Abstract
The effect of starter culture combinations on the quality of Turkish type dry fermented sausage (sucuk) were evaluated during ripening and storage periods. Sucuk formulations were produced without (control) and with three different starter culture combinations; i) Staphylococcus carnosus+Pediococcus pentosaceus, ii) Staphylococcus carnosus+ Lactobacillus sakei, and iii) Staphylococcus carnosus+Pediococcus pentosaceus+ Lactobacillus sakei. Analysis of microbiological, physico-chemical and lipase enzyme levels of samples were conducted until the 60th day. Interactions among the presence of lipolytic starter cultures, lipase enzyme levels and thiobarbituric acid reactive substances were also evaluated both in ripening and drying periods. There were apparent differences on microbiological and chemical properties between samples prepared with starters and control. It has been concluded that the use of lipolytic starter cultures in suitable combination would have positive effect on the acceleration of ripening and improvement of the quality of dry fermented sausages.
Introduction
Turkish dry-fermented sausage (sucuk) is a traditional, well-known meat product fermented by either natural microflora or the addition of starter cultures. Cattle, sheep and buffalo meat are used separately or in various combination by mixing with fat, salt, sugar, nitrite/nitrate, dry garlic and various spices such as black pepper, red pepper, cumin in sucuk production (Aksu and Kaya, Citation2004). Although, sucuk is traditionally produced by their natural flora (Bozkurt and Erkmen, Citation2002), today dry fermented sausages are produced by adding starter cultures mainly composed of lactic acid bacteria (LAB) and either staphylococci or micrococci (Hammes and Knauf, Citation1994) in industrial scale. Pediococcus pentosaceus, Staphylococcus carnosus and Lactobacillus sakei are among the most commonly used starter cultures in manufacturing of sucuk. These LAB have positive effect on hygienic properties of the product, inhibiting the growth of pathogenic and spoilage microflora by acidification and the production of antimicrobials. They contribute to the development of colour and flavour in dry fermented sausages (Cenci-Goga et al., Citation2008, Citation2012). They additionally influence the composition of nonvolatile and volatile compounds mainly by degrading free amino acids and inhibiting the oxidation of unsaturated free fatty acids (Søndergaard and Stahnke, Citation2002).
The typical flavour of dry sausages is due to the products originating from fermentation of carbohydrates, lipolysis/lipid oxidation, proteolysis, seasonings and curing salts. Traditionally, lipolysis was thought to be related to bacterial lipase activity (Kenneally et al., Citation1998). Lipolysis, together with proteolysis, is believed to play a central role in aroma formation (Viallon et al., Citation1996; Chizzolini et al., Citation1998) and could be affected from the curing salts or ingredients such as nitrite-nitrate (Martin et al., Citation2006). Some changes still occur and continue over chemical and physical properties of dry fermented sausages during storage period (PER), when sausages are dried under natural environmental conditions. However, quick fermentation with commercial starter cultures are not always compatible to natural fermentation flora; therefore, the use of starters could often cause losses of desirable sensory characteristics (Leroy et al., Citation2006; Samelis et al., Citation1998). Recent researches and legislations on additives regarding the negative health effects of chemical preservatives especially nitrite and nitrate in meat products, thus behaviour of natural starters against competitive natural flora in the absence of antimicrobial additive could be an advantage in ripening (Cenci-Goga et al., Citation2012). Therefore, it has been aimed to evaluate the effect of different starter culture combinations on lipolysis, ripening and the quality of sucuk in the absence of nitrite and nitrate, during both ripening periods (RIPs) and PERs.
Materials and methods
Preparation of sucuk
Meat used in experimental sucuk production was purchased from a local market in Istanbul. Sucuk dough was prepared from cattle meat (80%), mixed with body fat (cattle) and tail fat (sheep) (20%) as a base material. Then, salt (2.0%), glucose (0.5%), dry garlic (1.0%), black pepper (0.7%), red pepper (0.8%), hot red pepper (0.5%), cumin (0.5%) and corianders (0.02%) were added according to the traditional preparation of sucuk. The meat was minced to about 1.3-2.5 cm and the spices were added and mixed with the minced meat. Finally, the whole sucuk dough was divided into 4 equal groups. Each of the groups was treated separately without (Control) and with different starter culture combinations: Staphylococcus carnosus+Pediococcus pentosaceus (SP), Staphylococcus carnosus+Lactobacillus sakei (SL), and Staphylococcus carnosus+Pediococcus pentosaceus+Lactobacillus sakei (SPL). Staphylococcus carnosus subsp. utilis was used as lipolytic starter culture in formulations. All starter bacteria have been added to the dough at 10–6 CFU/g level with the ratio of 1:1, 1:1 and 2:1:1 in SP, SL and SPL, respectively.
Afterwards, the dough groups were filled into 40 mm fibrous casings until 400±20 g batons were produced for each sample, representing control, SP, SL and SPL groups. Sucuks were kept at controlled climatic conditions for 18 days and natural environmental conditions after the 18th day, which were: 90-95% relative humidity (RH) and 20-24°C at days 0-2, 85-90% RH and 18-20°C at days 3-4, 80-85% RH and 16-18°C at days 5-7, 75-80% RH and 16-18°C at days 8-12, 70-75% RH and 16-18°C at days 13-18 and variable atmospheric conditions, which were 45-70% RH and 16-19°C at days 19-60. The experimental sucuk manufacturing was duplicated at room temperature in two different dates for each group.
Analyses of sucuk
Microbiological [total aerobic plate counts (TAPC), LAB, coliforms and staphylococci] and physico-chemical (pH, aw, moisture, fat, protein, lipid oxidation, lipase enzyme levels) analyses were conducted at days 0, 1, 4, 7, 12, 18 of RIP and 25, 35, 45, 60 of PER. Three separate 400±20 g batons have been used at each sampling day for all analyses.
Microbiological analyses
Portion of sucuk samples (25 g) were transferred to a sterile stomacher bag with 225 mL of 0.1% peptone water (CM0009; Oxoid, Basingstoke, UK) and homogenised for 2 min. Serial decimal dilutions were prepared using the same diluents up to 10–6. A 0.1- or 1-mL inoculum of appropriate dilutions was spread on plate count agar (CM0325; Oxoid). Plates were incubated at 35ºC for 48 h for determination of TAPC (Harrigan, Citation1998).
Lactic acid bacteria counts were determined by plating with overlay on De Man Rogosa and Sharpe agar (CM0361; Oxoid) and incubated in anaerobic conditions at 35ºC for 48 h (Davidson and Cronin, Citation1973). Coliforms were examined in Violet Red Bile agar (CM0107; Oxoid) respectively by using pour plates with overlay added before incubation, and incubated at 35°C for 24 h. Numbers of Staphylococci were defined on Baird-Parker agar (CM0275; Oxoid) supplemented with egg yolk-tellurite emulsion (SR0054; Oxoid). Spread plates were incubated at 35°C for 24 h (Harrigan, Citation1998). All microbiological tests were carried out in duplicates, and the results were expressed as log10 CFU/g.
Physico-chemical analyses
The pH of sucuk was determined by using a Hanna pH meter (Hanna HI-9321; Hanna Instruments Woonsocket, RI, USA), water activity (aw) measurement by using a hygrometer (Lufft, Fellbach, Germany) at room temperature and moisture contents by drying a homogeneous mixture of the sample in an oven (Heraeus Holding GmbH, Hanau, Germany) at 105±2°C until a constant weight was obtained, according to AOAC procedures. Total protein content of sucuk was defined using the Kjeldahl method and total fat content of sucuk was carried out by the Soxhelet method (AOAC, Citation1984).
Thiobarbituric acid reactive substances values
Thiobarbituric acid reactive substances values of sucuk samples were evaluated according to the method of Shrestha and Min (Citation2006) by measuring the absorbance of colour developed at 530 nm using UV visual spectrophotometer (Chebios Optimum-One; Chebios, Rome, Italy) mixing 20% trichloroacetic acid in 2 M phosphoric acid solution at 4°C with 0.005 M thiobarbituric acid solution. Results were calculated according the percentage of malondialdehyde (MDA) which has a molecular weight of 72.06.
The thiobarbituric acid reactive substances (TBARS) value was calculated as follows:
Lipase enzyme levels of sucuk samples
Lipase enzyme that separates middle and long chain fatty acid esters is determined flourometrically by using commercial lipase test kits (Reflectoquant 1.05851.0001; Merck KGaA, Darmstadt, Germany). For this purpose, two disposable test tubes were used. One of them which included 1.0 mL Reagent Lip 1, 0.25 mL sample and 1.0 mL Reagent Lip 2, while the other included only 1.0 mL reagent Lip 1. After mixing the reagents carefully, the test strip was immersed in the sample reagent mixture for few seconds, then it was put immediately in the second test tube containing only the reagent Lip 1. After 15 min, the strip was removed and allowed the long edge of the strip into an absorbent paper. The strip was then inserted into the flourometer (RQflex10plus, 116955 photometer; Merck KGaA) for reading the results after the end of the reaction time (µg/L lipase).
Statistical analyses
Analysis of variance (ANOVA) was conducted for each variable to investigate the effect of starter cultures, ripening and storage time. The experimental sucuk production was performed twice at room temperature in two different dates for each group and the arithmetic means of the six sucuk samples for each batch were calculated. Then all the values [converted to logarithms of the number of colony forming units (log CFU/g) for microbiological analyses] were analysed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA) and graphs were obtained with means and standard deviations (SPSS, Citation2008).
Results and discussion
Microbiological changes during ripening and storage period
Changes in microbial populations are shown in . Results have been evaluated according to the two basic drying periods of sucuks: RIP between day 0-18, and PER between day 18-60. In general, differences between the samples were observed in control and starter cultured groups at RIP, while the differences were not remarkable during PER.
Total aerobic plate and LAB counts gradually increased until the 18th day and showed a slight decrease until 35th day and rapid decrease after the 45th day in Control, SP, SPL; while SL was almost constant regarding TAPC. Lactic acid bacteria counts increased steadily between days 0-4 in starter added groups; contrarily, Control has reached max. 7 log CFU/g. Starter culture groups remained at higher numbers than Control until the 25th day, than a fractional decrease was seen in all groups until the end of PER. It has been concluded that starter combination prepared with Lactobacillus sakei would provide more stable microflora in sucuks than those prepared with other starters, when considered prolonged PERs.
Staphylococci counts rose until the 4th day, and remained almost stable through 18th day for starter culture groups, while an evident decrease was observed in Control group after the 12th day. Nevertheless, Staphylococci numbers in starter culture groups started to decrease at 25th day. Prominently, staphylococci numbers in SL were always below the other groups including Control both in RIP and PER. This may occur due to the predominant effect of L. sakei against S. carnosus, while used in 1:1 combination in fermented meat products (Cocconcelli and Fontana, Citation2008).
A rapid decrease in coliforms below 1 log CFU/g was observed at 4th day in SL, SPL and at 7th day in SP until the increase period started at 25th day. Contrarily, coliforms stayed almost at constant level in Control group both in RIP and PER, which could be considered as a typical result of antibacterial (such as bacteriocins) or antagonistic effect of starter cultures on coliforms in sucuk fermentation.
Kaban and Kaya (Citation2006) stated that the Enterobacteriaceae count, which was 10–3 CFU/g in sucuk samples without starter cultures on the first day, was 10–2 CFU/g in sucuks with starter cultures, and dropped below detectable levels in sucuks with starter cultures on day 5 while it dropped under the same level in the control group on day 9. Similar results for coliform counts were obtained in our study, where they constantly decreased until day 25 ().
Staphylococci and micrococci seem to have a very complex lipolytic system or low substrate specificity, since they were shown to break down a wide variety of substrates (Kenneally et al., Citation1998). The growth of these bacteria was affected by acidification or by the inability of the starter to compete with the autochthonous microbes, which was in agreement with Lizaso et al. (Citation1999) and Samelis et al. (Citation1998) who considered acidification to be the main cause of micrococci-staphylococci inhibition in dry fermented sausages.
Bacterial lipases are produced during the exponential growth phase and the production is greatly influenced by growth conditions with maximum amounts formed at optimum temperatures and pH for growth. Starter cultures could be responsible for lipolysis in the early stages of ripening, when conditions of temperature, pH and NaCl% would be more favourable (Makhzoum et al., Citation1995; Kenneally et al., Citation1998). The majority of bacterial lipases showed highest activity in a neutral to alkaline pH range and between the 30-40°C temperature ranges (Kenneally et al., Citation1998).
The dominance of LAB counts in fermented meat products may affect the lipolytic activity of other microbial flora. The conditions required for the survival and lipolytic activity of starter cultures would pose contradiction to the conditions required for the activity of metabolic lipase. Moreover, cell quantity of starter culture could not ensure the expected lipolytic activity in fermented meat products.
Zhao et al. (Citation2011) stated that LAB populations were above 8 log CFU/g in cultured group and dominated the microflora due to the good adaptation to the meat environment from the beginning of the fermentation and maintained this level during ripening and storage. In parallel, LAB counts started at an initial count of 10–6 CFU/g, increased rapidly up to 10–7 CFU/g after 24 h, and 10–8 CFU/g after 4 days in the present study (). Similarly, Kenneally et al. (Citation1998) found that Lactobacilli counts were 5×10–7 CFU/g in all inoculated sausages and increased rapidly up to 10–8-10–9 CFU/g after 24 h, remaining almost constant up to 35th day. Spaziani et al. (Citation2009) also indicated that LAB increased very slowly until the 21th day of storage without starter culture addition, and a small increase of Micrococcaceae (~10– 5-10–6 CFU/g) was observed at about 21 days. They highlighted the low growth rate of LAB is consistent with the pH profile, whilst high acidification rates are usually accompanied by fast LAB growth rates.
Changes in physico-chemical properties of sucuk during ripening and storage period
Changes in physico-chemical properties are summarised in . The initial moisture levels were approximately 56% in all samples, and the moisture content ranged between about 55 to 24% with rapid decreases in RIP and ranged from 25 to 13% with more constant decreases in PER, where atmospheric conditions were natural (variable temperature and humidity). Moisture losses were grater in starter groups than control in drying period (after 18th day). A rapid fermentation of sucuks with starter addition could be the reason of the faster drying. Water activity levels were 0.96 at the beginning of RIP, between 0.8-0.9 at day 18 and below 0.8 at day 35 and until the end of PER, in all samples. Decreases in moisture and aw have been concluded as a typical consequence of drying process of sucuk. Spaziani et al. (Citation2009) emphasised that the sausages lost weight during processing and, as a consequence, aw value gradually decreased to 0.87-0.88 and sausages dried evenly, whereas pH values stayed around their initial values during processing. Zhao et al. (Citation2011) determined that the moisture content ranged between ~21 and 30% and aw was 0.88 in all with or without starter added sausages, at the end of ripening (day 7), while a decrease was observed to ~0.85 by day 35 compared to day 0.
The initial pH values in all samples were approximately 5.8; a dramatic decrease to ~4.5 was observed at 4th day of RIP in SP, SL, SPL, in contrast with the stable and constant pH values seen in Control until 12th day.
The pH fall was presumably caused by an accumulation of organic acids, mainly lactic, present in these types of sausages as a result of carbohydrate, especially glucose breakdown during fermentation (Bloukas et al., Citation1997). The low growth rate of LAB in these types of sausage is consistent with the pH profile. It is well known that high acidification rates are usually accompanied by fast LAB growth rates. However, increased lactic acid production by LAB, in the presence of glucose, has been noticed in spite of an unchanged specific growth rate, suggesting that the additional energy obtained from direct fermentation of glucose is used for functions other than growth (Guyot et al., Citation2000).
As the pH value of sucuks decreased sharply during the first few days of ripening due to the production of acid (LAB converts glucose to lactic acid), it became nearly constant after this period and the pH level of sucuks could even be more stable when starter cultures have been used in production (Bozkurt and Erkmen, Citation2002; Kayaardi and Gok, Citation2003; Bozkurt and Bayram, Citation2006; Cocconcelli and Fontana, Citation2008). In the present study, LAB counts reached over 10–8 CFU/g level at 4th day in SP, SL and SPL, while it was 12th day in Control, where rapid pH decreases were seen. Higher pH values in control samples could be due to the occurrence of autochthonous lactobacilli with low acidifying power as similarly stated by Casaburi et al. (Citation2007) and Hierro et al. (Citation1999).
The Turkish Standard Institute states that pH value for high quality ripened sausages should be in the range of 4.7-5.2 (Turkish Standard Institute, Citation1983). According to this standard, the pH values were below the recommended level in starter added sucuks during the whole drying period. It can be expected more acidic activity due to the absence of nitrite together with the activity of starter cultures. As stated by Scannell et al. (Citation2001), a greatest pH drop and more acidic activity are reported with L. sakei and low nitrite level in fermented salami.
Changes in thiobarbituric acid reactive substances and lipase enzyme levels of sucuk during ripening and storage period
Changes in TBARS and lipase enzyme levels of sucuk samples are shown in . Thiobarbituric acid reactive substances values gradually increased until the 12th day and started to decrease until the end of storage in SP, SL and SPL; while Control samples were almost constant during RIP and PER. The differences in sucuk samples were observed between control and starter culture groups at RIP and PER, and they were also remarkable in starter culture added samples during RIP and PER with higher levels of oxidation during RIP then PER, ranging from SL>SPL>SP.
Lipase enzyme levels of sucuk samples had undulating behaviour until the 7th day of RIP in Control and starter culture groups, then decreased until the 18th day of RIP, and remained constant till the 45th day of PER where a sharp increase was remarkable in all sucuks ranging from SP>SPL>SL>Control.
Lipolysis and lipid oxidation are related to flavour formation in dry sausages (Gandemer, Citation2002) due to the generation of flavour precursors, free fatty acids. However, until now it has not been shown how fat can act not only as a source but also as a solvent for flavour compounds in dry sausages and so, how both aspects affect consumer acceptance. It has been reported that high fat sucuks had higher TBARS values than low fat ones, where this highest amount of fat in sucuk produced increased lipolysis and lipid oxidation (Olivares et al., Citation2011). Although the lipid oxidation was triggered by the amount of fat used in the formulation, the fat used in the manufacturing of sucuks were the same for all groups in our study. The differences seen in TBARS levels would be the result of the formation of aroma compounds and pH decrease due to lactic activities of starter cultures. Moreover, it has also been concluded that the groups where L. sakei were used (SL and SPL) had relatively higher TBARS values between day 7 and 25, as a result of dominance and strong lactic activity of L. sakei and therefore decreased pH levels in ripening period. Kayaardi and Gok (Citation2003) stated that sucuks prepared with the addition of olive oil in place of animal fat were more susceptible to lipid oxidation and that maximum TBA value of sucuk was 1.63, which is above the acceptable range of <1 mg MDA/kg (Bloukas et al., Citation1997). Yildiz-Turp and Serdaroglu (Citation2008) indicated that after 12 days, all sucuk samples had TBA values within acceptable limits (<1.0), because of the antioxidant activity of the - tocopherol present in the added hazelnut oil in formulation. Bozkurt and Erkmen (Citation2002) observed that sucuks made without starter culture had higher TBA values than those made with starter cultures. Furthermore, they emphasised that the effects of nitrite and nitrate on TBA values were less than for the other additives (α-tocopherol, ascorbic acid and phosphates).
No direct correlation between lipase enzyme levels and TBARS values in RIP were evaluated in the present study, since TBARS values may vary depending on the several biochemical activities in fermentation. Thiobarbituric acid reactive substances decreased under the acceptable limit (>1.0 mg MDA/kg) after the 18th day in all starter cultured groups, and continued gradually afterwards. Similarly, Bozkurt (Citation2006, Citation2007) stated that the decrease and levelling of TBARS values after RIP could be due to the lost or decomposition of formed TBARS substances to volatile compounds.
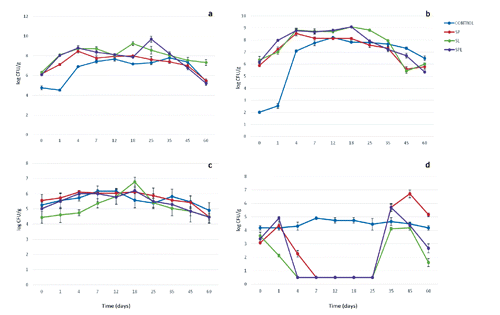
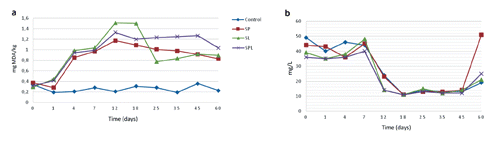
Table 1. Changes in physico-chemical properties of sucuk samples during ripening and storage period.
Conclusions
Since lipolytic activity and the controlled drop of pH are among the major quality attributes, the usage of lipolytic starter cultures in sausage fermentation and manufacturing is unavoidable. Even though SP would give more stable results in PER, the SL and SPL groups were found to be more favourable when microbiological and physico-chemical results were considered. Elevated lipase enzyme levels detected in SP and SPL groups after the 45th day would be the reason of secondarily dominance of lipolytic Staphylococcus in the sausages.
The usage of L. sakei and S. carnosus in suitable combination as a starter culture in sucuk formulation may provide an additional tool for the acceleration of ripening and the improvement of the quality of dry fermented sausages in prolonged storage. They would have a positive effect on controlling the growth and survival of potentially pathogenic bacteria and on contributing to the structural qualities of nitrite and nitrate free sucuks.
References
- Aksu M.I. Kaya M., 2004. Effect of usage Urtica dioica L. on microbiological properties of sucuk, a Turkish dry-fermented sausage. Food Control 15:591-595.
- AOAC, 1984. Official methods of analysis. Centennial edition. Association of Official Analytical Chemists, Washington, DC, USA.
- Bloukas J.G. Paneras E.D. Fournitzis G.C., 1997. Effect of replacing pork backfat with olive oil on processing and quality characteristics of fermented sausages. Meat Sci. 45:133-144.
- Bozkurt H., 2006. Utilization of natural antioxidants: green tea extract and Thymbra spicata oil in Turkish dry-fermented sausage. Meat Sci. 73:442-450.
- Bozkurt H., 2007. Comparison of the effects of sesame and Thymbra spicata oil during the manufacturing of Turkish dry-fermented sausage. Food Control 18:149-156.
- Bozkurt H. Bayram M., 2006. Colour and textural attributes of sucuk during ripening. Meat Sci. 73:344-350.
- Bozkurt H. Erkmen O., 2002. Formations of biogenic amines in Turkish style sausage during ripening and storage periods. J. Food Quality 25:317-332.
- Casaburi A. Aristoy M.C. Cavella S. Monaco R.D. Ercolini D. Toldra F., 2007. Biochemical and sensory characteristics of traditional fermented sausages of Vallo di Diano (Southern Italy) as affected by the use of starter cultures. Meat Sci. 76:295-307.
- Cenci-Goga B.T. Ranucci D. Miraglia D. Cioffi A., 2008. Use of starter cultures of dairy origin in the production of Salame nostrano, an Italian dry-cured sausage. Meat Sci. 78:381-390.
- Cenci-Goga B.T. Rossitto P.V. Sechi P. Parmegiani S. Cambiotti V. Cullor J.S., 2012. Effect of selected dairy starter cultures on microbiological, chemical and sensory characteristics of swine and venison (Dama dama) nitrite-free dry-cured sausages. Meat Sci. 90:599-606.
- Chizzolini R. Novelli E. Zanardi E., 1998. Oxidation in traditional Mediterranean meat products. Meat Sci. 49:87-99.
- Cocconcelli P.S. Fontana C., 2008. Characteristics and applications of microbial starters in meat fermentations. In: ToldráF. ( ed.) Meat biotechnology. Springer, New York, USA, pp 129-148.
- Davidson C.M. Cronin F., 1973. Medium for the selective enumeration of lactic acid bacteria from foods. J. Appl. Microbiol. 26:439-440.
- Gandemer G., 2002. Lipids in muscles and adipose tissues, changes during processing and sensory properties of meat products. Meat Sci. 62:309-321.
- Guyot J.P. Calderon M. Morlon-Guyot J., 2000. Effect of pH control on lactic acid fermentation of starch by Lactobacillus manihotivorans LMG18010T. J. Appl. Microbiol. 88:176-182.
- Hammes W.P. Knauf H.J., 1994. Starters in the processing of meat products. Meat Sci. 36:155-168.
- Harrigan W.F., 1998. Laboratory methods in foods microbiology. Academic Press Ltd., Waltham, MA, USA.
- Hierro E. De La HozL. Ordonez J.A., 1999. Contribution of the microbial and meat endogenous enzymes to the free amino acids contents of dry fermented sausages. J. Agr. Food Chem. 47:1156-1161.
- Kaban G. Kaya M., 2006. Effect of starter culture on growth of Staphylococcus aureus in sucuk. Food Control 17:797-801.
- Kayaardi S. Gok V., 2003. Effect of replacing beef fat with olive oil on quality characteristics of Turkish soudjouk (sucuk). Meat Sci. 66:249-257.
- Kenneally P.M. Leuschner R.G. Arendt E.K., 1998. Evaluation of the lipolytic activity of starter cultures for meat fermentation purposes. J. Appl. Microbiol. 84:839-846.
- Leroy F. Verluyten J. De VuystL., 2006. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 106:260-285.
- Lizaso G. Chasco J. Beriain J., 1999. Microbiological and biochemical changes during ripening of salchichon, a Spanish dry cured sausages. Food Microbiol. 6:219-228.
- Makhzoum A. Knapp J.S. Owusu R.K., 1995. Factors affecting growth and extracellular lipase production by Pseudomonas fluorescens 2D. Food Microbiol. 12:277-290.
- Martin B. Garriga M. Hugas M. Bover-Cid S. Veciana-Nogues M.T. Aymerich T., 2006. Molecular, technological and safety characterization of gram-positive catalasepositive cocci from slightly fermented sausages. Int. J. Food Microbiol. 107:148-158.
- Olivares A. Navarro J.L. Flores M., 2011. Effect of fat content on aroma generation during processing of dry fermented sausages. Meat Sci. 87:264-273.
- Samelis J. Metaxopoulos J. Vlassi M. Pappa A., 1998. Stability and safety of traditional Greek salami. A microbiological ecology study. Int. J. Food Microbiol. 44:69-82.
- Scannell A.G.M. Hill C. Ross R.P. Schwarz G. Arendt E.K., 2001. Effect of nitrite on a bacteriocinogenic Lactococcus lactis transconjugant in fermented sausage. Eur. Food Res. Technol. 213:48-52.
- Shrestha S. Min Z., 2006. Effect of lactic acid pretreatment on the quality of fresh pork packed in modified atmosphere. J. Food Eng. 72:254-260.
- Søndergaard A.K. Stahnke L.H., 2002. Growth and aroma production by Staphylococcus xylosus, S. carnosus and S. equorum. A comparative study in model systems. Int. J. Food Microbiol. 75:99-109.
- Spaziani M. Del TorreM. Stecchini M.L., 2009. Changes of physico-chemical, microbiological and textural properties during ripening of Italian low-acid sausages. Proteolysis, sensory and volatile profiles. Meat Sci. 81:77-85.
- SPSS, 2008. Statistical package for the social sciences, ver. 16.0. SPSS Inc., Chicago, IL, USA.
- Turkish Standards Institute, 1983. Turkish sucuk. Turkish Standards Institute Publ., Ankara, Turkey.
- Viallon C. Berdague J.L. Montel M.C. Talon R. Martin J.F. Kondjoyan N. Denoyer C., 1996. The effect of stage of ripening and packaging on volatile content and flavour of dry sausages. Food Res. Int. 29:667-674.
- Yildiz TurpG. Serdaroglu M., 2008. Effect of replacing beef fat with hazelnut oil on quality characteristics of sucuk. A Turkish fermented sausage. Meat Sci. 78:447-454.
- Zhao L. Jin Y. Ma C. Song H. Li H. Wang Z. Xiao S., 2011. Physico-chemical characteristics and free fatty acid composition of dry fermented mutton sausages as affected by the use of various combinations of starter cultures and spices. Meat Sci. 88:761-766.
