Abstract
Fillets and cooking yields, water holding capacity, textural properties, colour, proximate composition, collagen and fatty acids of five strains (1T1, 1T2, 1T3, USA, UK) of rainbow trout, Oncorhynchus mykiss, reared in three farms (F1, F2, F3), were measured before and after cooking. Physico-chemical parameter of the strains greatly differed both in raw and cooked state. 1T2 and USA recorded the highest yields. 1T2 distinguished from the other strains, showing lowest values of hardness, chewiness, gumminess and springiness. It also had brighter and less pigmented flesh with low fat, mainly in the raw state. USA strain showed the most valuable traits in terns of texture and colour, and had higher fat and collagen content in flesh. The physico-chemical profile of each strain was differently modified by cooking. USA strain maintained a positive texture and colour profile after cooking and its quality was the best.
Keywords:
Introduction
Rainbow trout, Oncorhynchus mykiss, is a Pacific trout of the Salmonidae family. It is widely farmed in many countries around the world due to its rapid growth and high nutritional value (Fallah et al., Citation2011). Oncorhynchus mykiss is the main freshwater fish species farmed in Italy (ISMEA, Citation2010), mainly in the North-East regions, where Trentino Alto Adige is historically a major area of traditional high-quality production. Since it is widely reported that genetic differences affect the sensory qualities of the flesh of various salmonids (Johnston et al., Citation2000; Johnston, Citation2001), the possibility of improving the commercial and physico-chemical quality of rainbow trout fillets by genetic selection has recently been studied (Kause et al., Citation2002, Citation2003, Citation2004; Quillet et al., Citation2005; Tobin et al., Citation2006). In the same vein, genetic selection programmes have been promoted in many countries. Italy, however, is not among these countries as no complete programme has been proposed yet.
Important quality characteristics of meat of land and aquatic species are the ability to retain water and textural and colorimetric attributes (Hyldig and Nielsen, Citation2001; Huff-Lonergan and Lonergan, Citation2005; Steine et al., Citation2005; Bugeon et al., Citation2010). In fish, these attributes are affected by factors such as nutritional status, water temperature, physical activity, muscle structure and composition, postmortem shrinkage and fibre proteolysis (Andersen et al., Citation1997; Hyldig and Nielsen, Citation2001; Ginés et al., Citation2004; Huff-Lonergan and Lonergan, Citation2005).
Unacceptable water holding retention causes loss of saleable weight and proteins (Huff-Lonergan and Lonergan, Citation2005). Water retention is also important for fish texture since higher water content in muscle reduces its mechanical strength (Hultmann and Rustad, Citation2002). Over-soft or mushy fillets are not favoured by consumers (Rasmussen, Citation2001) and softness is also a problem for the fish industry (Hultmann and Rustad, Citation2002). Lipids and collagen likewise play an important role in texture profile, influencing firmness, juiciness and palatability (Hyldig and Nielsen, Citation2001; Rasmussen, Citation2001; Fallah et al., Citation2011).
Besides these parameters, the uniformity of flesh colour is an important quality criterion, especially in salmonids with pigmented fillets (Bugeon et al., Citation2010). Consumers seem to prefer red flesh and it has been shown that redness significantly contributes to the overall enjoyment of cooked salmon (Steine et al., Citation2005).
Cooking is known to affect physico-chemical parameters of fish, causing disintegration of muscle fibre, water loss, pigment loss and pigment oxidation. Thermal changes to myofibrillar proteins increase toughness, whereas heat-induced transformation of collagen to gelatin [starting at 35 to 40°C, according to Schubring (Citation2008)] makes the flesh more tender since the layered myotomes tend to slide away in response to compression (Hyldig and Nielsen, Citation2001; Mørkøre et al., Citation2006; Aussanasuwannakul et al., Citation2010). For salmonids conflicting effects of cooking have been reported, namely a decline (Mørkøre et al., Citation2006; Aussanasuwannakul et al., Citation2010) and an increase (Ginés et al., Citation2004; Mørkøre et al., Citation2006; Larsen et al., Citation2011) in hardness after cooking.
The aim of the present study was to investigate major physico-chemical traits of five strains of rainbow trout farmed in Trentino-Alto Adige: three Italian strains and two genetically selected foreign strains. Considering the absence of a complete genetic selection programme in Italy, our research aimed to highlight similarities and differences between local and highly selected foreign strains to determine which of them had the best qualitative profile.
Materials and methods
Five rainbow trout strains were obtained from local (IT1, IT2, IT3) and foreign (UK and USA) suppliers. Eyed-stage eggs were bought and incubated until hatching. Juveniles of the five strains were transferred to three trout farms (FA, FB, FC) with different environmental and managing conditions in Trentino-Alto Adige, North-Eastern Italy. In each farm, every strain was reared in a different tank (). The water flow of each tank was individually regulated with the aim to maintain the dissolved oxygen (DO) level in outlet water always higher than 5 ppm, modifying it during the rearing phase, according to water temperature and metabolic needs of the fish biomass. All fish lots were kept at the same density (50 fish/m3) and fed the same commercial feed. In the finishing period, from live weight 350 g to marketable size (700-800 g), feed composition was as follows: moisture 9%, crude protein 42%, crude fat 24%, N-free extractives 17.2%, crude fibre 1.8%, and ash 6%. The content of feed in astaxanthin was 100 mg/kg. The fish were fed six days a week. When they reached marketable size, fish of each tank were slaughtered by asphyxia in the same farm where they were reared, then transported to the same plant where they were processed after about 2 h after catching. Ten fish of each strain and farm (50 fish per farm) were randomly sampled for analyses. Only females have been utilised and the animals did not show evident differences in their maturation state. The modified average daily gain (ADGm) of fish was calculated as final weight (g)/age (days) at slaughtering.
The morphometric traits measured on the whole fish were total weight (TW) and total length (TL); condition factor (CF) was calculated as 100×TW (g)/TL3 (cm). After automatic sectioning, head, frame and both fillets were obtained and weighed to calculate their percentage of TW. Fillet yield (FY) was calculated as 100×[fillet weight (g)/TW (g)]. The fillets were sent to the laboratory in refrigerated boxes and weighed 24 h after slaughtering. Left fillets were analysed raw, while right fillets were wrapped in aluminium foil, placed on a tray immersed in water in a fish-steamer, boiled (at 98-100°C) for 10 min and then cooled at room temperature and weighed. Cooking yield (CY) was calculated as 100×[cooked fillet weight (g)/raw fillet weight (g)]. Water holding capacity (WHC) and colorimetric and texture analyses were carried out on raw and cooked fillets. Analyses of proximate composition, total lipids and collagen were performed on raw and cooked fillets without skin, homogenised and freeze-dried prior to analysis.
Water holding capacity, texture and colour
Water holding capacity was measured by the compression test on filter paper according to Grau and Hamm (Citation1953), measuring the area of released fluid.
Texture analyses were carried out using a Zwick Roell® 109 texturometer (Zwick Roell, Ulm, Germany) with Text Expert II software, equipped with a 200 N load cell. The Warner-Bratzler (WB) shear test was performed on a sample of 5×5 cm surface area, taken from the central part of the fillet (one measurement for each fillet), including both the hypaxial and epaxial regions. A straight blade (width of 7 cm), perpendicular to muscle fibre direction, was used at a crosshead speed of 30 mm/min to 50% of total deformation. Maximum shear force, defined as maximum resistance of the sample to shearing (Veland and Torrissen, Citation1999) was determined.
Texture profile analysis (TPA) was carried out on a sample of 5×5 cm surface area, taken from the epaxial part of the fillet, from the cranial insertion point of the dorsal fin. Two compression test cycles were conducted using a 10 mm diameter cylindrical probe at a constant speed of 30 mm/min to 50% of total deformation. Six texture parameters were calculated, as suggested by Veland and Torrissen (Citation1999) and Ayala et al. (Citation2010): hardness (peak force of the first compression cycle), cohesiveness (ratio of positive area of the force during the second compression compared to that obtained during the first compression), resilience (ratio of upstroke area to downstroke area during the first compression cycle), springiness (height of sample recovered between the two compression cycles), gumminess (hardness multiplied by cohesiveness) and chewiness (hardness multiplied by cohesiveness multiplied by springiness). All measurements were made at room temperature. All parameters were determined from the plot of force (N) compared with deformation (%).
A Spectro-color®116 colorimeter (Bell Technology Ltd., Auckland, New Zealand), using Spectral qc 3.6 software, was utilised for colorimetric measurement in the CIELab system (Commission Internationale de l’Éclairage, Citation1976). In this system, lightness (L*) is expressed on a 0 to 100 scale from black to white; redness index (a*) ranges from red (+60) to green (-60) and yellowness index (b*) ranges from yellow (+60) to blue (-60). Colour was measured in duplicate on epaxial, ventral and caudal positions on fillets and expressed as mean.
Proximate composition and collagen
Moisture, crude protein (Nx6.25) and ash contents were determined according to AOAC (Citation2000) 950.46, 976.05, and 920.153 methods, respectively. Total lipid extraction was performed according to a modified Folch et al. (Citation1956) method. Freeze-dried samples, reconstituted fresh by adding distilled water, were homogenised with a 2:1 chloroform-methanol (v/v) solution and filtered. The filter was washed several times, and distilled water with 0.88% KCl was added to the filtrate up to a [chloroform:methanol]:water ratio of 4:1. Tubes were stirred and a biphasic system was obtained by standing overnight. The lower phase containing lipids dissolved in chloroform was siphoned off and recovered. Total lipid content was determined gravimetrically, after removal of the solvent (chloroform) by evaporation under vacuum and lipid resuspension in a known volume of chloroform (5 mL). Lipid content was weighed in a crucible (gross weight minus tare) after complete evaporation of chloroform. The hydroxyproline content necessary to quantify total collagen was obtained by hydrolysing samples with 70% perchloric acid (HClO4) for 4 h at 100°C and diluting it into a flask, as suggested by Galasinski et al. (Citation1978) and Bonnet and Kopp (Citation1984). Quantities for hydrolysis (1.5 g out of 7.5 mL) and dilution volume of hydrolysed sample (50 mL) were one-half those suggested by Bonnet and Kopp (Citation1984). Diluted samples were then filtered with 413-VWR no. 516-0816 filter papers. For the colorimetric reaction, hydroxyproline standard solution, with concentrations ranging from 2 to 20 g/mL, was included. Aliquots of 0.1 mL of standard and filtered samples were transferred to Eppendorf tubes (2 mL) and 0.2 mL acetate/citrate buffer (pH 6) was added. Samples were neutralised with 1.8 M NaOH. According to Bergman and Loxley (Citation1963), an oxidant solution composed of 1 volume of Chloramine-T at 7% (w/w) and 4 volumes of acetate/citrate buffer (pH 6) was added to the tubes and left to react for 25 min at room temperature. Erlich’s reagent solution was prepared according to Bonnet and Kopp (Citation1984) by dissolving 20 g p-dimethylaminobenzaldehyde in 30 mL HClO4 and mixing 3:13 (v/v) with isopropanol. One mL of this solution was added to the tubes. Tubes were held at 60°C for 25 min in a water bath (Bergman and Loxley, Citation1963). Absorbance was measured at 558 nm using a spectrophotometer (Perkin Elmer-Lambda EZ 150; Perkin Elmer, Waltham, MA, USA). Total collagen content was calculated assuming a conversion factor of 17.8 (Montero et al., Citation1990).
Statistical analysis
Data was analysed using SAS Proc GLM (SAS, Citation2007) with the following model:
where: Y=kth observation of the ith strain and the jth farm; S=strain effect (i=1…5); F=farm effect (j=1…3); X=independent variable (body weight); E=random error effect.
For the texture parameters, fillet thickness was used as a further covariate. Differences between least square means were tested with Student’s t-test. All the statistical analyses on meat traits were performed separately for raw and cooked fillets.
The coefficients of the residual (after the above model) correlation between physical and chemical traits were also calculated. Physical and chemical parameters also underwent Principal Component Analysis (PCA) (Naes et al., Citation1996), using SAS Proc FACTOR (SAS, Citation2007) with Varimax Rotation and the first three factors underwent ANCOVA analysis with the above model. Finally, discriminant analysis (SAS, Citation2007) was performed to discriminate fish of different strains or farms on the basis of physical and chemical traits.
Results and discussion
Characteristics of raw and cooked fillets
Since fish from each tank were sampled at marketable size, the age of fish when they were sampled differed between strains and farms (). Total weight and ADGm also differed between strains and farms and consequently all parameters analysed were covaried by TW.
As shown in , the morphometric traits and yields proved to be greatly influenced by genetic and rearing factors. Specifically, IT2 and USA strains recorded the highest FY, while USA also had the highest CY. On the contrary, the lowest FY and CY were found in fish of IT1 strain, characterised by a significantly higher head incidence, and UK strain, slightly shorter and stockier in shape as highlighted by CF. However, a significant strain×farm interaction was found for all morphometric traits, with changes sometimes occurring in rank of genotypes in the different farms. However, UK and USA always registered the highest and the lowest CF respectively, while USA showed the lowest head incidence and the highest FY in two of the three farms analysed.
The results of physical parameter analyses of raw and cooked fillets are shown in and . The five strains differed significantly in all parameters and their pattern often varied in relation to farm, as the significance of interactions indicated. Such genetic differences are confirmed by the literature. In particular, genetic factors are known to affect muscle structure, since many authors report that cell size and fibre diameter vary between populations of salmonids (Valente et al., Citation1998, Citation1999; Johnston et al., Citation2000). Genetic diversity may therefore influence water loss from cell compartments (Huff-Lonergan and Lonergan, Citation2005), endurance of force (Hurling et al., Citation1996; Hyldig and Nielsen, Citation2001) and optical properties (Johnston et al., Citation2000; Johnston, Citation2001) of fillet muscle.
shows WHC and texture parameters in raw and cooked fillet in greater detail. Strain did not significantly influence WHC values recorded in raw muscle. After cooking the differences became significant (P<0.01): IT1 recorded the lowest water losses, although it did not show systematically the lowest value in all farms. UK and USA strains had the highest water losses in two of the farms. Heating induced an increase in the amount of water released by disintegrating cell structures in all strains, as reported by Ofstad et al. (Citation1993) and Rørå et al. (Citation2003).
As raw fillet is concerned, differences among strains were registered in WB-shear force values, with lower values in USA and IT2 that were the strains with the highest ADGm (). The previous strains were the lowest and the highest, respectively, for the resilience. Any differences for hardness and cohesiveness among strains were found while for the gumminess, mathematically derived from these two texture parameters, a clear influence by strains was highlighted. Despite the differences observed for springiness, the strains had similar chewiness. Strain×farm interaction still led to changes in rank position of the strains in each farm, even if UK always had the highest gumminess, while IT2 the highest resilience and the lowest gumminess in two farms out of three.
By inducing myofibril disintegration, cooking can determine variation in texture parameters. In cooked fillets, WB-shear force and hardness followed a similar trend, although significant differences (P<0.001) between strains were only detected for hardness. On average, the IT2 strain had the softest flesh and IT3 the hardest even if this behaviour was detected in two of the farms, while differences were not significant in one farm. Differences in resilience, gumminess and springiness among strains were maintained after heat treatment, that determined also a difference in chewiness, not found in raw samples. Cooked fillets of IT2 had the lowest gumminess, springiness and chewiness, showing the minimal values in two farms out of three.
With regard to the relatively constant cohesiveness values both in raw and cooked fillet, certain authors (Bhattacharya et al., Citation1993; Larsen et al., Citation2011) observed that unlike other parameters, this parameter did not change with different cooking temperature and methods. shows the colour parameters of raw and cooked fillets. The five strains differed significantly in colour characteristics but the results were still dependent on the farm, as revealed by the interaction. Raw fillets of IT2 strain showed a different colorimetric profile from the other strains, being brighter and less pigmented in two of the farms while in the third farm the differences among strains were not significant. After cooking all strains showed a brighter and yellower appearance and differences were highlighted even between fillets with similar colour when raw, however the strains were no longer differentiated in red component. The yellowest flesh was that of UK strain in all the three farms and the least yellow was IT1 strain. Increased L* and b* components in cooked compared to raw fillets were presumably due to heat-induced oxidation of conjugated double bonds of carotenoid molecules, which leads to discoloring of flesh (Choubert and Baccanaud, Citation2006). Cooking led to a downward trend in a* component, in line with the results of various authors (Mørkøre et al., Citation2001; Choubert and Baccanaud, Citation2010). In line with our findings, Larsen et al. (Citation2011) found that cooked salmon fillets were lighter and more yellow than when raw. Protein aggregation probably increases opacity and the light that enters the surface has less chance of being selectively absorbed (Larsen et al., Citation2011). Conversely, Choubert and Baccanaud (Citation2010) found a decrease in L* and b* after dry and moist cooking of rainbow trout and associated it with loss of yellow component. It has been demonstrated that colour attributes are influenced by pigment deposition in the flesh of salmonids (Storebakken and Kyoon No, Citation1992). Pigmentation may have been partly genetically determined, since it has been demonstrated that salmonid strains differing in growth rate, sexual maturation, age at slaughter, structure and chemical composition of muscle, show variations in pigment deposition (Storebakken and Kyoon No, Citation1992; Bjerkeng, Citation2000). Ytrestøyl et al. (Citation2006) found that in salmon the fast growth was associated with lower muscle concentrations of astaxanthin due to lower pigment digestion. Indeed, the slowest-growing strains (), IT1 and UK, were the reddest (). IT2, which is among the fastest growing strains (), differed from the others in having faintly coloured flesh ().
Proximate composition of raw and cooked fillets is shown in . Raw fillets from IT2 strain were the leanest, while those from IT1 had the highest lipid content in two of the farms. No significant differences in collagen content were detected between strains, although IT1 had marginally less and USA more (). Variations in lipids, ash and moisture content among strains were maintained after cooking.
In IT2 strain, the leaness of the flesh may be a concomitant factor influencing low redness and yellowness indexes, since carotenoids are lipid-soluble. Although many authors have reported a positive relationship between lipid and L* in salmonids (Rørå et al., Citation1998; Mørkøre et al., Citation2001; Bugeon et al., Citation2010), IT2 showed significantly higher L* component but the leanest flesh. Presumably, other important factors including anatomical structure (Larsen et al., Citation2011) and surface ultrastructure of the muscle, carotenoid deposition, neutral lipid accumulation, and oxidation/oxygenation of muscle pigments exerted a stronger influence on light absorption than fat content.
Correlation
shows the residual correlation coefficients between physical and chemical traits of raw and cooked fillets. The coefficients express the link between traits within the main factors strain and farm, as reported in the statistical model. Water holding capacity was not significantly correlated with the other traits in raw and cooked fillets. The absence of a link between WHC and TPA parameters is in disagreement with the results of Hultmann and Rustad (Citation2002) in salmon and cod. These authors found that the amount of water released from muscle reduced its mechanical strength.
Regarding the relationship between TPA parameters, high positive relationships were evident, partially due to the mathematical link between certain parameters (i.e. gumminess with hardness and cohesiveness; chewiness with gumminess and springiness). In detail, shear force was positively correlated with hardness only in raw flesh and springiness was the most independent trait. Though cohesiveness was well below one () indicating that only part of the deformation induced by compression was recovered, flesh that recovered original fillet thickness better also showed a higher speed and force of recovery as expressed by resilience (r=0.65 and 0.78, in raw and cooked fillets, respectively).
Concerning the link between texture and chemical composition, the results showed that the lipid percentage in muscle was not significantly correlated with texture parameters in raw and cooked fillets, except for shear force and hardness that were positively correlated. The latter did not agree with the findings of several other studies which revealed that rainbow trout fillets with high fat content have a softer consistency compared to fillets with lower fat content (Andersen et al., Citation1997; Mørkøre et al., Citation2001, Citation2006; Aussanasuwannakul et al., Citation2010). Comparison of strains () partially confirmed this lack of relationship, since both the fattest strains, IT1 and USA, and the leanest strain, IT2, had the softest flesh.
A significant negative correlation was found between total protein and shear force (r=-0.34), hardness (r=-0.32), resilience (r=-0.22) and chewiness (r=-0.19) of raw flesh and with cohesiveness (r=-0.26) and resilience (r=-0.27) of cooked fillets. According to Li et al. (Citation2005) there is no significant relationship between total hydroxyproline content and hardness of raw salmon fillets, suggesting a negligible contribution of total collagen to texture compared to collagen cross-links. In line with this, we found a non-significant correlation between texture parameters and collagen content in raw fillets. Cooking weakens muscle structure by converting collagen to gelatin (Aussanasuwannakul et al., Citation2010). Although the contribution of connective tissue to texture is known to be negligible in cooked fish (Hatae et al., Citation1986), in this study we found a negative correlation between TPA parameters and collagen, which was not significant except for chewiness (r=-0.34). The influence of collagen on chewiness can be explained by the fact that cooked flesh of fish having higher collagen content dissolved readily into flakes, becoming softer and providing lower resistance to mastication. An inverse relationship between cooked flesh firmness and collagen content was also observed by Bugeon et al. (Citation2010).
As for colour, the intensity of redness and yellowness increased with decreasing L* (r=-0.67 and r=-0.31, respectively) in raw fillets, showing a pattern partially in line with that observed by Einen and Skrede (Citation1998); a positive correlation was found between yellowness index and redness index (r=0.63) and lipid content (r=0.19), as reported by Einen and Skrede (Citation1998), Rørå et al. (Citation1998) and Mørkøre et al. (Citation2001, Citation2006) in Atlantic salmon.
In this study, redness index was correlated with protein content in raw and cooked fillets because the reddish pigments, carotenoids and myoglobin, are primarily associated with muscle protein (Storebakken and Kyoon No, Citation1992; Bjerkeng, Citation2000). Consequently, redness index was negatively correlated with some texture parameters (shear force, hardness, resilience and chewiness in raw fillets; and cohesiveness and resilience in cooked fillets).
Principal Component Analysis
In order to analyse the joint behaviour of physico-chemical traits, PCA was applied to the dataset using the Varimax Rotation that optimises and balances variance partition between defined factors (SAS, Citation2007). Because of the reduced number of samples analysed for collagen content, collagen was excluded from PCA. reveals that the first three Factors explained about 57% of the total variance of the parameters in raw fillets and almost 49% in cooked fillets.
In raw fillets, Factor1 associated the variables colour, moisture and lipids. In particular, lipids, a* and b* were positively associated and showed similar loading values, in the opposite direction to moisture and L*. No significant association was found between the abovementioned parameters and the texture indicators that influenced Factor2, where shear force, hardness and chewiness were linked and showed similar high loading values. These parameters were closely linked to protein and a* but in the opposite direction. Significantly, resilience, cohesiveness and springiness were not linked to other textural parameters, and were the parameters that most influenced Factor3. In cooked fillets, Factor1 was influenced by the texture parameters which were all positively associated, unlike in raw fillets, while increase in moisture and decrease in protein, lipids and a* value were associated in Factor2 together with decrease in resilience. For this reason, contrary to what is found in the raw fillet, Factor1 became a descriptor of texture attributes and Factor2 became, primarily, a descriptor of composition. Factor3 combined high values of L*, b* and protein content with low gumminess. Unlike in the raw state, the chromaticity indexes a* and b* were no longer linked, probably because the red component was due to carotenoid content, while the yellow component was indicative of carotenoid loss during cooking (Birkeland et al., Citation2006).
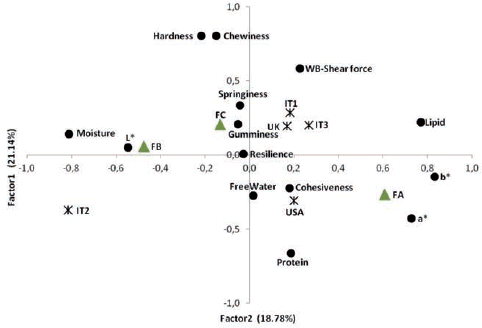
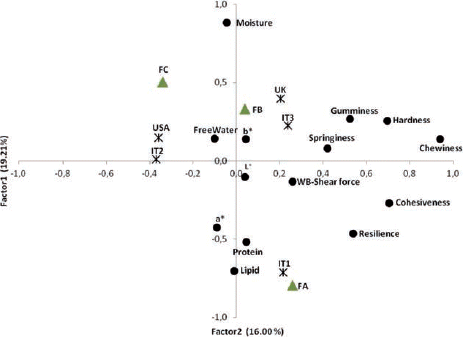
As shown in all three Factors were affected by strain and farm, both in raw and cooked fillets. In raw fillets, Factor1 differentiated IT2 from the other strains. IT2 and USA showed similar values for Factor2, which was influenced mainly by parameters associated with texture. The similarity between these strains was enhanced by cooking, as shown by the values of all three Factors.
Concerning the effect of farm, in raw fillets all farms differed from each other in Factor1, whereas FA was characterised as different from FC in Factor2 that associated textural properties. The difference in texture between these farms was confirmed in cooked fillets, as the pattern of Factor1 shows. After cooking, FB and FC showed no differences in Factor2, whereas the specificity of FA for the parameters synthesised in this Factor was confirmed.
The plot of the first two Factors highlighted the spatial distribution of the loading values and the averages for each strain and farm. shows the pattern for raw fillets. IT2 strain plotted in the third quadrant of the figure, next to L* and opposite lipids, confirming the greater lightness and leanness of fillets of this strain. IT1, IT3 and UK showed similarities in quality traits, first of all texture, since they clustered on the positive axis of Factor2 where certain textural parameters had a positive loading. With regard to farm effect, FA was differentiated from FB and FC, being located in the positive part of Factor1 and in the same area as lipids, a* and b*.
In the plot of the first two Factors of cooked fillets () a different response to cooking was evident between strains. IT1strain differed sharply from the others. It plotted at the bottom of the second quadrant, in the negative sector of Factor2 next to lipids, protein and a*. IT2 and USA were well defined by the first Factor and plotted opposite texture parameters. This means that the two strains developed a similar softer texture after cooking through a decrease in all textural parameters. Concerning farms, FA also differentiated greatly from FB and FC after cooking. Like raw fillets, FA plotted next to lipids and a*, suggesting that fish reared in FA had higher lipid content and redness index after cooking.
Discriminant analysis () of physico chemical parameters revealed that the different strains could be clearly distinguished from each other. For raw fillets, the correct classification percentage for strain in the whole sample was 58%. The best classified strains were IT2 and USA, which were correctly classified in more than 66% of cases, indicating their good separation in terms of physico-chemical parameters. Again for raw fillets, overall classification accuracy of the discriminant functions for farm was 83.3%, even higher than for strains, indicating that physico-chemical parameters were also affected by specific rearing conditions of these farms (). In particular, fish reared in FC was more clearly distinguished from fish reared in the other farms (86% correct classification). Cooking enhanced the accuracy of classification, since the correct classification percentages were higher, both for strain (67%) and farm (86%). In contrast to raw fillets, the best scores for strain classification were obtained for IT1 and IT3, whereas the best score for farm classification was obtained for fish from FB.
Table 1. Rearing conditions in each farm.
Table 2. Morphometric characteristics and marketable yields of fish estimated at an average weight of 775.3 g.
Table 3. Water holding capacity and texture parameters in raw and cooked fillets estimated at an average weight of 775.3 g.
Table 4. Colorimetric attributes of raw and cooked fillets estimated at an average weight of 775.3 g.
Table 5. Chemical composition (% on wet basis) of raw and cooked fillets estimated at an average weight of 775.3 g.
Table 6. Residual correlation coefficient between physical and chemical parameters of raw (above the diagonal) and cooked fillets (below the diagonal).
Table 7. Results of factor analysis for physico-chemical parameters in raw and cooked fillets.
Table 8. Effect of strain and farm on the three factors in raw and cooked fillets.
Table 9. Classification results using linear discriminant function for strain and farm (% correctly classified).
Conclusions
This study shows that genetic differences among rainbow trout strains, affecting growth performance and efficiency of feed utilisation, had a strong influence on qualitative traits of fillets. The five strains gave different responses depending on the farm they were reared in. IT2 and USA proved to be the most valuable strains in terms of market traits, since they recorded the best FY and CY. Despite this, raw and cooked fillets of IT2 strain differed from USA, showing mediocre texture and colorimetric profile, considering that in general consumers prefer firm and elastic flesh and a reddish tint. However, the leanness and low collagen content of IT2 fillets are a positive characteristic of this strain, since a need to lower the lipid content of farmed fish and to avoid the unpleasant softening effect induced by gelatinisation of collagen is recognised. Such advantages over the USA strain should be investigated by sensory analysis to determine whether differences in chemical parameters between strains can be spotted by consumers.
Acknowledgements
The authors gratefully acknowledge the financial support from Edmund Mach Foundation, Agricultural and Food Supply Chain Department, S. Michele all’Adige (TN), Italy.
References
- AndersenU.B. ThomassenM.S. RøråA.M.B., 1997. Texture properties of farmed rainbow trout (Oncorhynchus mykiss): effects of diet, muscle fat content and time of storage on ice. J. Sci. Food. Agr. 74:347-353.
- AOAC, 2000. Official methods of analysis. 17th ed. Association of Official Analytical Chemists, Washington, DC, USA.
- AussanasuwannakulA. KenneyP.B. BrettK.P. WeberG.M. YaoJ. SliderS.D. ManorM.L. SalemM., 2010. Effect of sexual maturation on growth, fillet composition, and texture of female rainbow trout (Oncorhynchus mykiss) on a high nutritional plane. Aquaculture 317:79-88.
- AyalaM.D. AbdelI. SantaellaM. MartìnezC. PeriagoM.J. GilF. BlancoA. Lopez AlborsO., 2010. Muscle tissue structural changes and texture development in sea bream, Sparus aurata L., during postmortem storage. Food Sci. Technol.-Leb. 43:465-475.
- BergmanM. LoxleyR., 1963. Two improved and simplified methods for the spectrophotometric determination of hydroxyproline. Anal. Chem. 35:1961-1964.
- BhattacharyaS. ChoudhuryG.S. StudebakerS., 1993. Hydrothermal processing of Pacific Chum salmon: effects on texture and in-vitro digestibility. J. Food Quality 16:243-251.
- BirkelandS. HaarstadI. BjerkengB., 2006. Effects of salt-curing procedure and smoking temperature on astaxanthin stability in smoked salmon. J. Food Sci. 69:198-203.
- BjerkengB., 2000. Carotenoid pigmentation of salmonids fishes. Recent progress. pp 71-89 in Proc. 5th Int. Symp. Aquat. Nutr., Mérida, Mexico.
- BonnetM. KoppJ., 1984. Dosage du collagène dans les tissus conjoctifs, la viande et les produits carnes. Cah. Tech. INRA 5:19-30.
- BugeonJ. LefevreF. CardinalM. UyanikA. DavenelA. HaffrayP., 2010. Flesh quality in large rainbow trout with high or low fillet yield. J. Muscle Foods 21:702-721.
- ChoubertG. BaccanaudM., 2006. Colour changes of fillets of rainbow trout (Oncorhynchus mykiss W.) fed astaxanthin or canthaxanthin during storage under controlled or modified atmosphere. Food Sci. Technol.-Leb. 39:1203-1213.
- ChoubertG. BaccaunaudM., 2010. Effect of moist or dry heat cooking procedures on carotenoid retention and colour of fillets of rainbow trout (Oncorhynchus mykiss) fed astaxanthin or canthaxanthin. Food Chem. 119:265-269.
- Commission Internationale de l’Éclairage, 1976. Official recommendations on uniform colour space, colour difference equations and metric colour terms. CIE ed., Paris, France.
- EinenO. SkredeG., 1998. Quality characteristics in raw and smoked fillets of Atlantic salmon, Salmo salar, fed high-energy diets. Aquacult. Nutr. 4:99-108.
- FallahA.A. Siavash Saei-DehkordiS. NematollahiA., 2011. Comparative assessment of proximate composition, physicochemical parameters, fatty acids profile and mineral content in farmed and wild rainbow trout (Oncorhynchus mykiss). Int. J. Food Sci. Tech. 46:767-773.
- FolchJ. LeesM. Sloane-StanleyG.H., 1956. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497-509.
- GalasinskiW. GadekA. RatkiewiczA. RzeczyckiW., 1978. A convenient modification of the method for hydroxyproline determination in proteins. Anal. Biochem. 85:550-555.
- GinésR. ValdimarsdottirT. SveinsdottirK. ThorarensenH., 2004. Effects of rearing temperature and strain on sensory characteristics, texture, colour and fat of Arctic charr (Salvelinus alpinus). Food Qual. Prefer. 15:177-185.
- GrauR. HammR., 1953. Eine einfache methode zur bestimmung der wasserbindung im muskel. Naturwissenschaften 40:29-30.
- HataeK. TobimatsuA. TakeyamaM. MatsumotoJ.J., 1986. Contribution of the connective tissues on the texture differences of various fish species. B. Jpn. Soc. Sci. Fish. 52:2001-2008.
- Huff-LonerganE. LonerganS.M., 2005. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 71:194-204.
- HultmannL. RustadT., 2002. Textural changes during iced storage of salmon (Salmo salar) and cod (Gadus morhua). J. Aquat. Food Prod. T. 11:105-123.
- HurlingR. RodellJ.B. HuntH.D., 1996. Fiber diameter and fish texture. J. Texture Stud. 6:679-685.
- HyldigG. NielsenD., 2001. A review of sensory and instrumental methods used to evaluate the texture of fish muscle. J. Texture Stud. 32:219-242.
- ISMEA, 2010. Check up ittico 2010. Istituto di Servizi per il Mercato Agricolo Alimentare ed., Roma, Italy.
- JohnstonI.A., 2001. Genetic and environmental determinants of muscle growth patterns. In: JohnstonI. (ed.) Fish physiology. Gulf Professional Publ., Oxford, UK, pp 141-186.
- JohnstonI.A. AldersonR. SandhamC. DingwallA. MitchellD. SelkirkC. NickellD. BakerR. RobertsonB. WhyteD. SpringateJ., 2000. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture 189:335-349.
- KauseA. RitolaO. PaananenT., 2004. Breeding for improved appearance of large rainbow trout in two production environments. Aquac. Res. 35:924-930.
- KauseA. RitolaO. PaananenT. EskelinenU. MäntysaariE., 2003. Big and beautiful? Quantitative genetic parameters for appearance of large rainbow trout. J. Fish Biol. 62:610-622.
- KauseA. RitolaO. PaananenT. MäntysaariE. EskelinenU., 2002. Coupling body weight and its compositions: a quantitative genetic analysis in rainbow trout. Aquaculture 211:65-79.
- LarsenD. QuekS. EyresL., 2011. Evaluating instrumental colour and texture of thermally treated New Zealand King salmon (Oncorhynchus tshawytscha) and their relation to sensory properties. Food Sci. Technol.-Leb. 44:1814-1820.
- LiX. BickerdikeR. LindsayE. CampbellP. NickellD. DingwallA. JohnstonI.A., 2005. Hydroxylysyl pyridinoline cross-link concentration affects the textural properties of fresh and smoked Atlantic salmon (Salmo salar L.) flesh. J. Agr. Food Chem. 53:6844-6850.
- MonteroP. BorderiasJ. TurnayJ. LeyzarbeM.A., 1990. Characterization of hake (Merluccius merluccius L.) and trout (Salmo irideus Gibb) +collagen. J. Agr. Food Chem. 38:604-609.
- MørkøreT. HansenA.Å. UnanderE. EinenO., 2006. Composition, liquid leakage, and mechanical properties of farmed rainbowtrout: variation between fillet sections and the impact of ice and frozen storage. J. Food Sci. 67:1933-1938.
- MørkøreT. ValletJ.L. CardinalM. Gomez-GuillenM. MonteroP. TorrissenO.J. NortvedtR. SigurgisladottirS. ThomassenM.S., 2001. Fat content and fillet shape of Atlantic salmon: relevance for processing yield and quality of raw and smoked products. J. Food Sci. 66:1348-1354.
- NaesT. BaardsethP. HelgesenH. IsakssonT., 1996. Multivariate techniques in the analysis of meat quality. Meat Sci. 43:135-149.
- OfstadR. KidmanS. MyklebustR. HermanssonA.M., 1993. Liquid holding capacity and structural changes during heating of fish muscle: cod (Gadus morhua L.) and salmon (Salmo salar). Food Struct. 12:163-174.
- QuilletE. Le GuillouS. AubinJ. FauconneauB., 2005. Two-way selection for muscle lipid content in pan-size rainbow trout (Oncorhynchus mykiss). Aquaculture 245:49-61.
- RasmussenR.S., 2001. Quality of farmed salmonids with emphasis on proximate composition, yield and sensory characteristics. Aquac. Res. 32:767-786.
- RøråA.M.B. KvåleA. MørkøreT. RørvikK. HallbjoørnS. ThomassenS., 1998. Process yield, colour and sensory quality of smoked Atlantic salmon (Salmo salar) in relation to raw material characteristics. Food Res. Int. 31:601-609.
- RøråA.M.B. RegostC. LampeJ., 2003. Liquid holding capacity, texture and fatty acids profile of smoked fillets of Atlantic salmon fed diets containing fish oil or soybean. Food Res. Int. 36:231-239.
- SAS, 2007. SAS/STAT user’s guide, version 9.1. SAS Inst. Inc. Cary, NC, USA.
- SchubringR., 2008. Comparative study of the DSC pattern, color, texture and water-binding capacity of rainbow trout muscle during heating. J. Food Process. Pres. 32:190-218.
- SteineG. AlfnesF. RøråA.M.B., 2005. The effect of color on consumer WTP for farmed salmon. Mar. Resour. Econ. 20:211-219.
- StorebakkenT. Kyoon NoH., 1992. Pigmentation of rainbow trout. Aquaculture 100:209-229.
- TobinD. KauseA. MäntysaariE.A. MartinS.A.M. HoulihanD.F. DoblyA. KiesslingA. Rungruangsak-TorrissenK. RitolaO. RuohonenK., 2006. Fat or lean? The quantitative genetic basis for selection strategies of muscle and body composition traits in breeding schemes of rainbow trout (Oncorhynchus mykiss). Aquaculture 261:510-521.
- ValenteL.M.P. GomesE.F.S. FauconneauB., 1998. Biochemical growth characterization of fast and slow-growing rainbow trout strains: effect of cell proliferation and size. Fish Physiol. Biochem. 18:213-224.
- ValenteL.M.P. RochaE. GomesE.F.S. SilvaM.W. OliveiraM.H. MonteiroR.A.F. FauconneauB., 1999. Growth dynamics of white and red muscles in fast and slow growing strains of rainbow trout. J. Fish Biol. 55:675-691.
- VelandJ.O. TorrissenO.J., 1999. The texture of Atlantic salmon (Salmo salar) muscle as measured instrumentally using TPA and Warner-Bratzler shear test. J. Sci. Food. Agr. 79:1737-1746.
- YtrestøylT. StruksnøsG. RørvikK.A. KoppeW. BjerkengB., 2006. Astaxanthin digestibility as affected by ration levels for Atlantic salmon, Salmo salar. Aquaculture 261:215-224.