Abstract
The present study was undertaken to test the effect of two vulva injections of D-cloprostenol on day 7, 9 and 10 of oestrous cycle on the duration of the interestrous interval in gilts. Following a pre-treatment oestrous cycle, 87 gilts were assigned to receive vulva injections of 75 μg D-cloprostenol at 08:00 and 14:00 h on day 7 (D7; n=30), day 9 (D9; n=29) or day 10 (D10; n=28) of their second observed oestrous cycle. Across the treatments, the duration of the oestrous cycle with D-cloprostenol treatment (19.1±0.1 d) was not different from that of the previous oestrous cycle (20.1±0.4 days). Plasma progesterone concentrations were evaluated 6 h before and 24 and 72 h after D-cloprostenol treatment in the D9 group. Compared to pretreatment levels (9.6±0.4 ng/mL), plasma progesterone concentrations were reduced (P<0.05) at 24 h (6.3±1.0 ng/mL) and 72 h after treatment but complete luteolysis did not occur. These data indicate that in gilts double vulva administration of D-cloprostenol is not able to induce a complete luteolisys and hence the duration of the oestrous cycle is not modified.
Keywords:
Introduction
In pig production, gilts replace 40 to 60% of the breeding herd annually. The gilt replacement has a major impact on achievement of breeding targets. Typically, large groups of randomly cycling gilts are maintained in the gilt pool to ensure that sufficient females are in oestrus each breeding week. Having replacement females exhibit oestrus at predictable times would allow for a smaller gilt pool, as well as more efficient use of gestation and farrowing facilities. Methods of oestrus synchronisation include boar exposure and injection of gonadotrophins, but are effective only in prepubertal animals and the oestrus response can be unpredictable. In cycling gilts, an effective protocol to synchronise oestrus is oral administration of allyl trenbolone for 14 to 18 days. Oestrus responses and insemination can be made to take place about 5 to 9 d after last feeding of allyl trembolone but this protocol requires daily treatments and is relatively expensive.
In many domestic species, prostaglandin F2α (TOF2α) administration is able to induce luteolysis during much of the luteal phase and is utilised for oestrus synchronisation and timed artificial insemination in cows (De Rensis and Peter, Citation1999), ewes (Menchaca et al., Citation2004), and buffaloes (De Rensis and Lopez-Gatius, Citation2007). In contrast, in female pigs a single administration of PGF2α is not effective for inducing luteolysis until after day 12 of the oestrous cycle (Diehl and Day, Citation1974; Guthrie and Polge, Citation1976) and, therefore, cannot be utilised for oestrus synchronisation. The reason for the species difference in luteolytic activity of exogenous PGF2α is unclear.
As reported by Gadsby et al. (Citation1990, Citation1993), receptors for PGF2α are present in the porcine corpora lutea (CL) by 5 d of the oestrous cycle and thus an absence of receptors is not the reason for the lack of luteolytic capacity before 12 d (De Rensis et al., Citation2012). The pattern of PGF2α administration may have an effect since luteal regression is caused by PGF2α secreted from the endometrium in a series of discrete pulses (Kindhal et al., Citation1981; Inskeep and Murdoch, Citation1980). In swine, PGF2α pulses generally occur at 6 to 8 h intervals (Gleeson et al., Citation1974) suggesting that multiple injections of PGF2α could be more effective for inducing luteolysis than a single injection. Indeed, two PGF2α injections administered 6 h apart were more effective for farrowing induction than the traditional single injection (Kirkwood and Aherne, Citation1998) and in gilts, injection of 12.5 mg PGF2α every 12 h on days 5 to 10 of the oestrous cycle induced luteolysis and reduced the interoestrous interval by 6 d (Estill et al., Citation1993, Citation1995). Interestingly, Dclopsotenol, a PGF2α analogue with a 10-fold potency compared to the commercial racemic mixture of DL-cloprostenol (Re et al., Citation1994), induces luteal PGF2α production but this autoamplification occurred only in CL with luteolytic capacity. However, in miniature pigs, two vulvar injections of a high dose (3.0 mg) of cloprostenol with a 12 h interval on day 7 of their oestrous cycle induced luteolysis and a return to oestrus on day 12 (Kuge et al., Citation2006), suggesting that administration of high doses may eliminate the need for auto-amplification. Further, this early luteolytic effect was not evident following intramuscular injection which supports the earlier observation that vulva injections are effective at 25% of the label luteolytic dose (Kirkwood et al., Citation1996) with the corollary that any dose injected into the vulva will be equivalent to a higher dose injected intramuscular. Taken together, it is evident that the luteolytic effect of PGF2α in pigs is dependent on the day of the oestrous cycle administered, as well as dose and frequency of injection. The present study was undertaken to test the effect of two vulva injections of D-cloprostenol on day 7, 9 or 10 of oestrous cycle on the duration of the interestrous interval in gilts.
Materials and methods
The experiment was conducted on a commercial swine farm located in Parma province, Italy, in compliance with the requirements of the University of Parma Animal Care Committee.
A total of 87 white crossbred gilts were housed in groups of approximately 5 per pen and were fed a commercial ration formulated to provide 15% crude protein. Water was available ad libitum. At the start of the study, gilts were 138.0±8.6 kg body weight and 232.0±3.8 days of age and had exhibited an oestrous cycle of normal length. The duration of the previous oestrous cycles served as the control comparison for the duration of the subsequent treatment oestrous cycle. Gilts received vulva injections of 75 μg D-cloprostenol (Dalmazin®; FATRO, Ozzano dell’Emilia, Italy) at 08:00 h and again at 14:00 h on day 7 (D7; n=30), day 9 (D9; n=29) and day 10 (D10; n=28). After D-cloprostenol administration, gilts were subject to twice daily contact with a mature boar to facilitate detection of oestrus. Oestrus detection was performed daily until oestrus occurred or until day 16 if gilts remained anoestrus.
Blood samples were collected via jugular venipuncture from ten D9 gilts 6 h before the first cloprostenol injection and at 24 h and 72 h after the second cloprostenol injection for determination of plasma progesterone concentrations using a commercial radioimmunoassay kit (DSL 3900; Diagnostic System Laboratories, Webster, TX, USA) previously validated for pigs (Macchi et al., Citation2010). Assay sensitivity and intra-assay coefficient of variation were 0.09 ng/mL and 5%, respectively.
Statistical analysis
Differences within groups for the duration of the oestrous cycles were compared using Wilcoxon test. As data of plasma progesterone concentration were normally distributed (Kolmogorov-Smirnov test), statistical analysis was performed by the parametric t test for paired data.
Results and discussion
To our knowledge, this is the first study to evaluate the effect of two vulva injections of a high dose of the PGF2α analogue D-cloprostenol on plasma progesterone levels before day 12 of their oestrous cycle in commercial gilts; a time when the CL are considered insensitive to single prostaglandin injection. The results show that there was no significant effect of the day of injection on oestrous responses (). Across the treatments, the duration of the oestrous cycle with D-cloprostenol treatment (19.1±0.1 d) was not different from the preceeding oestrous cycle (20.1±0.4 d). These results were contradictory with data previously reported for miniature pigs, where the duration of oestrous cycles were reduced after D-cloprostenol administration at day 7 of the oestrous cycle (Kuge et al., Citation2006).
Compared to pre-treatment concentrations (9.6±0.4 ng/mL), plasma progesterone concentrations were reduced (4.17±1.0 ng/mL; P<0.05) at 24 and 72 h (6.3±1.0 ng/mL) following D-cloprostenol injection () but, differently from the mini pig (Kuge et al., Citation2006) a complete luteolysis was not observed. The reason for this difference between commercial gilts and mini pigs is unknown, although an association between the dose of cloprostenol and the metabolism of the animal may be involved. Although speculative, the absence of a complete luteolytic effect may be related to the number of CL, with a larger number of CL requiring a higher cloprostenol dose to ensure a complete luteolysis.
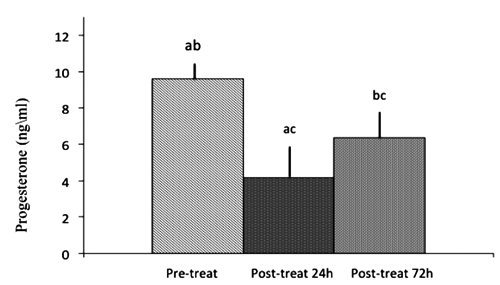
Table 1. The effects of D-cloprostenol injection on days 7, 9 or 10 of the oestrous cycle on interestrous interval length in gilts (mean±standard deviation).
Conclusions
In conclusion, the data of this study indicate that in commercial gilts a double vulvar high dose D-cloprostenol administration at day 7, 9 and 10 of the oestrous cycle decreases plasma progesterone levels but complete luteolysis does not occur. Future development of a longer lasting potent analogue of PGF2α may allow a more consistent luteolytic effect permitting the development of protocols for oestrus synchronisation.
Acknowledgements
The authors thank Dr. Carlo Gazza (FATRO, Ozzano dell’Emilia, Italy) for the D- cloprostenol and Dr. A. Negri for technical support.
References
- De RensisF. Lopez-GatiusF., 2007. Protocols for synchronizing estrus and ovulation in buffalo (Bubalus bubalis): a review. Theriogenology 67:209-216.
- De RensisF. PeterA., 1999. The control of follicular dynamics by PGF2α, GnRH, hCG and estrus synchronization in cattle. Reprod. Domest. Anim. 34:49-59.
- De RensisF. SaleriR. TummarukP. TechakumphuM. KirkwoodR.N., 2012. Prostaglandin F2α and control of reproduction in female swine: a review. Theriogenology 77:1-11.
- DiehlJ.R. DayB.N., 1974. Effect of prostaglandin Fa on luteal function in swine. J. Anim. Sci. 39:392-396.
- EstillC.T. BrittJ.H. GadsbyJ.E., 1993. Repeated administration of prostaglandin F2α during the early luteal phase causes premature luteolysis in the pig. Biol. Reprod. 49:181-185.
- EstillC.T. BrittJ.H. GadsbyJ.E., 1995. Does increased PGF2α induce luteolysis during early diestrus in the pig? Prostaglandins 49:255-267.
- GadsbyJ.E. BalapureA.K. BrittJ.H. FitzT.A., 1990. Prostaglandin F2 alfa receptors on enzyme dissociated pig luteal cells throughout the estrous cycle. Endocrinology 126:787-795.
- GadsbyJ.E. LovdalJ.A. BrittJ.H. FitzT.A., 1993. Prostaglandin F2 alpha receptor concentrations in corpora lutea of cycling, pregnant, and pseudopregnant pigs. Biol. Reprod. 49:604-608.
- GleesonA.R. ThorburnG.D. CoxR.I., 1974. Prostaglandin F concentration in the utero-ovarian venous plasma of the sow during the luteal phase of the estrous cycle. Prostaglandins 5:521-529.
- GuthrieH.D. PolgeC., 1976. Luteal function and oestrus in gilts treated with a synthetic analogue of prostaglandin F2α (ICI79.939) at various times during the oestrus cycle. J. Reprod. Fertil. 48:423-425.
- InskeepE.K. MurdochW.J., 1980. Relation of ovarian functions to uterine and ovarian secretion of prostaglandins during the estrous cycle and early pregnancy in the ewe and cow. Int. Rev. Physiol. 22: 325-356.
- KindahlH. LindellandJ.O. EdqvistL.E., 1981. Release of prostaglandin F2α during the oestrus cycle. Acta Vet. Scand. 77:143-158.
- KirkwoodR.N. AherneF.X., 1998. Increasing the predictability of cloprostenol-induced farrowing in sows. Swine Health Prod. 6:57-59.
- KirkwoodR.N. ThackerP.A. AherneF.X. GoonewardeneL.A., 1996. The effect of dose and route of administration of prostaglandin F2α on the parturient response of sows. Swine Health Prod. 4: 123-126.
- KugeT. IwataH. KuwayamaT. DomekiI. ShioyaY. MonjiY.I., 2006. Induction of estrus by prostaglandin F2 alfa administration in the vagina vestibules of miniature pig. J. Reprod. Develop. 52:391-396.
- MacchiE. CucuzzaA. BadinoP. OdoreR. ReF. BevilacquaL. MalfattiA., 2010. Seasonality of reproduction in wild boar (Sus scrofa) assessed by fecal and plasmatic steroids. Theriogenology 73:1230-1237.
- MenchacaA. MillerV. GilJ. PinczakA. LacaM. RubianesE., 2004. Prostaglandin F2α treatment associated with timed artificial insemination in ewes. Reprod. Domest. Anim. 39:352-355.
- ReG. BadinoR NovelliA. VallisneriA. GirardiC., 1994. Specific binding of DL-cloprostenol and D-cloprostenol to PGF2αlpha receptors in bovine corpus luteum and myometrial membranes. J. Vet. Pharmacol. Ther. 17:455-458.
