Abstract
The aim of this experiment was to determine the effect of polyethylene glycol (PEG) on in vitro gas production (GP) kinetics of Prosopis cineraria leaves at different growth stages. The contents of total phenol (TPH), total tannin (TT) and condensed tannin (CT) were determined. Effects on in vitro organic matter digestibility (OMD), metabolisable energy (ME) and effective dry matter digestibility were assessed by PEG tannin bioassay. No significant differences (P>0.05) were observed for TPH content; however, the stage of flowering had the highest (P<0.05) content of both TT and CT. No interaction effects (P>0.05) were observed between the growth stage and PEG addition for in vitro GP and its parameters. Addition of PEG increased (P<0.05) GP, OMD and ME in all stages. In conclusion, adding PEG to P. cineraria leaves can improve their nutritive value and could be considered as a potential feed for ruminants.
Introduction
Tree legume forages play an important role in livestock nutrition in many parts of the tropics. One of the commonly used tree species in south of Iran is Prosopis spp. (mesquite). The genus Prosopis belongs to the family Leguminosae (subfamily Mimosaceae). About 43 species of this genus are known; three of them are commonly found in Iran. These species are P. cineraria (Iranian Kahor), P. koelziana (Valley Kahor) and P. juliflora (Pakestanian Kahor). Mesquite trees and shrubs are vigorous, drought- and heat-tolerant plants that are able to survive in many arid parts of the world. In some of these regions mesquite leaves and pods are principal sources of forage during dry seasons (Felker, Citation1979).
Prosopis cineraria is found in the arid areas of Arabia, Indian sub-continent, Iran and Afghanistan where it provides fodder, fuel, shade and improvement of soil and stabilises sand dunes. Kahor leaves and pods are consumed by many animal species. However, Prosopis sp. has been reported to contain levels of anti-nutrients such as tannins. Therefore, the nutritive value of these leaves as feed for ruminants is offset by tannin potential negative effect on protein utilisation. Tannins are known to affect the availability of nutrients by formation of soluble and insoluble complexes. Their effects on the digestibility of nutrients will vary depending on tannin content and astringency (McNeill et al., Citation1998). As it would take a considerable effort to screen for all possible anti-nutritive factors by conventional chemical methods, there is interest in the possible use of simple bio-assays as potential screening methods. The use of polyethylene glycol (PEG) to neutralise condensed tannins (CTs) has proved useful in further elucidating the specific nutritional consequences of dietary CT as it displaces protein-tannin complexes. As a consequence, CTs interact more strongly with PEG than they do with protein (Mangan, Citation1988). Thus, supplementation with PEG has been used to alleviate the negative effects of tannins on livestock (Landau et al., Citation2000). Palmer and Jones (Citation2000) have shown that PEG improved the in vitro digestibility of nitrogen in Calliandra and most other legumes containing tannins. The treatment with tannin binding agents can be highly effective in overcoming the anti-nutritive effects of tannins leading to improved animal performance. However, their effects can be variable which may relate to the nature of the tannins and the nature of the tannin-feedstuff complexes which can form.
The in vitro gas production (GP) method is a relatively simple and inexpensive tool to study potential effect, mechanism of action and fate of phytochemicals in the rumen (Makkar, Citation2005). This method coupled with PEG uses as a specific binding agent and provides useful information on the biological activity of tannins (Ammar et al., Citation2004). However, the in vitro GP must be completed by other measures of end-products fermentation such as the amount of degraded matter for obtaining more complete information. This experiment was carried out to assess the effect of adding PEG [molecular weight (MW) 6000] on in vitro GP of P. cineraria, rich in tannins, at different growth stages.
Materials and methods
Forages were collected from Sardoeeiye of Jiroft, located in the South-West of Iran. Sardoeeiye region is situated at 57°19’E and 29°9’N. Its average altitude is 2700 m asl. Fresh mesquite leaves were collected from trees planted. Leaves were removed from branches, pooled to five samples per different growth stages. The samples were dried at 60°C in forced air oven for 48 h, except for those samples used for tannins determination that were air-dried in the shade to minimise changes in tannin content and activity (Makkar and Singh, Citation1991).
Chemical analysis
Standard methods as described in AOAC (Citation1990) were used for determination of dry matter (DM) (method #930.15), ash (method #924.05) and N (method #984.13). Ash-free neutral detergent fibre (NDF) was determined using sodium sulfite according to the method of Van Soest et al. (Citation1991), while ash-free acid detergent fibre (ADF) (method #973.18) was determined based on AOAC (Citation1990).
Total phenols determination
Samples were analysed for CT using the method of Makkar (Citation2000). Briefly, dried plant material (200 mg) was extracted with acetone:water (10 mL; 70:30, v/v) in ultrasonic bath for 20 min. Contents were centrifuged (4°C, 10 min, 3000×g), then the supernatant was kept on ice until analysis. Total phenols were determined with the Folin-Ciocalteau reagent, and detected at 725 nm. A calibration curve was prepared using tannic acid (Merck GmbH, Darmstadt, Germany). Total phenols were calculated as tannic acid equivalents and expressed as equivalents g/kg DM.
Tannins determination
Samples were analysed for total extractable tannin (TT) using HCL-butanol as described in Makkar (Citation2000). Briefly, an aliquot from the above acetone:water extract (0.5 mL; although this extract occasionally needed diluting with the extract of acetone:water, if final absorbance at 550 nm exceeded 0.6 absorbance units) plus HCL-butanol (3 mL) and ferric ammonium sulphate (0.1 mL) reagents were heated in a boiling water bath for 60 min. Absorbance was read at 550 nm. The colorimetric data (in absorbance units) were converted to leucocyanidin equivalents based on the assumption that the color yield of CT, E 1%,550 nm, is 460 (Porter et al., Citation1986).
Non-tannin phenols (NTP) were determined using absorption to insoluble polyvinylpyrrolidone (PVPP). The insoluble PVPP (100 mg) was weighed in 100×12 mm test tubes. Distilled water (1 mL) and 1 mL tannin containing extract were added and vortexed. The tube was kept at 4°C for 15 min, vortexed again, then centrifuged (3000×g) for 10 min, and then the supernatant was collected. The phenolic content of the supernatant was measured by the Folin-Ciocalteau reaction and this was accepted as the NTP (Makkar, Citation2000).
Total tannins (TT) were calculated as the difference between TPH and NTP. The results were expressed as gallotannin. Protein perceptiblephenilics were determined according to Makkar (Citation2000) and results were expressed as tannic acid equivalent.
Gas production
Rumen fluid for the in vitro digestibility tannin bioassay was obtained from two mature male native cattle with live weight of 346±11.5 kg fitted with permanent 70 mm rumen cannulae. Cattle were fed a ration divided into equal meals at 08:00 a.m. and 04:00 p.m. daily. Cattle had free access to water throughout the experiment. Rumen fluid was obtained from the two cattle in the morning before feeding (07:00 a.m.), flushed with CO2, filtered through three layers of cheesecloth and mixed (1:2, v/v) with an anaerobic mineral buffer solution as described by Makkar et al. (Citation1995) and revised by Makkar (Citation2000). Preparation of an in vitro mineral buffer media for the gas test was completed as described by Menke and Steingass (Citation1988). Reduced buffer medium composition, per liter, was 70.0 g of NaHCO3, 4.00 g of NH4HCO3, 5.7 g of Na2HPO4, 6.2 g of KH2PO4, 0.6 g of MgSO4·7H2O, 0.52 g of Na2S, 13.2 g of CaCl2·H2O, 10.00 g of MnCl2·4H2O, 1.00 g of CoCl2·6H2O, 0.01 g of sodium resazurin, 60 mL of freshly prepared reduction solution containing 580 mg of Na2S·9H2O, and 3.7 mL of 1 M NaOH. The mixture was kept stirred, under CO2 flushing at 39°C, using a magnetic stirrer fitted on a hotplate.
Effects of tannins on in vitro organic matter digestibility (OMD) were assessed by incubating approximately 375 mg (DM bases) of triplicate test feed samples with or without 750 mg PEG with MW 6000 (Merck Schuchardt OHG, Hohenbrunn, Germany). Feed samples were incubated in 100 mL glass syringes based on Menke and Steingass (Citation1988) procedures. The PEG tannin bioassay was performed according to Makkar et al. (Citation1995) and revised by Makkar (Citation2000). Petroleum jelly was applied to the piston to ease movement and prevent escape of gas. Syringes were pre-warmed (39°C) for 1 h before addition of 30±0.5 mL of rumen buffer mixture into each syringe, and incubated in a water bath maintained at 39±0.1°C as described by Blümmel and Ørskov (Citation1993). Syringes were gently shaken every hour during the first 8 h of incubation. Gas production readings (mL) were recorded at 2, 4, 6, 8, 10, 12, 16, 24, 48, 72 and 96 h for PEG-treated and -untreated samples. Total gas values were corrected for blank with a hay standard with a known GP.
For a more precise estimation of GP throughout the duration of in vitro fermentation, a nonlinear equation was used to analyse the kinetic data (France et al., Citation2000).
Cumulative GP data were fitted to the exponential equation Y=b (1-e-ct), where b is the GP from the fermentable fraction (mL), c the GP rate constant for b, t the incubation time (h), and Y the gas produced at time t.
Feed OMD (g/kg OM) and metabolisable energy (ME) in MJ/kg DM were estimated by equations of Menke and Steingass (Citation1988), based on 24 h GP (mL) and crude protein (CP) content (g/kg DM) as: OMD (g/kg OM)=148.8+8.89 net GP+4.5 CP+0. 651 XA. ME (MJ/kg DM)=2.20+0.136 net GP+0.057 CP+0.0029 CP2. Where CP is in g/100 g DM; XA ash in g/100 g DM. Net GP data were converted from 375 to 200 mg after 24 h of incubation
Statistical analysis
Data of chemical composition and tannin content were subjected to analysis using the General Linear Model procedure of SAS (Citation2002), based on the statistical model: Yij=µ+ Si+eij, where Yij is the general observation on chemical composition and tannin content, µ is the general mean, Si is the ith effect of growth stage on the observed parameters, and eij is the random standard error. Means were tested using Duncan test.
For in vitro GP and estimated parameters of digestibility and ME, the following statistical model was fitted: Yijk=µ+Si+Pj+(SP)ij+Eijk where Yijk is the observation on GP and digestibility estimates, Si is the i th effect of growth stage and Pj is the j th effect of PEG on the observed parameters. The (SP)ij term represents ith and jth interaction effects of growth stage and PEG on in vitro GP, and Eijk is the random standard error.
Results
Primary and secondary compounds
The chemical composition of P. cineraria at different growth stages is shown in . There were variations in chemical compositions between phenological stages of P. cineraria. The CP content of the different stages ranged (P<0.05) from 9.26 to 11.79% for seed ripping and vegetative stages, respectively. The NDF and ADF content ranged (P<0.05) from 45.12 to 46.51 and 28.14 to 30.48%, respectively for seed ripping and vegetative stages.
The content of TPH was almost the same (P>0.05) between different stages. However, the stage of flowering had the highest (P<0.05) content of both TT and CT compared to seed ripping and vegetative stages, respectively ().
Gas production
No interaction effects (P>0.05) were observed between the growth stage and PEG addition for in vitro GP and GP parameters (, ). The cumulative volume of GP increased (P<0.05) with increasing time of incubation (, ). Addition of PEG to the stage of maturity increased (P<0.05) in vitro GP only during the first 8 h of incubation without significant (P>0.05) effects from 8 to 96 h of incubation. However, the addition of PEG increased GP (P<0.05) over the time. The insoluble but fermentable fraction (b), the rate of GP during incubation (c), OMD, dry OMD (DOMD), and ME all not affected (P>0.05) by stage of growth with significant (P<0.05) response for PEG addition (, ).
Discussion
In our study, leaves of Prosopis cineraria had CP content more than 80 g/kg DM (range: 92.6 to 117.9 g/kg DM), which according to Norton (Citation2003) should provide ruminal ammonia levels above the minimum required by rumen microorganism to support optimum growth for maintenance, production and optimum activity. However, the CP content of leaves of seed ripping stage was lower than required by microorganisms in the rumen to support optimum activity (Norton, Citation2003).
In tree leaves, tannins are tightly bound to the cell wall (NDF and ADF) and cell protein (Reed et al., Citation1990). Tannins may form a less digestible complex with dietary proteins and may bind and inhibit the endogenous protein, such as digestive enzymes (Kumar and Sing, Citation1984). Tannin can also adversely affect the microbial and enzyme activities (Silanikove et al., Citation1996a; Makkar et al., Citation1995).
There was a relationship between TPH and TT which is similar to findings of Makkar et al. (Citation1993) who noted a high positive correlation between content of TT and TPH compounds. Levels of TPH and TT in the growth stages were higher for flowering than vegetative stage compared to seed ripping stage. The variation in the phenolic compound contents was probably due to growth stage (Makkar and Singh, Citation1993).
However, in ruminants dietary CT 20 to 40 g/kg DM has been shown to have beneficial effects because they reduce the wasteful protein degradation in the rumen by the formation of a protein-tannin complex (Min et al., Citation2003). Getachew et al. (Citation2002) concluded that samples containing TPH and TT (tannic acid equivalent/kg DM) up to 40 and 20 g/kg DM, respectively, are not expected to induce an increase in GP with addition of PEG. On the other hand, high levels of CT in tree leaves have been reported to restrict the nutrient utilisation and decrease voluntary food intake, nutrient digestibility and N retention (Kumar and Vaithiyanathan, Citation1990; Silanikove et al., Citation1996b). Total tannin content of forages in the range of 60 to 100 g/kg DM depresses intake and growth of animals (Barry et al., Citation1984). The tannin content of leaves obtained in this experiment fell into this range. Therefore, supplementation of PEG can be recommended to reduce the detrimental effect of tannin in leaves. Pritchard et al. (Citation1998) showed that giving PEG to sheep fed with mulga markedly increased feed intake, weight gain and wool growth. Gilboa et al. (Citation2000) also showed that a single daily oral dose of PEG substantially improve feed intake and efficiency of utilisation by sheep and goats consuming tannin rich forages (Gurbuz, Citation2007). The effects of tannins seem to be dependent on several factors including forage species, chemical nature and structure of tannins, and biochemical interaction among tannins and proteins, than the tannins level itself. On the other hand, in palm leaves, addition of PEG had small effect despite their relatively high tannin content (Arhab et al., Citation2009).
The OMD was higher when PEG was added to the different growth stages. Increased in vitro GP and OMD due to addition of PEG suggest a negative influence of tannins on digestibility (Makkar, Citation2003). Inactivation of tannins through PEG binding increases availability of nutrients resulting in increased microbial activity and GP (Makkar, Citation2003).
Addition of PEG caused different increments in GP between different growth stages. These variable responses of GP could be due to variations in tannin content between different stages. The increase in the GP in the presence of PEG can be due to an increase in the availability of nutrients to rumen microorganisms, especially N (Bakhshizadeh and Taghizadeh, Citation2013). Increased degradability of samples at different growth stages treated with PEG was reflected in greater GP at different time intervals.
The leaves of P. cineraria at the stage of flowering provide more soluble fractions, which is a fermentable energy source within time. Gas production is associated with volatile fatty acids production following fermentation of substrate; therefore, more fermentation of a substrate will result in a greater GP (Blümmel and Ørskov, Citation1993). Differences between GP could be explained by the differences in total VFA production and molar proportion of VFA as a result of fermentation (Beuvink and Spoelstra, Citation1992). Doane et al. (Citation1997) found a significant correlation between GP and VFA production. Quickly soluble carbohydrates produce relatively higher propionate as compared to acetate and vice versa when slowly fermentable carbohydrates are fermented (Getachew et al., Citation1998).
The increased GP when samples were incubated with PEG were also reported for different forages by other authors. Basha et al. (Citation2013) noted that the addition of PEG overcomes the inhibitory effect of tannins on rumen microbes. Arhab et al. (Citation2009) evaluated the influence of tannins present in arid zone forages from North Africa including Aristida plumosa, Astragalus gombiformis, Genista saharae, two date palm fractions (leaves and racemes), and vetch-oat hay taken as control on in vitro GP. They found that addition of PEG resulted in an overall increase in GP (20.2%), with the exception of Danthonia and Aristida. However, PEG addition did not influenced the rate of GP. Moreover, the increase in GP when samples were incubated with PEG were also reported by others (Baba et al., Citation2002; Rubanza et al., Citation2005; Singh et al., Citation2005). Bakhshizadeh and Taghizadeh (Citation2013) determine the effect of PEG inclusion during in vitro incubation on GP kinetics, OMD and the ME of red grape pomace. Total phenol, TT, CT and hydrolysable tannins contents in grape pomace were 4.23, 2.23, 1.16 and 0.89%. Addition of PEG resulted in an increased GP at all incubation times than control; however, there was no significant increase in GP within levels of PEG.
Arhab et al. (Citation2009) stated that addition of PEG can affect partitioning factor (PF) of tannin contain forage. They showed that PEG addition can promote GP but not OMD with decreasing PF of Aristida plumose and palm leaves forages. Whereas, addition of PEG did not alter PF in Genista saharae, Danthonia forskahlii and vetch-oat hay. They returned the different responses between these types of forages to the limited ability of PEG to completely inhibit the negative effects of tannins (Baba et al., Citation2002; Frutos et al., Citation2004), which depends mainly both on stereochemistry and chemical structure of tannins. Other factors than tannins, like limited available N for ruminal microbiota, the higher NDF, ADF and lignin contents, and the saponins may limit fermentation (Ndlovu and Nherera, Citation1997). Blümmel et al. (Citation2003) suggested selection of forages for high degradability but proportionally low GP. The theoretical range for PF values for tannins free plants was suggested by Blümmel et al. (Citation1997) to be between 2.75 and 4.41. According to Blümmel and Becker (Citation1997), plants with high PF are in general highly digestible and the values correlate well with DM intake in ruminants. Thus, these results could suggest that these forages had a potential nutritive value which tends to enhance microbial synthesis rather than GP.
The effect of PEG addition is more pronounced on potential GP, measured at 96 h of incubation. The effects of tannins on nutrient degradability depends essentially on the formation of complexes between tannins and the components of diets, primarily proteins and to a lesser extent with amino acids, polysaccharides and minerals, as well as on their effects on the microbial population and on its enzymatic activity (McSweeney et al., Citation2001). Guimarães-Beelen et al. (Citation2006) noted that if the rate of GP is reduced, the bacteria colonisation is restricted. This could suggest that complexes forming between tannins and PEG generate steric obstruction which does not permit and/or limit the fixation of adherent bacteria to the feeds.
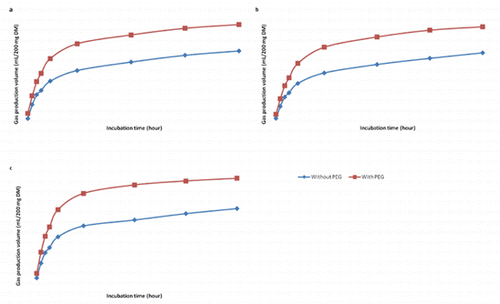
Table 1. Chemical composition and phenolic compounds of Prosopis cineraria at different growth stages.
Table 2. Gas production characteristics of Prosopis cineraria leaves without or with polyethylene glycol at different growth stages
Conclusions
The addition of PEG, which has a relatively low cost in Iran, improves nutritional value of Prosopis cineraria testified by increased GP and OMD. It appears that a PEG/GP assay is a useful first screen for evaluating nutritional value of tannin-rich feeds. However, further research is needed to assess their impact on animal performance.
Acknowledgments
This work has been realised in relation to a research project of the Special Animal Research Institute, University of Zabol.
References
- AmmarH. LopezS. GonzalezJ.S. RanillaM.J., 2004. Distribution of condensed tannins (proanthocyanidins) in various fibre fractions in young and mature leaves of some oaks species. Anim. Feed Sci. Tech. 32:253-260.
- AOAC, 1990. Official method of analysis. 15 th ed., Association of Official Analytical Chemists, Washington, DC, USA.
- ArhabR. MacheboeufD. AggounM. BoussebouaH. VialaD. BesleJ.M. 2009. Effect of polyethylene glycol on in vitro gas production and digestibility of tannin containing feedstuffs from north African arid zone. Available from: http://www.veterinaria.uady.mx/ojs/index.p hp/TSA/article/view/200/168
- BabaA.S.H. CastroF.B. OrskovE.R. 2002. Partitioning of energy and degradability of browse plants in vitro and the implication of blocking the effects by the addition of polyethylene glycol. Anim. Feed Sci. Tech. 95:93-104.
- BakhshizadehS. TaghizadehA. 2013. The effect of polyethylene glycol (6000) supplementation on in vitro kinetics of red grape pomace. Available from: http://ecisi.com/wp-content/uploads/2013/05/523-528.doc.pdf
- BarryT.N. ManleyT.R. DuncanJ.S. 1984. The role of condensed tannins in the nutritional value of Lotus pedunculatus for sheep intake. Brit. J. Nutr. 51:485-491.
- BashaN.A. ScogingsP.F. NsahlaiI.V. 2013. Effects of season, browse species and polyethylene glycol addition on gas production kinetics of forages in the subhumid subtropical savannah, South Africa. J. Sci. Food Agr. 93:1338-1348.
- BeuvinkJ.M.W. SpoelstraS.F. 1992. Interactions between substrate, fermentation end products, buffering systems and gas production upon fermentation of different carbohydrates by mixed rumen micro-organisms in vitro. Appl. Microbiol. Biot. 37:505-509.
- BlümmelM. BeckerK. 1997. The degradability offifty-four roughages and neutral detergentfibre as described by gas production and their relationship to voluntary feed intake. Brit. J. Nutr. 77:757-768.
- BlümmelM. KarsliA. RusselJ.R. 2003. Influence of diet on growth yields of rumen microorganisms in vitro and in vivo: influence of variable carbon fluxes to fermentation products. Brit. J. Nutr. 90:625-635.
- BlümmelM. ØrskovE.R. 1993. Comparison of an in vitro gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Anim. Feed Sci. Tech. 40:109-119.
- DoaneP.H. SchofieldP. PellA.N. 1997. Neutral detergent fibre disappearance, gas and volatile fatty acids production during the in vitro fermentation of six forages. J. Anim. Sci. 75:3342-3352.
- FelkerP. 1979. Mesquite: an all-purpose leguminous arid land tree. In: Ritchie G.A. ( ed.) New agricultural crops. Westview Press, Boulder, CO, USA, pp 88-132.
- FranceJ. DijkstraJ. DhanoaM.S. LopezS. BanninkA. 2000. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Brit. J. Nutr. 83:143-150.
- FrutosP. HervásG. GiráldezF.J. MantecōnA.R. 2004. An in vitro study on the ability of polyethylene glycol to inhibit the effect of quebracho tannins and tannic acid on rumen fermentation in sheep, goats, cows, and deers. Aust. J. Agr. Res. 55:1125-1132.
- GetachewG. BlümmelM. MakarH.P.S. BeckerK. 1998. In vitro gas measuring techniques for assessment of nutritional quality of feeds. Anim. Feed Sci. Tech. 72:261-281.
- GetachewG. MakkarH.P.S. BeckerK. 2002. Tropical browses: contents of phenolic compounds, in vitro gas production and stoichiometric relationship between short chain fatty acid and in vitro gas production. J. Agric. Sci. 139:341-352.
- GilboaN. PerevolotskyA. LanduaS. NitsanZ. SilanikoveN. 2000. Increasing productivity in goats grazing Mediterranean woodland and scrubland by supplementation of polyethylene glycol. Small Ruminant Res. 38:183-190.
- Guimarães-BeelenP.M. BerchielliT.T. BeelenR. MedeirosA.N. 2006. Influence of condensed tannins from Brazilian semiarid legumes on ruminal degradability, microbial colonization and ruminal enzymatic activity in Saanen goats. Small Ruminant Res. 61:35-44.
- GurbuzY. 2007. Determination of nutritive value of leaves of several Vitis vinifera varieties as a source of alternative feedstuff for sheep using in vitro and in situ measurements. Small Ruminant Res. 71:59-66.
- KumarR. SingM. 1984. Tannins: their adverse role in ruminant nutrition. J. Agr. Food Chem. 32:447-453.
- KumarR. VaithiyanathanS. 1990. Occurrence, nutritional significance and effect on animal productivity of tannins in tree leaves. Anim. Feed Sci. Tech. 30:21-38.
- LandauS. PerevolotskyA. BonfilD. BarkaiD. SilanikoveN. 2000. Utilization of low quality resources by small ruminants in Mediterranean agro-pastoral systems: the case of browse and aftermath cereal stubble. Livest. Prod. Sci. 64:39-49.
- MakkarH.P.S., 2000. Quantification of tannins in tree foliage. A laboratory manual. FAO/IAEA ed., Vienna, Austria.
- MakkarH.P.S., 2003. Quantification of tannins in tree and shrub foliage. A laboratory manual. Kluwer Academic Publ., Dordrecht, The Netherlands.
- MakkarH.P.S., 2005. Use of nuclear and related techniques to develop simple tannin assays for predicting and improving the safety and efficiency of feeding ruminants on tanniniferous tree foliage. Summary of the FAO/IAEA Coordinated Research Project. Anim. Feed Sci. Tech. 122:3-12.
- MakkarH.P.S. SinghB. 1991. Distribution of condensed tannins (proanthocyanidins) in various fraction of young and mature leaves of some oak species. Anim. Feed Sci. Tech. 32:253-260.
- MakkarH.P.S. SinghB. 1993. Effect of storage and urea addition on detannification and in sacco dry matter digestibility of mature oak (Quercus incana) leaves. Anim. Feed Sci. Tech. 41:247-259.
- MakkarH.P.S. BlümmelM. BeckerK. 1995. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and their implication in gas production and true digestibility in vitro techniques. Brit. J. Nutr. 73:897-913.
- MakkarH.P.S. BlümmelM. BorowyN.K. BeckerK. 1993. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J. Sci. Food Agr. 61:161-165.
- ManganJ.L. 1988. Nutritional effects of tannins in animal feeds. Nutr. Res. Rev. 1:209-231.
- McNeillD.M. OsborneN. OsborneN. KomolongM.K. NankervisD. 1998. Condensed tannins in the genus Leucaena and their nutritional significance for ruminants. pp 205-214 in Proc. Conf. Leucaena: adaptation, availability and farming system, Hanoi, Vietnam.
- McSweeneyC.S. PalmerB. McNeillD.M. KrauseD.O. 2001. Microbial interaction with tannins: nutritional consequences for ruminants. Anim. Feed Sci. Tech. 91:83-93.
- MenkeK.H. SteingassH. 1988. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28:7-55.
- MinB.R. BarryT.N. AttwoodG.T. McnabbW.C. 2003. The effect of condensed tannins on the nutrition of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Tech. 106:3-19.
- NdlovuL.R. NhereraF.V. 1997. Chemical composition and relationship to in vitro gas production of Zimbabwean browsable indigenous tree species. Anim. Feed Sci. Tech. 69:121-129.
- NortonB.W. 2003. The Nutritive value of tree legumes. In: GutteridgR.C. SheltonH.M. ( eds.), Forage tree legumes in tropical agriculture. Available from: http://www.fao.org/ag/AGP/AGPC/doc/Publicat/Gutt-shel/x5556e0j.htm
- PalmerB. JonesR.J. 2000. The effect of PEG addition in vitro on dry matter and nitrogen digestibility of Calliandra calothyrsus and Leucaena leucocephala leaf. Anim. Feed Sci. Tech. 85:259-268.
- PorterL.J. HirstichL.N. ChanB.G. 1986. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25:223-230.
- PritchardD.A. StocksD.C. O’SullivanB.M. MartinP.R. HurwoodI.S. O’RourkeP.K. 1998. The effect of polyethylene glycol (PEG) on wool growth and live weight of sheep consuming a mulga (Acacia aneura) diet. Proc. Aust. Soc. Anim. Prod. 17:290-293.
- ReedJ.D. SollerH. WoodA. 1990. Fodder tree and straw diets for sheep: intake, growth, digestibility and the effect of phenolics on nitrogen utilization. Anim. Feed Sci. Tech. 30:39-50.
- RubanzaC.D.K. ShemM.N. OtsyinaR. BakengesaS.S. IchinoheT. FujiharaT., 2005. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Tech. 119:129-142.
- SAS, 2002. SAS user’s guide: statistics. Version 9.0. SAS Inst. Inc., Cary, NC, USA.
- SilanikoveN. GilboaN. NirI. PerevolotskyZ. NitsanZ. 1996a. Effect of a daily supplementation of polyethylene glycol on intake and digestion of tannin-containing leaves (Quercus calliprinos, Pistacia lentiscus, Ceratonia siliqua) by goats. J. Agr. Food Chem. 44:199-205.
- SilanikoveN. GilboaN. PerevolotskyZ. NitsanZ. 1996b. Goats fed tannin containing leaves do not exhibit toxic syndromes. Small Ruminant Res. 21:195-201.
- SinghB. SahoA. SharmaR. BhatT.K. 2005. Effect of polyethylene glycol on gas production parameters and nitrogen disappearance of some tree forages. Anim. Feed Sci. Tech. 123-124:351-364.
- Van SoestP.J. RobertsonJ.B. LewisB.A. 1991. Methods for dietary fibre, neutral detergent fibre, and non-starch carbohydrates in relation to animal nutrition. J. Dairy Sci. 74:3583-3597.
