1 d and dehydrated in a graded series of alcohol solutions. Finally, each segment was embedded in paraffin wax using standard techniques. Eight transverse sections from each intestinal segment cut at a thickness of 4 m were fixed in each slide and stained with hematoxylin-eosin and the subsequent values were measured using an image analyser (Nikon Cosmozone 1 S; Nikon Co., Tokyo, Japan). Images were used to measure villus height, villus area, cell area and cell mitosis.
Measurement of villus height
Two villi having a lamina propria were randomly selected per transverse section. The villus were measured from the tip to the base excluding the crypt. The average villi heights from the three birds (16 villi from eight different sections in each segment, per bird) were expressed as a mean villus height for one treatment group.
Measurement of villus area
Two villi with lamina propria were chosen from each section and the width of the villus was measured at the basal and apical parts. The widths of 16 villi at the basal and apical parts were measured from different sections in each bird. The villus area was calculated from the villus height, basal width and apical width as follows:
The mean villus areas from the three birds (16 calculations of the villus area from eight different sections in each segment, per bird) were expressed as a mean villus area for one group.
Measurement of epithelial cell area
The area of the epithelial cell layer was randomly measured at the middle part of the villus and the cell nuclei within the cell layer were counted. Then, the area of the layer was divided by the number of cell nuclei. A total calculation of cell areas was measured from four different sections per bird and these four values were expressed as a mean cell area in one bird. These four mean cell areas from the four birds were expressed as a mean cell area for one treatment group.
Measurement of cell mitosis in the crypt
Mitotic cells having homogenous, intensely stained, basophilic nuclei with hematoxylin (Tarachai and Yamauchi, 2000) were counted. Total mitosis numbers were counted from four different sections per bird and an average of these values was expressed as a mean cell mitosis number for each bird. Finally, these mean cell mitosis numbers from the three birds were expressed as a mean cell mitosis number for one group.
Scanning electron microscopy examination
A 2-cm long section of the intestine was slit longitudinally, opened and washed with 0.1 M phosphate buffered saline (pH 7.4). The tissue sample was pinned flat and fixed in this flattened position in a mixture of 3% glutaraldehyde and 4% paraformaldehyde fixative solution in 0.1% cacodylate buffer (pH 7.4) for 1 h at room temperature, cut into 4×4 mm squares. It continued this fixing for 1 h. The pieces were rinsed with 0.1 M sodium cacodylate buffer and were post-fixed with 1% osmium tetroxide in a 0.1 M ice-cold sodium cacodylate buffer for 2 h. The specimens were dried in a critical point drying apparatus. The dried specimens were coated with platinum and observed at 8 kV with SEM (Hitachi S-4300SE/N; Hitachi Ltd.). Morphological alterations of the epithelial cells on the villus apical surface were compared between each treatment group.
Statistical analysis
Statistical analysis of the laying performance, egg quality, phosphorus, calcium and magnesium of the eggshell, blood component and light microscopy examination (villus and villus area, absorptive epithelial cell area and cell mitosis) were statistically analysed by oneway analysis of variance (ANOVA) with the Statistical Package for the Social Sciences (SPSS) software (version 10.0 for Windows; SPSS Inc., Chicago, IL, USA). Differences among the treatment groups were tested by Tukey’s studentised range test and differences were considered significant at P<0.05.
Results and discussion
Laying performance and egg quality
The effect of dietary Pantoea-6® and plant extracts on laying performance and egg quality are presented in . Compared with the control, the egg weight, albumen weight and eggshell breaking strength were significantly higher in the Pantoea-6® and plant extracts groups (P<0.05). Eggshell weight was significantly higher in the Pantoea-6® group (P<0.05).
Phosphorus, calcium and magnesium in eggshell
The effect of dietary Pantoea-6® and plant extracts on the phosphorus, calcium and magnesium contents in eggshell is shown in . There were no significant (P>0.05) differences in phosphorus, calcium and magnesium of eggshell among the dietary treatments.
Blood component
The effect of dietary Pantoea-6® and plant extracts on total protein, albumin, A/G, T-BIL, AST, ALT, ALP, LD, glyceride, T-cho, BUN, creatinin, calcium, inorganic phosphorus and glucose is given in . In this case too, there were no significant (P>0.05) differences in these blood components among the dietary treatments.
Eggshell examination
The effect of dietary Pantoea-6® and plant extracts on eggshell structure is shown in . Eggshell structure (air cell, equator and sharp end) can be clearly seen in all groups: it tended to have greater thickness in both dietary Pantoea-6® and plant extracts groups than in the control.
Light microscopic examination
Villus height and area, cell area and mitosis in all intestinal segments tended to be higher in all laying hens in the dietary Pantoea-6® and plant extracts groups than in the control (). The duodenum and jejunum of both treatment groups showed higher cell area values than those of the control (P<0.05).
Scanning electron microscopy examination
In the duodenum, in the villus apex of the control (), flat cell areas (small arrows) were found. In treatment groups (,), such flat cells developed into clearly protuberated cells (large arrows). The same can be seen on the jejunal villus apical surface of the control group (). On the ileal villus apex surface of the control () flat cell areas (small arrows) were observed too. However, the cell protuberances cells (large arrows) continued to be present in the Pantoea-6® and plant extracts groups (,).


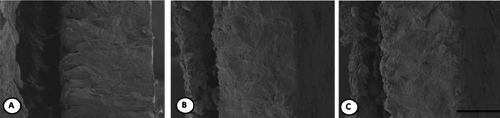
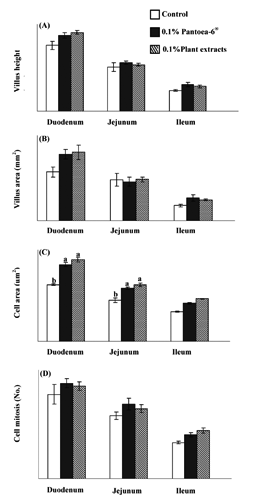
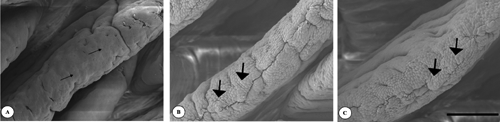
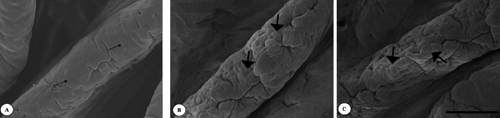
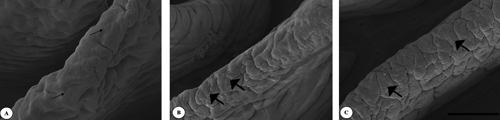
Table 1. Composition and analysis of basal diet.
Table 2. Laying performance and egg quality in control, 0.1% Pantoea-6®, and 0.1% plant extracts (mean±SE, n=11) (8 weeks after feeding).
Table 3. Phosphorus, calcium and magnesium in eggshell in control, 0.1% Pantoea-6®, and 0.1% plant extracts (mean±SE, n=2).
Table 4. Blood component in hens fed control, 0.1% Pantoea-6®, and 0.1% plant extracts (mean±SE, n=5).
General remarks
The results of the present study showed that supplementation of the diet with Pantoea-6® and plant extracts did not negatively influence egg production, feed intake, feed efficiency, final body weight, eggshell thickness, yolk weight, yolk colour and Haugh units of the laying hens. These data correspond with no changes to final body weight (Qureshi et al., Citation1983; Abdullah et al., Citation2010) and feed conversion ratio in broiler chicks fed dietary garlic (Konjufca et al., Citation1997; Abdullah et al., Citation2010). Interestingly, the egg weight, albumen weight and eggshell breaking strength of the treatment groups were significantly higher than the control. Eggshell weight of the Pantoea-6® group was significantly higher than in the other groups. As the nutritional composition of diets in all groups was almost the same, the tendency to better egg quality seems to have been induced by Pantoea-6® and plant extracts. In this study, Pantoea-6® was made from extraction of fermented wheat flour with Pantoea agglomerans, which is gram-negative bacteria (Hebishima et al., Citation2010). Rajput et al. (Citation2013) reported that LPS is a structural component of the outer membrane of gram-negative bacteria. Xie et al. (Citation2000) found that LPS increased the total plasma protein concentration in chickens. Kohchi et al. (Citation2006) reported that feeding LPS serves to strengthen the prophylactic ability. Moreover, LPS activates macrophages and the innate immune system. Thus, the improvement in egg and albumen weight might be attributed to the effect of LPS on nutrient digestibility. However, studies on the effects of LPS on poultry are limited compared with studies using mammals. Therefore, further study is being carried out to address this issue.
In previous studies, garlic supplementation improved egg (Yalcin et al., Citation2006; Mahmoud et al., Citation2010) and albumen weight (Qatramiz, Citation2006; Mahmoud et al., Citation2010). The mechanisms of garlic have been accredited to its effective antioxidant action (Yang et al., Citation1993), and its ability to stimulate immunological responsiveness (Reeve et al., Citation1993). Ramakrishna et al. (Citation2003) reported that garlic supplementation probably enhanced the activities of the pancreatic enzymes and provided a micro-environment for better nutrient utilisation in rats. Supplementation with black cumin (Nigella sativa L.) can increase the shell strength of eggs (Aydin et al., Citation2008). Radwan et al. (Citation2008) indicated that feeding dietary Curcuma Longa to laying hens could increase the eggshell weight and thickness because it could improve the micro environment in uterus (site of calcium deposition). Hernandez et al. (Citation2004) and Jang et al. (Citation2004) reported that dietary feeding of essential oil extracted from herbs improved the secretion of digestive enzymes thus improving the digestibility of the feeds. In the current study, the eggshell structure (air cell, equator and sharp end) can be clearly seen in both Pantoea-6® and plant extracts groups. Density of mamillary knobs tended to be lower in both treatment groups. Van Toledo et al. (Citation1982) reported that weak eggshells generally have a higher mamillary knobs density. Moreover, the eggshell structure tended to have greater thickness in both the dietary Pantoea-6® and plant extracts groups than in the control. Increased thickness of the eggshell by the addition of Pantoea-6® and plant extracts may be attributed to the components of these supplements which have antioxidant activities (Abdullad et al., Citation2010).
The morphological differences among the intestinal parts would be induced by the nutrients in the diets (Mekbungwan et al., Citation2003; Yamauchi et al., Citation2006) and the intestinal absorptive function of each segment (Rattanavut and Yamauchi, Citation2012). The present results show that most light microscopy parameters (villus height and area, cell area and mitosis) in all intestinal segments tended to have higher values in both treatment groups than in the control. In particular, the cell area in the duodenum and jejunum of the Pantoea-6® and plant extracts groups had higher values than the control. Under normal circumstances, the major absorption of nutrients occurs in the duodenum and jejunum (Noy and Sklan, Citation1995). Based on the results, we conclude that the absorptive function of cells in the duodenum and jejunum was sufficient in both treatment groups, whereas the ileum did not play a significant role in absorption. The results reported in this study are in complete agreement with what has been reported in the literature. Santin et al. (Citation2001) reported that when birds were treated with 0.2% of yeast cell walls, the villus height increased. Adibmoradi et al. (Citation2006) reported that garlic administration enhanced the villus height and crypt depth and decreased the epithelial thickness and goblet cells number in the duodenum, jejunum and ileum of birds; similar results were reported by Nusairate (Citation2007). Garlic administration stimulated the selective population of intestinal cells (Abdullah et al., Citation2010). Gupta and Sandhu (Citation1998) found that feeding garlic agglutinin to rats caused lengthening intestinal villi due to cellular hypertrophy and hyperplasia. Increased villus size indicates an increased villus length (Lauronen et al., Citation2000) and provides a greater surface area for the absorption of available nutrients (Onderci et al., Citation2006). The ingested nutrients are absorbed into the blood or lymphatic system after being transported from the intestinal lumen through the mucosal epithelial cell barrier (Caspary, Citation1992).
The epithelial cells react morphologically to ingested diets more quickly than the intestinal villi (Tarachai and Yamauchi, Citation2000; Maneewan and Yamauchi, Citation2004), because the histological reaction to ingested diets differs between the micro (epithelium) and macro (villi) levels. In the present study, protuberated cells were observed on the villus apical surface in both the dietary Pantoea-6® and plant extracts groups. It is possible that both dietary Pantoea-6® and plant extracts are able to stimulate gut cellular proliferation by direct interaction with a selective population of intestinal cells or indirectly via gut endocrine cells. The present dietary amounts of Pantoea-6® and plant extracts would also increase the light microscopy parameters and protuberated cells, resulting in increased digestion and absorption.
Conclusions
This study shows that supplementing Pantoea-6® and plant extracts at a 0.1% level might have a beneficial effect on egg and albumen weight, eggshell quality and eggshell structure parameters, as well as on small intestine histological parameters.
References
- AbdullahA.Y. MahmoudK.Z. NusairatB.M. QudsiehR.I., 2010. Small intestinal histology, production parameters, and meat quality as influenced by dietary supplementation of garlic (Allium sativum) in broiler chicks. Ital. J. Anim. Sci. 9:e80.
- AdibmoradiM. NavidshadB. SeifdavatiJ. RoyanM., 2006. Effect of dietary garlic meal on histological structure of small intestine in broiler chickens. J. Poult. Sci. 43:378-383.
- AydinR. KaramanM. CicekT. YardibiH., 2008. Black cumin (Nigella sativa L.) supplementation into the diet of the laying hen positively influences egg yield parameters, shell quality, and decrease egg cholesterol. Poultry Sci. 87:2590-2595.
- CasparyW.F., 1992. Physiology and pathophysiology of intestinal absorption. Am. J. Clin. Nutr. 55:299-308.
- DunnI.C. BainM. EdmondA. WilsonP.W. JosephN. SolomonS. DeketekaereB. De BaerdemaekerJ. SchmutzM. PreisingerR. WaddingtonD., 2005. Heritability and genetic correlation of measurements derived from acoustic resonance frequency analysis; a novel method of determining eggshell quality in domestic hens. Brit. Poult. Sci. 46:280-286.
- GuptaA. SanduhR.S., 1998. Effect of garlic agglutinin and garlic extracts on the rat. Nutr. Res. 18:841-850.
- HebishimaT. MatsumotoY. SomaG. KohchiC. WatanabeG. TayaK. HayashiY. HirotaY., 2010. Immune recovery effects of immunopotentiator from Pantoea agglomerans (IP-PA1) on low antibody productions in response to Salmonella enteritidis vaccine and sheep red blood cells in dexamethasone-treated chicken models. J. Vet. Med. Sci. 72:435-442.
- HernandezF. MadridJ. GarciaV. OrengoJ. MegiasM.D., 2004. Influence of two plant extracts on broiler performance, digestibility and digestive organ size. Poultry Sci. 83:169-174.
- HuntonP., 2005. Research on eggshell structure and quality: an historical overview. Braz. J. Poult. Sci. 7:67-71.
- JangI.S. KoY.H. YangH.Y. HaJ.S. KimJ.Y. KangS.Y. YooD.H. NamD.S. KimD.H. LeeC.Y., 2004. Influence of essential oil components on growth performance and the functional activity of the pancreas and small intestine in broiler chickens. Asian Austral. J. Anim. 17:394-400.
- KohchiC. InagawaH. NishizawaT. YamaguchiT. NagaiS. SomaG., 2006. Applications of lipopolysaccharide derived from Pantoea agglomerans (IP-PA1) for health care based on macrophage network theory. J. Biosci. Bioeng. 102:485-496.
- KonjufcaV.H. PestiG.M. BakalliR.I., 1997. Modulation of cholesterol levels in broiler meat by dietary garlic and copper. Poultry Sci. 76:1264-1271.
- LauronenJ. PakarinenM.P. KuusanmakiP. SavilahtiE. VentoP. PaavonenY. HalttunenJ., 2000. Intestinal adaptation after massive proximal small bowel resection in the pig. Brit. Poult. Sci. 41:416-423.
- MahmoundK.Z. GharaibehS.M. ZakariaH.A. QatramizA.M., 2010. Garlic (Allium sativum) supplementation: influence on egg production, quality and yolk cholesterol level in layer hens. Asian Austral. J. Anim. 23:1503-1509.
- ManeewanB. YamauchiK., 2004. Intestinal villus recovery in chickens refed semipurified protein-, fat-, or fibre-free pellet diets. Brit. Poult. Sci. 45:163-170.
- MeimandipourA. Hair-BejoM. ShuhaimiM. AzharK. SoleimaniA.F. RastiB. YazidA.M., 2010. Gastrointestinal tract morphological alteration by unpleasant physical treatment and modulating role of Lactobacillus in broilers. Brit. Poult. Sci. 51:52-59.
- MekbungwanA. YamauchiK. ThongwittayaN., 2003. Histological alterations of intestinal villi in growing pigs fed soybean and pigeon pea seed meals. Can. J. Anim. Sci. 83:755-760.
- NoyY. SklanD., 1995. Digestion and absorption in the young chick. Poultry Sci. 74:366-373.
- NusairateB.M., 2007. Dietary supplementation of garlic (Allium Sativum): influence on performance parameters, meat quality and humoral immune response in broiler chicks. Degree Diss., Jordan University of Science and Technology, Irbid, Jordan.
- OnderciM. SahinN. SahinK. CikimG. AydinA. OzercanI. AydinS., 2006. Efficacy of supplementation of a-amylase-producing bacterial culture on the performance, nutrient use and gut morphology of broiler chickens fed a corn-based diet. Poultry Sci. 85:505-510.
- QatramizA., 2006. Effect of garlic (Allium sativum) supplementation on egg quality, yolk cholesterol and humoral immune response in layer hens. Degree Diss., Jordan University of Science and Technology, Irbid, Jordan.
- QureshiA.A. AbuirmeilehN. DinZ.Z. ElsonC.E. BurgerW.C., 1983. Inhibition of cholesterol and fatty acid biosynthesis in liver enzymes and chicken hepatocytes by polar fractions of garlic. Lipids 18:343-348.
- RadwanN.L. HassenR.A. QotaE.M. FayekH.M., 2008. Effect of natural antioxidant on oxidative stability of eggs and productive and reproductive performance of laying hens. Int. J. Poult. Sci. 7:134-150.
- RajputN. NaeemM. AliS. ZhangJ.F. ZhangL. WangT., 2013. The effect of dietary supplementation with the natural carotenoids curcumin and lutein on broiler pigmentation and immunity. Poultry Sci. 92:1177-1185.
- RamakrishnaR.R. PlatelK. SrinivasanK., 2003. In vitro influence of spices and spice-active principles on digestive enzymes of rat pancreas and small intestine. Nahrung 47:408-412.
- ReeveV.E. BosnicM. RosinovaE. Boehm-WilcoxC., 1993. A garlic extract protects from ultraviolet B (280-320 nm) radiation-induced suppression of contact hypersentivity. Photochem. Photobiol. 58:813-817.
- RuttanavutJ. YamauchiK., 2012. Growth performance and intestinal histomorphology in egg-type growing roosters fed recycled food waste containing effective microorganisms. Turk. J. Vet. Anim. Sci. 36:509-518.
- SantinE. MaiorkaA. MacariM. GreccoM. SancheezJ.C. OkadaT.M. MyasakaA.M., 2001. Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. J. Appl. Poultry Res. 10:236-244.
- StadelmanW.J., 2000. Eggs and egg products. In: Francis F.J. ( ed.) Encyclopedia of food science and technology. John Wiley & Sons, New York, NY, USA, pp 593-599.
- SuzukiJ. NishizawaT. InagawaH. OkutomiT. MorikawaA. SomaG.-I. MizunoD., 1992. Homeostasis as regulated by activated macrophage. IX. Enhancement effect of LPSw (a lipopolysaccharide from wheat flour) on hen egglaying and breaking strength of eggshell. Chem. Pharm. Bull. 40:1274-1276.
- TarachaiP. YamauchiK., 2000. Effects of luminal nutrient absorption, intraluminal physical stimulation and intravenous perenteral alimentation on the recovery responses of duodenal villus morphology following feed withdrawal in chickens. Poultry Sci. 79:1578-1585.
- TasaiW.T. YangJ.M. LaiC.W. ChengY.H. LinC.C. YehC.W., 2006. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresource Technol. 97:488-493.
- Van ToledoB. ParsonsA.H. CombsG.F., 1982. Role of ultrastructure in determining eggshell strength. Poultry Sci. 61:569-572.
- XieH. RathN.C. HuffG.R. HuffW.E. BalogJ.M., 2000. Effect of salmonella typhimurium lipopolysaccharide on broiler chickens. Poultry Sci. 79:33-40.
- YalcinS. OnbasilarE.E. ReisliZ. YalcinS., 2006. Effect of garlic powder on the performance, egg traits and blood parameters of laying hens. J. Sci. Food Agr. 86:1336-1339.
- YamauchiK. BuwjoomT. KogeK. EbashiT., 2006. Histological intestinal recovery in chickens refed dietary sugar cane extract. Poultry Sci. 85:645-651.
- YamauchiK. IncharoenT. YamauchiK., 2010. The relationship between intestinal histology and function as shown by compensatory enlargement of remnant villi after midgut resection in chickens. Anat. Rec. 293:2071-2079.
- YangG.C. YasaeiM.P. PageS.W., 1993. Garlic as antioxidant and free radical scavenger. J. Food Drug Anal. 1:357-364.
