Abstract
The aim of this study was to determine the nutritional characteristics of Zapoteca tetragona (Willd.) H. Hern to assess the suitability of this plant for ruminant nutrition. The nutritional evaluation consisted of in vitro and in vivo trials. Secondary compounds including total phenols, condensed tannin and non-protein amino acids (NPAA) were determined. Two stage in vitro digestibility was conducted using substrates with increasing levels of Z. tetragona replacing elephant grass (Pennisetum purpureum) as control feed. The inclusion of 30% Z. tetragona was compared to 100% elephant grass by in vitro gas production technique and in vivo digestibility trial using sheep. Forage from Z. tetragona was appreciably high in crude protein (CP) and lower in neutral detergent fibre. Moreover, it was rich in Ca and P. Total phenols, condensed tannin and NPAA contents were very low. In vitro gas production technique showed that after 48 h incubation, the gas produced from Z. tetragona was higher than elephant grass (P<0.05). Increasing level of Z. tetragona led to better dry matter (DM) and CP digestibility compared to elephant grass. In vivo trial showed no difference in DM intake between the two tested feed, however higher CP intake was reported when sheep fed Z. tetragona as well as for CP digestibility and N retention (P<0.05). It can be concluded that Z. tetragona has a strong potential as forage crop with valuable nutritional quality. Moreover, Z. tetragona could represent an alternative feedstuff to conventional forage and a promising substitute fodder in tropical ecosystem.
Introduction
There is currently an increasing interest in the production of alternative forage crops for ruminant feeding. This reflects both the potentially lower production costs per unit of energy associated with some alternative forage crops and the ability of some of these crops to increase total dry matter (DM) intake and improve animal productions (Khan and Habib, Citation2012; Tufarelli et al., Citation2012). Leaves of browse species are potential source of nutrients that could be used to improve the production of ruminants consuming tropical pastures of low nutritive value (Barakat et al., Citation2013). Tree leaves are richer in crude protein (CP), minerals and digestible nutrients than grasses (Olafadehan, Citation2013).
Zapoteca tetragona (Willd.) H. Hern. is a tropical leguminous shrub widely cultivated in the forest buffers area of Indonesia. This shrub originally came from Latin or Central America and brought to Indonesia by the forestry people. Previously, Z. tetragona was called white Calliandra since the flower has white colour, if compared to the red Calliandra (Calliandra calothyrsus) having red flower. Z. tetragona is a fast growing species and regrows well after several cuttings. Compared to other tropical shrub species, such as Acacia angustisima, Calliandra callothysus and Leucaena diversifolia, the DM biomass production of Z. tetragona is higher (Sajimin and Purwantari, Citation2006). The protein content of Z. tetragona is quite high and it represents a valuable protein source compared to other tropical shrubs (Laudadio et al., Citation2009; Cazzato et al. Citation2013). However, to date the information of Z. tetragona forage as alternative feed for ruminants are limited, and little attention has been given to the nutritional evaluation of Z. tetragona in ruminant feeding. Moreover, since the Z. tetragona leaves contain several secondary compounds which may influence the animal production, it is necessary to assess the nutritional value of Z. tetragona through not only chemical analyses but also by in vitro techniques and in vivo trials.
Materials and methods
Study area and sampling
Z. tetragona and elephant grass (Pennisetum purpureum) were locally produced from mature crops after harvesting of plants in the research facility of the Indonesian Research Institute for Animal Production, Bogor, Indonesia (7°5’S latitude, 107°40’E longitude). The crops were harvested, wilted, and transported to the research facility. The feeding trail was conducted at the animal research facilities of Indonesian Research Institute for Animal Production, Bogor, Indonesia.
In vivo digestibility trial
A metabolism trial was conducted lasted for 21 d in metabolism cages using eight healthy sheep of 24±2.2 kg live body weight (BW) with facility of quantitative collection of faeces and urine separately. Each animal received the two diets in different periods, following a Latin square design. Each period consisted of 14 days of adaptation to diet in individual stalls with a concrete floor, two days of adaptation to faecal collection bags applied on bucks, and five days of faeces collection (Givens et al., Citation2000). Daily feed intake was also recorded. The diets consisted of a mixture of elephant grass and Z. tetragona in the ratio of 70:30 w/w and a control diet of 100% of elephant grass, both diets at a level of 4% BW per day. The proximate composition of elephant grass and Z. tetragona was reported in . Samples of feed offered, orts, faeces and urine voided were collected every morning. A 0.1 portion of faeces and urine, respectively, were pooled over the 5-day collection period and finally sub-sampled to obtain representative samples for analysis. Rumen liquor (50 mL) was taken from each sheep in the morning before feeding, using an oesophageal tube under mild vacuum from the reticulum near the reticuloomasal orifice, and filtered through two layers of cheesecloth. An aliquot of the filtered rumen liquor was collected for protozoal and bacterial counts (Ogimoto and Imai, Citation1981), and another aliquot of filtered rumen liquor was collected for the determination of the ammonia concentration (Conway and Byrne, Citation1933).
Two-stage in vitro digestibility
In order to determine the best level of Z. tetragona, different percentages (15, 30, 45 and 100%, respectively) were tested by in vitro fermentation assay. The Tilley and Terry (Citation1963) rumen fluid/pepsin two-stage in vitro technique was used to estimate the in vitro DM digestibility and CP digestibility. Results were compared with control incubations (i.e. samples without Z. tetragona) using elephant grass. Rumen fluid was collected from sheep via rumen cannulae before the morning meal was pooled, placed in a prewarmed (39°C) vacuum flask and transported immediately to the laboratory. The rumen fluid was diluted (1:2 v/v) with a culture medium containing macro- and micromineral solutions, resazurin and bicarbonate buffer solution prepared as described by Menke and Steingass (Citation1988). The medium was kept at 39°C and saturated with CO2. Samples (0.5 g) of each forage (control or with one of the Z. tetragona levels) were weighed in triplicate and placed in bottles, to which were added 40 mL of rumen fluid/artificial saliva solution. The bottles were kept for 48 h in a 39° C water bath. The in vitro test bottles from the same ingredient were split in two groups to measure rumen degradation and to perform the gastric digestion.
In vitro gas production technique
The estimates of gas production were obtained through the method described by Menke and Steingass (Citation1988). The gas production effects were evaluated using Z. tetragona (30%) and elephant grass (100%). The rumen fluid was obtained as described in the previous section. Incubations were conducted in 100 mL syringes, which were rinsed (distilled water) and dried before each study, then flushed with CO2 prior to the addition of substrate containing 200 mg DM of each sample and 30 mL of buffered rumen fluid. The treatments were elephant grass (control) and Z. tetragona added in syringe, with four syringes per treatment. The syringes were plugged with the pistons smeared by vaseline and kept in a 39°C water bath. Gas production was recorded after 2, 4, 6, 8, 10, 12, 16, 24, 30 and 48 h of incubation by pressure readings. The results were corrected to blank samples (flasks containing buffered rumen fluid without the forages) and standard samples to 24 h of incubation and jointly with the levels of forage chemical components.
Chemical analysis
Samples of Z. tetragona, elephant grass and dry faeces were ground in a hammer mill with a 1 mm screen and analysed in triplicate for DM, ash (967.05), CP (Kjeldahl N×6.25; 990.03), ether extract (945.16), Ca and P according to AOAC (Citation2000). The neutral detergent fibre (NDF), acid detergent fibre (ADF) and lignin were analysed according to Mertens (Citation2002), AOAC (Citation2000; 973.187), and Van Soest et al. (Citation1991), respectively, using the sequential procedure and the filter bag system (Ankom Technology, Macedon, NY, USA). The NDF and ADF fractions include residual ash. Total phenols and condensed tannin analysis were carried out following the methods described by FAO/IAEA (Citation2000) and non-protein amino acids (NPAA) according to the AOAC (Citation2000). Rumen ammonia was analysed by the Kjeldahl method (990.03; AOAC, Citation2000).
Statistical analysis
Data on the intake, digestibility, N retention and ruminal parameters in sheep were analysed for the fixed effect of diet and random effects of feeding period and sheep, using PROC MIXED procedure of the SAS (SAS, Citation2004). The DM and CP digestibility data were compared using t-test (Steel et al., Citation1997). Similar design was used also for the two-stage in vitro digestibility and in vitro gas production.
Results and discussion
The effects of dietary Z. tetragona in ruminant diets have not been previously reported, and the cross referencing in the discussion of findings in this report will be based on findings from other tropical leguminous shrubs. Therefore, this subject should be considered in new investigations.
Z. tetragona forage had a higher content of CP (23.5 vs 17.5% DM) and lower one of NDF (27.6 vs 56.2% DM) compared with elephant grass (). Moreover, Z. tetragona was rich in Ca (1.7% DM) and P (0.33% DM), and it also contained an adequate amount of phenols (3.31%) and low levels of condensed tannin and NPAA (0.73 and 0.6%, respectively). The total condensed tannin in Z. tetragona could result insufficient to bind protein causing less degradation (Waghorn, Citation2008). Secondary compounds in Z. tetragona did not seem to affect the activity of rumen microbes and rumen fermentation in degrading forage. Moreover, the CP may be soluble or easily degradable by rumen microbes.
The replacement of elephant grass with Z. tetragona in the sheep ration resulted in a significant increase of CP intake (P<0.001), whereas DM intake resulted similar between the two treatments (). However, all in all, the intake of all nutrients increased consistently with the inclusion of Z. tetragona in the ration, indicating that Z. tetragona is palatable for sheep. The amount (g/day) of N retained in the body increased significantly (P<00.01) from 2.34 to 14.45 g/day with the substitution of elephant grass with Z. tetragona (). This findings suggest that the additional N supplied by Z. tetragona forage was efficiently utilised and resulted in a greater N retention as also recently found by Khan and Habib (Citation2012) using peanut (Arachis hypogaea) forage.
Apparent in vivo digestibility of DM and CP showed that in sheep fed Z. tetragona the CP digestibility was improved (P=0.003), whereas DM digestibility was unaffected by dietary treatment. Our results are in line with previous findings of Khamseekhiew et al. (Citation1991) in another tropical leguminous species Gliricidia sepium, as protein source replacing elephant grass, indicated that total DM and CP digestibility using 40% Gliricidia in ruminant ration were higher compared to control ration. Further, the CP digestibility of Z. tetragona was also higher than another tropical leguminous forage as red calliandra (Calliandra calothyrsus) (Palmer and Jones, Citation2000). Therefore, we can state that Z. tetragona resulted a species highly degradable in the rumen.
Ruminal ammonia concentration, and total bacteria and protozoa counts were unaffected by dietary treatments, resulting similar in sheep fed elephant grass or Z. tetragona. These positive findings could be relate to the secondary compounds in Z.tetragona that not showed any negative effect on total bacteria or protozoa in the sheep rumen. This also explains that the high total bacteria and protozoa will provide an high degradation rate in the rumen.
Data on the effect of substituting elephant grass with different levels of Z. tetragona on two-stage in vitro DM and CP digestibility are reported in . Digestibility of DM was higher (P<0.05) for Z. tetragona at all levels of inclusion compared to the control elephant grass. Similarly, the CP digestibility was higher (P<0.05) for Z. tetragona, in particular at 15% of inclusion when incubated using rumen liquor and pepsin. The improved digestibility in the Z. tetragona as compared to elephant grass is consistent with the previous studies involving Guinea grass with Ficus religiosa (Bamikole, Citation2003) or Pterocarpus erinaceus combined with Gamba grass (Andropogon gayanus; Olafadehan, Citation2013). It seems that Z. tetragona resulted in improved rumen ecology and a faster degradation of the diets compared to the elephant grass. The higher nutrient digestibility of our alternative could be attributed to lower fibre contents of the Z. tetragona than elephant grass. The lower fibre of Z. tetragona could result in fast degradation. Moreover, legumes are generally retained in the rumen for a shorter time than grasses (Khan et al., Citation2012).
The in vitro gas production technique trial showed that starting from the first 3 h of incubation that the gas produced by 30% Z. tetragona was higher than 100% of elephant grass, and after 48 h of incubation the total gas production was 83.35 mL for 30% Z. tetragona and 72.2 mL for elephant grass, respectively (). The higher volume of gas produced when Z. tetragona was included reflects the higher ruminal degradability of this tropical leguminous shrub. Moreover, this increase suggests that more soluble carbohydrates and proteins were made available to microorganisms as supplementation increased (Arhab et al. Citation2009). This may also explain the increase in cumulative gas production of Z. tetragona at 48 h as previously reported by Chenost et al. (Citation2001).
Conclusions
Forage from Z. tetragona was significantly high in CP and low in NDF compared with conventional tropical forages. Due to the high nutritional value, the use this tropical shrub improved intake, digestibility and N retention in sheep. These findings show that including Z. tetragona in ruminant diet can sustain animal production and minimise the production losses during the feed shortage periods in tropical areas.
Acknowledgments
The authors would like to thank the Indonesian Research Institute for Animal Production, Bogor, Indonesia, and Prof. J.B. Liang for their support. Part of this work was presented at the 4th International Conference on Sustainable Animal Agriculture for Developing Countries (SAADC2013), 27-31 July 2013, Lanzhou, China.
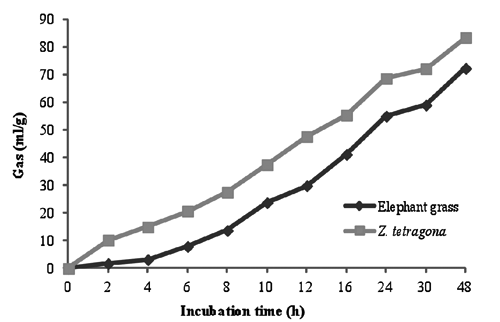
Table 1. Proximate chemical composition of forage from Z. tetragona and elephant grass.
Table 2. Dry matter and crude protein intake, in vivo digestibility, N retention and rumen parameters in sheep fed elephant grass (100%) and Z. tetragona (30%).
Table 3. In vitro dry matter and crude protein digestibility of elephant grass and Z. tetragona at different inclusion levels.
References
- AOAC, 2000. Official methods of analysis. 17th ed., Association of Official Analytical Chemists, Arlington, VA, USA.
- ArhabR. MacheboeufD. AggounM. BoussebouaH. VialvaD. BesleJ.M., 2009. Effect of polyethylene glycol on in vitro gas production and digestibility of tannin containing feedstuffs from North African Arid Zone. Trop. Subtrop. Agroecosyst. 10:475-486.
- BamikoleM.A., 2003. Macro-minerals bioavailability study in goats fed forages of nitrogen fertilized Guinea grass and Guinea grass-Verano stylo mixture. Available from: http://www.lrrd.org/lrrd15/12/bami1512.htm
- BarakatN.A.M., LaudadioV. CazzatoE. TufarelliV., 2013. Potential contribution of retama raetam (Forssk.) Webb & Berthel as a forage shrub in Sinai, Egypt. Arid Land Res. Manag. 27:257-271.
- CazzatoE. TufarelliV. LaudadioV. StellacciA.M. SelvaggiM. LeoniB. TroccoliC., 2013. Forage yield and quality of emmer (Triticum dicoccum Schübler) and spelt (Triticum spelta L.) as affected by harvest period and nitrogen fertilization. Acta Agr. Scand. B-S. P. 63:571-578.
- ChenostM. AufrereJ. MacheboeufD., 2001. The gas-test technique as a tool for predicting the energetic value of forage plants. Anim. Res. 50:349-364.
- ConwayE.J. ByrneA., 1933. An absorption apparatus for the micro-determination of certain volatile substances. The micro-determination of ammonia. Biochem. J. 27:419-429.
- FAO-IAEA, 2000. Quantification of tannins in tree foliage. FAO-IAEA ed., Vienna, Austria.
- GivensD.I. OwenE. AxfordR.F.E. OmedH.M., 2000. Forage evaluation in ruminant nutrition. CABI Publishing, Wallingford, UK.
- KhamseekhoewB. LiangJ.B. WongC.C. JalaniZ.A., 1991. Ruminal and intestinal digestibility of some tropical legume forages. Asian Austral. J. Anim. 14:321-325.
- KhanN.A. HabibG., 2012. Assessment of Grewia oppositifolia leaves as crude protein supplement to low-quality forage diets of sheep. Trop. Anim. Health Pro. 44:1375-1381.
- LaudadioV. TufarelliV. DarioM. HammadiM. SeddikM.M. LacalandraG.M. DarioC., 2009. A survey of chemical and nutritional characteristics of halophytes plants used by camels in Southern Tunisia. Trop. Anim. Health Pro. 41:209-215.
- MenkeK.H. SteingassH., 1988. Estimation of the energetic feed value from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 28:47-55.
- MertensD.R., 2002. Gravimetrical determination of amylase-treated neutral detergent fiber in feeds with refluxing in beakers or crucibles: collaborative study. J. AOAC Int. 85:1217-1240.
- OgimotoK. ImaiS., 1981. Atlas of rumen microbiology. Japan Scientific Society Press, Tokyo, Japan.
- OlafadehanO.A., 2013. Feeding value of Pterocarpus erinaceus for growing goats. Anim. Feed Sci. Tech. 185:1-8.
- PalmerB. JonesR.J., 2000. The effect of PEG addition in vitro on dry matter and nitrogen digestibility of Calliandra calothyrsus and Leucaena leucocephala leaf. Anim. Feed Sci. Tech. 85:259-268.
- SajiminY.C. PurwantariN.D., 2006. Forage production of some legume tree. pp 952-957 in Proc. Nat. Conf. Anim. Vet. Technol., Puslitbang Peternakan, Bogor, Indonesia.
- SAS, 2004. SAS/STAT 9.13 user’s guide. SAS Inst. Inc., Cary, NC, USA.
- SteelR.G.D., TorrieJ.H. DickyD.A., 1997. Principles and procedures of statistics: a biometrical approach. WCB/McGraw-Hill, New York, USA.
- TilleyJ.M.A., TerryR.A., 1963. A two-stage technique for the in vitro digestion of forage crops. J. Brit. Grassland Soc. 18:104-111.
- TufarelliV. KhanR.U. LaudadioV., 2012. Evaluating the suitability of field beans as a substitute for soybean meal in early-lactating dairy cow: production and metabolic responses. Anim. Sci. J. 83:136-140.
- Van SoestP.J. RobertsonJ.B. LewisB.A., 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583-3592.
- WaghornG., 2008. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production. Progress and challenges. Anim. Feed Sci. Tech. 147:116-139.
