Abstract
Previous studies showed that monochromatic green light stimuli during embryogenesis accelerated posthatch body weight and pectoral muscle growth of broilers. In this experiment, we further investigated whether the regulation of broiler embryonic or posthatch growth by green light stimulus during incubation is associated with the changes of some important hormones at different ages of embryos and broiler chickens. Fertile broiler eggs (Arbor Acres, n=880) were pre-weighed and randomly assigned 1 of 2 incubation treatment groups: i) dark condition (control group), and ii) monochromatic green light group (560 nm). The monochromatic lighting systems sourced from light-emitting diode lamps were equalised at the intensity of 15 lux (lx) at eggshell level. The dark condition was set as a commercial control from day one until hatching. After hatch, 120 day-old male chicks from each group were housed under white light with an intensity of 30 lx at bird-head level. Compared with the dark condition, chicks incubated under the green light showed significantly higher growth hormone (GH) levels from 19 d of embryogenesis (E19) to 5 d of posthatch (H5), and higher plasma insulinlike growth factor (IGF-I) levels from both E17 to E19 and H3 to H35. No significant differences were found in plasma thyroxine, triiodothyronine, and testosterone in embryos or hatched birds between the 2 groups. These results indicate that somatotropic axis hormones (GH and IGF-I) may be the most important contributor to chicken growth promoted by green light stimuli during embryogenesis.
Introduction
It is widely recognised that neuroendocrine hormones has many metabolic effects in the regulation of the somatic (skeletal muscle and bone) growth and body composition in avian species. The hormones of both the somatotrophic axis [growth hormone (GH); insulinlike growth factors] and the thyrotrophic [triiodothyronine (T3) and thyroxine (T4)] axis are considered to be prerequisite for normal growth and development (Decuypere and Buyse, Citation2005). Growth hormone has been shown to increase protein synthesis and to decrease proteolysis and protein oxidation rates, resulting in a net increase in proteic anabolism (Jorgensen et al., Citation1994; Bell et al., Citation1998). Postnatal growth of broilers is improved by injecting mammalian (ovine and bovine) GH (Hargis et al., Citation1989; Dean et al., Citation1993) or chicken GH (Kocamis et al., Citation1999) in fertile broiler breeder eggs, whereas most of the attempts to use mammalian (bovine and porcine) or chicken GH preparations to promote growth of hatched chicks, or to promote egg production of both pullets and mature hens were unsuccessful (Carter et al., Citation1955; Cogburn et al., Citation1989; Moellers and Cogburn, Citation1995). Thus, the circulating GH in chick embryos seems to play an important role in embryo development and postnatal growth. It is well established that the anabolic effects of GH on muscle are mediated by insulin-like growth factor-I (IGF-I), which stimulates myoblast and satellite cell proliferation and terminal myogenic differentiation (Banes et al., Citation1995; Florini et al., Citation1996), and induces hypertrophy and block atrophy in adult skeletal muscle (Adams and McCue, Citation1998; Chakravarthy et al., Citation2000). In ovo administration of recombinant human IGF-I improves the feed efficiency, tissue (breast and leg) growth of posthatch broilers without adverse affects on meat tenderness (Kocamis et al., Citation1998). Thyroid hormones are also crucial endocrine regulators of the embryonic development, hatching process, and posthatch metabolisms of broilers (Decuypere et al., Citation2000; Decuypere and Buyse, Citation2005). Lu et al. (Citation2007) found that both plasma T3 and T4 levels showed significant positive correlations with chick embryonic body weight (BW), suggesting that thyroid hormones might be important for maintaining normal growth. As an important gonadal hormone, plasma testosterone concentrations have positive correlation with growth rates of male broilers raised under intermittent white light provided by incandescent lamps (Kühn et al. Citation1996). In ovo injection of testosterone prior to incubation reduces 12-d-old female embryo weight and protein content of pectoralis superficialis muscle (Henry and Burke, Citation1999). However, monochromatic green light after posthatch was effective in stimulating testosterone secretion and myofiber growth of broilers (Cao et al., Citation2008).
Light stimulation is one of the effective ways to modulate the reproduction and growth in poultry. Rozenboim et al. (Citation2003, Citation2004) reported that intermittent monochromatic green light photostimulation during embryogenesis enhanced the embryo development and posthatch growth in broilers and turkeys. Recently, Zhang et al. (Citation2012, Citation2014) also found that continuous monochromatic green LED light stimuli during embryogenesis accelerated BW and posthatch pectoral muscle growth of broilers. However, whether this growth-promoting effect is mediated through regulation of endocrine axes (somatotrophic, thyroid hormone and gonadal axes) is still unknown. Thus, the present study was set up to investigate the dynamic changes of plasma GHs, thyroid hormones, and testosterone levels in late embryos and broiler chickens incubated under monochromatic green light in the attempt to study the possible endocrine changes.
Materials and methods
Light treatments and animal management
All the experimental procedures were approved by the Animal Care and Use Committee of the Feed Research Institute of the Chinese Academy of Agricultural Sciences, Beijing, China.
Eight hundred and eighty fertile broiler eggs (Arbor Acres) were purchased from a local hatchery (Beijing Huadu Poultry Breeding Co. Ltd., Beijing, China) and preweighed, selected for an average weight of 68.5 g (range=65 to 70 g). Eggs were randomly assigned to one of 2 treatment groups in 2 modified commercial incubators: i) control group (dark condition), and ii) monochromatic green light group (560 nm). The continuous green light system was provided by LED lower-power lamps [IF(forward current)=20 mA, VF(forward voltage)=2.1-2.5V, p(peak wavelength)=565 nm, IV(luminous intensity)=240 mcd, VR(reverse voltage)=5V; Zhongshan Silsmart Optoelectronics Co., Ltd., Zhongshan, China], and was equalised with an average intensity of 15 lux (lx) (13 to 18 lx from the middle to the air cell end of eggs) at eggshell level from the first day of incubation until hatching. The illumination was measured with a digital luxmeter (Mastech MS6610; Precision Mastech Enterprises, Hong Kong, China). Two identical, microcomputer automatic K12SS-1-BO7 incubators (Dezhou Zhicheng Incubation Equipment Co., Ltd., Shandong, China) were calibrated prior to hatching the experimental eggs. The capacity of incubator was 528 eggs with 6 hatching trays each, and the eggs were placed in the trays. There were no differences within the two incubators, as evidenced by the observed growth-promoting effect of green light stimuli in either incubator when we repeated the experiment with treatments applied to alternate incubators. Incubation conditions were 37.8±0.1°C and 60% relative humidity, and the eggs were turned through 270° every 1.5 h until d 19. Then, eggs were moved to a hatcher and incubated at 37.6°C, relative humidity 70%, in a stationary position until hatch. On d 10 of embryogenesis, all eggs were candled, and infertile eggs were recorded and then removed. The fertilisation rate of eggs was 93.38% in our present study.
After hatch, each bird was weighed, sexed, and tagged. One hundred and twenty male chicks from each incubation group were placed in 6 replicates with 20 birds each. All of the birds were housed under white light (30 lx at bird-head level) with a light schedule of 23 h light and 1 h dark according to Rozenboim et al. (Citation2004) and lights off from 21:00 h to 22:00 h as described by Zhang et al. (Citation2012). All the birds were allowed ad libitum access to a commercial diet for broiler chickens and 24-h access to water. The diet met the requirements of broilers recommended by the National Research Council (Citation1994). All birds were housed in an environmentally controlled rearing house. The temperature in the chicken house was set at 33°C for the first 7 d and was reduced by 3°C each consecutive week until it reached 24°C. The stocking densities of broilers were 20 to 15 birds/m2 from the first to 21 d and 15 to 13 birds/m2 from 22 to 42 d, respectively.
Sampling procedure
All blood samples were taken at approximately the same time of day (between 0800 and 1000 h) to avoid physiological fluctuations in circulating hormone levels. On d 15 of embryogenesis (E15), E17 and E19, twelve embryos (2 embryos form each set layer, and 6 layer of each incubator means 6 replicates per treatment) from each group were randomly selected. Eggs were opened at the air cell end and the entire embryos (including yolk sac and membrane) were removed. Immediately, about 0.6 to 0.8 mL blood was obtained with a 1-mL heparinised syringe from umbilical artery. Two blood samples from the same replicate were merged into a heparinised tube and mixed to ensure enough plasma for hormone analysis. The tubes were centrifuged at 1800×g for 10 min to obtain plasma samples. On d 1, 3, 5, 7, 21, 35 and 42 of hatch (H1, H3, H5, H7, H21, H35 and H42), after an 8-h fast, blood was drawn from each bird (1 bird per replicate) by heart puncture, placed into heparinised tubes, centrifuged and then at 1800×g for 10 min to obtain plasma. All plasma samples were stored at -20°C for later analysis of selected hormones.
Hormone analysis
Plasma GH, T3, T4 and testosterone concentrations were analysed using homologous available 125I-labelled RIA kits (Beijing North Institute of Biological Technology, Beijing, China). These commercial RIA kits were validated for measuring chicken samples (Li et al., Citation2007; Cao et al. Citation2008; Rao et al., Citation2009). All samples were analysed within 1 batch assay to avoid inter-assay variations. The minimum detectable dose was 0.5 ng/mL for GH, 50 pg/mL for T3, 3 ng/mL for T4, and 20 pg/mL for testosterone. Intra-assay coefficients of variation (CVs) were 6.7% for GH, 8.2% for T4, 7.9% for T3, and 11.8% for testosterone.
Plasma IGF-I concentrations were measured using a commercial Elisa kit of anti-chicken IGF-I (USCN Life Science and Technology Company, Wuhan, China; Liu et al., Citation2010) according to the manufacturer’s protocol. Each sample was evaluated in duplicate. The IGF-I concentrations were determined using a standard curve. The minimum detectable concentration was 1.56 ng/mL. The intra-assay and inter-assay CVs were lower than 5.3 and 8.9%, respectively.
Statistical analysis
Data were presented as mean±standard error. All statistical analyses were performed by a Student’s t-test using JMP version 5 software (SAS, Citation2002). The significance level was set at P<0.05.
Results
Somatotrophic axis hormones (growth hormone and insulin-like growth factor-I)
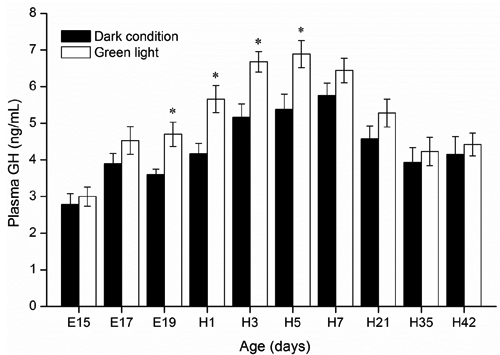
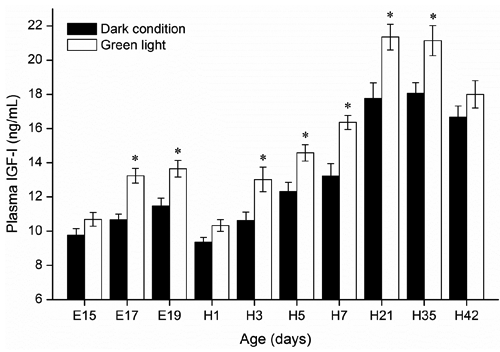
As shown in , the lowest plasma GH level (2.8 ng/mL) was detected in 15-d-old embryos in the control group, and a highest level (6.9 ng/mL) was detected in 5-d-old birds incubated under green light. Compared to the control group, significantly higher plasma GH levels in embryos and hatched birds incubated under green light were observed from E19 to H5 (P<0.05). In both groups, plasma GH levels gradually declined with age in chickens from H7 to H42. Dynamic changes in plasma IGF-I in embryos and hatched chickens are shown in . Green light stimuli during incubation significantly increased plasma IGF-I levels of embryos from E17 to E19, and of birds from H3 to H35 than those incubated under dark condition (P<0.05). Compared with E19, the significant decrease in plasma IGF-I in day-old chicks of both control (E19=11.48 ng/mL; H1=9.35 ng/mL; P<0.05) and green group (E19=13.65 ng/mL; H1=10.29 ng/mL; P<0.05) was observed.
Thyrotrophic axis hormones (thyroxine and triiodothyronine)
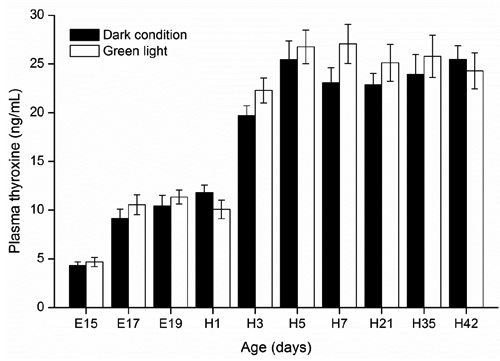
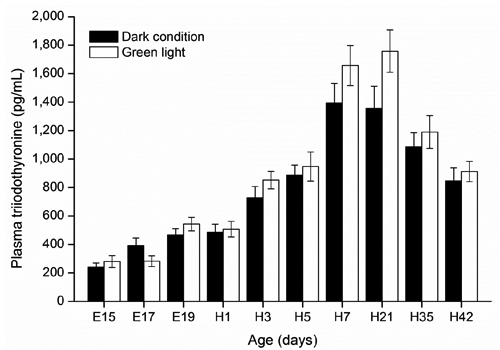
There were no significant changes in plasma T4 levels in embryos or hatched chickens incubated under dark condition or green light (P≥0.05; ). In both groups, plasma T4 dramatically increased from E15 (4.3 ng/mL) to H3 (22.3 ng/mL) except for a decrease in green light group on day H1, and then reached a plateau at H3 and remained still until H42 (27.1 ng/mL). Both plasma T4 and T3 levels in chick embryos and hatched chickens were not affected by green light stimuli during incubation ( and ). In addition, green light group showed a trend in increasing plasma T3 levels in hatched birds from H7 (P=0.09) to H21 (P=0.06) compared with the control group.
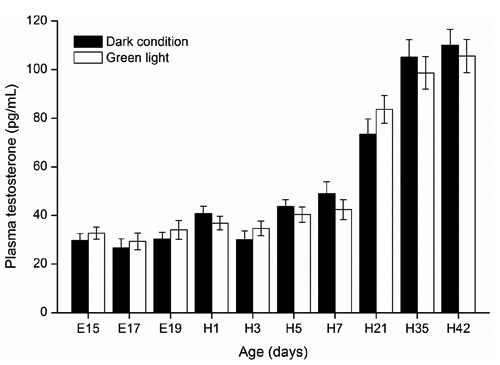
Gonadal axis hormone (testosterone)
shows dynamic changes in plasma testosterone in embryos and hatched birds. Plasma testosterone levels were low and stable in late embryos and early newly hatched chicks from E15 to H7, and then dramatically increased from H7 to H42. No significant differences were found in plasma testosterone between the green light and the dark light groups in embryos and hatched chickens.
Discussion
In a previous study, Lu et al. (Citation2007) reported there were no significant effects of sex on plasma hormones levels in chick embryos. Therefore, we did not sex embryos in the current study. In avian species, circulating GH is required for the normal growth and development of chick embryos and hatched birds. Some of the previous studies have focused on the light management and the growth performance and secretion of GH in poultry. Lighting stimuli enhanced GH secretion from pituitary somatotroph cells of male broilers, and compensatory growth in broilers was associated with an amplification of GH secretory burst mass (Kühn et al., Citation1996; Buyse et al., Citation1997). Our present study found that the plasma GH increased in chick embryos, and then gradually decreased in 7- to 42-d-old broilers, which confirms the findings of Harvey et al. (Citation1979) who showed an increase in plasma GH from embryonic d 17 to the peak and then decreased progressively since d 5 posthatch. A significantly higher plasma GH level in birds incubated under green light was observed from E19 to H5 in the present study, implicating that the light-sensitive phase of GH secretion from pituitary somatotrophs to continuous green light stimuli during incubation occurred in late embryogenesis, and its effect could last to early posthatch period.
Insulin-like growth factor-I is an important regulating factor in cellular proliferation and differentiation, and tissue growth (Florini et al., Citation1996). Plasma IGF-I is detectable as early as embryonic d 10, it increases until a plateau is reached from embryonic d 13 to d 18, and it decreases before hatching (Lu et al., Citation2007). In the present study, the changes of plasma IGF-I levels during the embryonic period are similar to those of Lu et al. (Citation2007). Besides, we observed that plasma IGF-I also dramatically increased and reached a plateau with age after hatching. Liu et al. (Citation2010) found that circulating IGF-I levels were higher in chicks reared under monochromatic green light concomitantly with the rise in satellite cell proliferation vs white and red lights. Therefore, IGF-I plays a central role for green light illumination promoting chick satellite cell myogenic processes during early posthatch stages (Liu et al., Citation2010). Johnson et al. (Citation1990) reported that there was no correlation between plasma IGF-I levels and the pulsatile pattern of GH secretion in broiler chickens. However, McMurtry et al. (Citation1997) demonstrated that IGF-I synthesis is both GH-dependent and GH-independent, and autocrine-paracrine IGFs action plays an important role in the process. In the present study, an increasing trend of plasma IGF-I levels in corresponding with the plasma GH levels during E15 to E19 suggested that circulating IGF-I is GH-dependent for embryo birds during late embryogenesis stage. The decline in plasma GH preceded the decline in circulating IGF-I, suggesting that IGF-I and GH are weakly coupled during late posthatch development of the chicken. Besides, we found a significant decrease in plasma IGF-I in day-old chicks, though Lu et al. (Citation2007) reported that plasma IGF-I levels increased in day-old chicks. Feed intake can decrease plasma levels of IGF-I in day-old chicks (Guernec et al., Citation2003), which might be the reason for the dramatically decrease in plasma IGF-I of day-old broilers in the current study. Our present study also showed that green light stimuli during incubation significantly increased plasma IGF-I levels of embryos from E17 to E19, and of chickens from H3 to H35 than those incubated under dark condition. IGF-I stimulates the proliferation and DNA synthesis in chicken muscle satellite cells (Banes et al., Citation1995; Florini et al., Citation1996), enhances glucose and amino acid uptake and protein synthesis, and inhibits protein degradation in chicken muscle cells (Duclos et al., Citation1999), and subsequently induces the hypertrophy of adult skeletal muscle (Adams and McCue, Citation1998; Chakravarthy et al., Citation2000), which may partially explain why green light stimuli during embryogenesis enhanced embryo development and posthatch growth in broiler chickens in the previous studies (Rozenboim et al., Citation2004; Zhang et al., Citation2012, Citation2014).
The elevated plasma T4 level before hatching in embryos of both green light and control groups in this study is in agreement with the previous reports by McNabb (Citation2000), Reyns et al. (Citation2003) and Lu et al. (Citation2007), suggesting that T4 is important for stimulating a variety of developmental and metabolic processes necessary for successful hatching. We also observed a rise in plasma T3 levels at the late embryo developmental stage. This result is in line with the studies of Decuypere et al. (Citation2000), McNabb (Citation2000) and Reyns et al. (Citation2003) which showed a sharp rise in plasma T3 level when the embryo switches to lung respiration at the late embryonic stage. During this stage, embryo survival rates improved when concentrations of embryonic thyroid hormones increased (Christensen and Davis, Citation2004). In the current study, both plasma T4 and T3 levels in embryo chicks were not affected by continuous green light stimuli during incubation, which may be linked to the absence of significant difference in death of the embryos and hatchability between the green light and control (Zhang et al., Citation2012; Citation2014). In addition, previous studies showed that white light provided by incandescent lamps stimuli during incubation elevated egg core temperature, which further accelerated embryo development and shortened hatching time (Shutze et al., Citation1962; Siegel et al., Citation1969). However, both low-power LED lamps and microcomputer automatic temperature control incubator can adjust the core temperature to maintaining a set value (37.8±0.1°C), which also offers an explanation why no significant difference in hatching time was found in our previous study (Zhang et al., Citation2012).
Light colour (wavelength) was reported to have an influence on the plasma testosterone levels in broilers in previous studies (Cao et al., Citation2008). However, our current result indicated that green light stimuli during embryogenesis did not enhance testis development and testosterone secretion in broiler embryos and hatched male chickens. Thus, we speculate that the growth-promoting effects caused by monochromatic green light stimuli during embryogenesis in our previous studies (Zhang et al., Citation2012, Citation2014) may have no direct relationship with testosterone secretion.
Conclusions
Continuous monochromatic green light stimuli during embryogenesis stimulated systemic secretion of GH and IGF-I of embryos and hatched chickens. However, this light strategy did not affect the secretion of thyroid hormones and testosterone in embryos and hatched birds until 42 days of age. These data suggest that somatotropic axis hormones (GH and IGF-I) may be the most important contributor to chicken growth promoted by green light stimuli during embryogenesis.
Acknowledgements
This study was financed by the National Natural Science Foundation of China (31072048), the Beijing Natural Science Foundation in China (6102022), the China Agriculture Research System Poultry-related Science, and the Technology Innovation Team of Peking (CARS-PSTP).
References
- AdamsG.R. McCueS.A., 1998. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J. Appl. Physiol. 84: 1716-1722.
- BanesA.J. TsuzakiM. HuP. BrigmanB. BrownT. AlmekindersL. LawrenceW.T. FischerT., 1995. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J. Biomech. 28:1505-1513.
- BellA.W. BaumanD.E. BeermannD.H. HarrellR.J., 1998. Nutrition, development and efficacy of growth modifiers in livestock species. J. Nutr. 128:360-363.
- BuyseJ. DecuypereE. VeldhuisJ.D., 1997. Compensatory growth of broiler chickens is associated with an enhanced pulsatile growth hormone (GH) secretion: preferential amplification of GH secretory burst mass. Brit. Poultry Sci. 38:291-296.
- CaoJ. LiuW. WangZ. XieD. JiaL. ChenY., 2008. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poultry Res. 17:211-218.
- CarterR.D. RisnerR.N. YacowitzH., 1955. Some effects of growth hormone preparations in pullets and mature hens. Poultry Sci. 34:1407-1414.
- ChakravarthyM.V. DavisB.S. BoothF.W., 2000. IGF-I restores satellite cell proliferative potential in immobilized old skeletal muscle. J. Appl. Physiol. 89:1365-1379.
- ChristensenV.L. DavisG.S., 2004. Maternal dietary iodide influences turkey embryo thyroid function. Poultry Sci. 3:550-557.
- CogburnL.A. LiouS.S. RandA.L. McMurtryJ.P., 1989. Growth, metabolic and endocrine responses of broiler cockerels given a daily subcutaneous (s.c.) injection of natural or biosynthetic chicken growth hormone. J. Nutr. 119:1213-1222.
- DeanC.E. HargisB.M. BurkeW.H. HargisP., 1993. Alterations in thyroid metabolism are associated with improved posthatch growth of chickens administered bovine growth hormone in ovo. Growth Develop. Aging 57:59-72.
- DecuypereE. BuyseJ., 2005. Endocrine control of postnatal growth in poultry. J. Poult. Sci. 42:1-13.
- DecuypereE. BuyseJ. BuysN., 2000. Ascites in broiler chickens: exogenous and endogenous structural and functional causal factors. World. Poultry Sci. J. 56:367-376.
- DuclosM.J. BeccavinC. SimonJ., 1999. Genetic models for the study of insulin-like growth factors (IGF) and muscle development in birds compared to mammals. Domest. Anim. Endocrin. 17:231-243.
- FloriniJ.R. EwtonD.Z. CoolicanS.A., 1996. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr. Rev. 17:481-517.
- GuernecA. BerriC. ChevalierB. Wacrenier-CereN. Le Bihan-DuvalE. DuclosM.J., 2003. Muscle development, insulin-like growth factor-I and myostatin mRNA levels in chickens selected for increased breast muscle yield. Growth Horm. IGF Res. 13:8-18.
- HargisP.S. PardueS.L. LeeA.M. SandelG.W., 1989. In ovo growth hormone alters growth and adipose tissue development of chickens. Growth Develop. Aging 53:93-99.
- HarveyS. DavidsonT.F. ChadwickA., 1979. Ontogeny of growth hormone and prolactin secretion in the domestic fowl (Gallus Domesticus). Gen. Comp. Endocr. 39:270-273.
- HenryM.H. BurkeW.H., 1999. The effects of in ovo administration of testosterone or an antiandrogen on growth of chick embryos and embryonic muscle characteristics. Poultry Sci. 78:1006-1013.
- JohnsonR.J. McmurtyJ.P. BallardF.J., 1990. Ontogeny and secretory patterns of plasma insulin like growth factor-I in meat type line chickens. Endocrinology 124:81-86.
- JorgensenJ.O. MullerJ. MollerJ. WolthersT. VahlN. JuulA. SkakkebaekN.E. ChristiansenJ.S., 1994. Adult growth hormone deficiency. Horm. Res. 42:235-241.
- KocamisH. Kirkpatrick-KellerD.C. KlandorfG. KilleferJ., 1998. In ovo administration of recombinant human insulin-like growth factor-I alters postnatal growth and development of the broiler chicken. Poultry Sci. 77:1913-1919.
- KocamisH. YeniY.N. Kirkpatrick-KellerD.C. KilleferJ., 1999. Postnatal growth of broilers in response to in ovo administration of chicken growth hormone. Poultry Sci. 78:1219-1226.
- KühnE.R. DarrasV.M. GysemansC. DecuypereE. BerghmanL.R. BuyseJ., 1996. The use of intermittent lighting in broiler raising. 2. Effects on the somatotrophic and thyroid axes and on plasma testosterone levels. Poultry Sci. 75:595-600.
- LiX.J. PiaoX.S. KimS.W. LiuP. WangL. ShenY.B. JungS.C. LeeH.S., 2007. Effects of chito-oligosaccharide supplementation on performance, nutrient digestibility, and serum composition in broiler chickens. Poultry Sci. 86:1107-1114.
- LiuW.J. WangZ.X. ChenY.X., 2010. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period. Anat. Rec. 293:1315-1324.
- LuJ.W. McMurtryJ.P. CoonC.N., 2007. Developmental changes of plasma insulin, glucagon, insulin-like growth factors, thyroid hormones, and glucose concentrations in chick embryos and hatched chicks. Poultry Sci. 86:673-683.
- McMurtryJ.P. FrancisG.L. UptonZ., 1997. Insulin-like growth factors in poultry. Domest. Anim. Endocrin. 14:199-229.
- McNabbF.M.A., 2000. Thyroids. In: WhittowG. ( ed.) Sturkie’s avian physiology. Academic Press, New York, NY, USA, pp 461-471.
- MoellersR.F. Cogburn,LA., 1995. Chronic intravenous infusion of chicken growth hormone increases body fat content of young broiler chickens. Comp. Biochem. Phys. A 110:47-56.
- National Research Council, 1994. Nutrient requirements of poultry. 9th rev. ed. National Academy Press, Washington, DC, USA.
- RaoK.Q. XieJ.J. YangX.J. ChenL. GrossmannR. ZhaoR.Q., 2009. Maternal low-protein diet programmes offspring growth in association with alterations in yolk leptin deposition and gene expression in yolk-sac membrane, hypothalamus and muscle of developing Langshan chicken embryos. Brit. J. Nutr. 102:848-857.
- ReynsG.E. VenkenK. de EscobarG.M. KühnE.R. DarrasV.M., 2003. Dynamics and regulation of intracellular thyroid hormone concentrations in embryonic chicken liver, kidney, brain, and blood. Gen. Comp. Endocr. 134:80-87.
- RozenboimI. HuisingaR. HalevyO. El HalawaniM.E., 2003. Effect of embryonic photostimulation on the posthatch growth of turkey poults. Poultry Sci. 82:1181-1187.
- RozenboimI. PiestunY. MobarkeyN. BarakM. HoyzmanA. HalevyO., 2004. Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poultry Sci. 83:1413-1419.
- SAS, 2002. JMP-statistics and graphics guide, version 5. SAS Inst. Inc., Cary, NC, USA.
- ShutzeJ. LauberJ. KatoM. WilsonW., 1962. Influence of incandescent and coloured light on chicken embryos during incubation. Nature 196:594-595.
- SiegelP.B. IsaksonS.T. ColemanF.N. HuffmanB.J., 1969. Photoacceleration of development in chick embryo. Comp. Biochem. Physiol. 28:753-758.
- ZhangL. ZhangH.J. QiaoX. YueH.Y. WuS.G. YaoJ.H. QiG.H., 2012. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poultry Sci. 91:1026-1031.
- ZhangL. ZhangH.J. WangJ. WuS.G. QiaoX. YueH.Y. YaoJ.H. QiG.H., 2014. Stimulation with monochromatic green light during incubation alters satellite cell mitotic activity and gene expression in relation to embryonic and posthatch muscle growth of broiler chickens. Animal 8:86-93.
