Abstract
This study was designed to compare Cortalap® extender with tris-citric acid-glucose (TCG), Lepus® and Merk III®, to assess the in vitro preservability of rabbit spermatozoa stored for 72 h at 5°C. The best extender identified in vitro was then compared with fresh semen to determine fertility and prolificacy rates in vivo. Eight semen pools were split into four subsamples and each of them was diluted to a ratio of 1:10 (volume:volume) with four different extenders (Lepus®, Cortalap®, TCG and Merk III®) and stored at 5°C for 72 h. Sperm motility, viability, sperm membrane and acrosome integrity were evaluated in both fresh and chilled semen at 4, 24, 48 and 72 h of storage. The results showed that Cortalap® was the best extender to preserve the quality of rabbit semen in vitro over 72 h in comparison with the other extenders (P<0.05). Then, Cortalap® was used for an artificial insemination (AI) trial to be compared with fresh semen. Two groups of does (n=30 each) were inseminated with fresh and chilled semen. The fertility and prolificacy rates for the does inseminated with chilled semen were significantly lower compared with those inseminated with fresh semen (P<0.05). In conclusion, although Cortalap® proved to be the best extender to preserve semen quality in vitro after 72 h at 5°C, the in vivo results showed that its use is not recommended in AI programmes.
Introduction
The demand for stored semen to be used in artificial insemination (AI) programmes of livestock animals is increasing (Vyt et al., Citation2004; Aurich et al., Citation2007; Xu et al., Citation2009; Zhao et al., Citation2009; Paulenz et al., Citation2010; Rosato and Iaffaldano, Citation2011).
The farming systems for commercial rabbit meat production are based almost exclusively on AI programmes, which are currently performed with fresh diluted semen within 6-12 h from semen collection, and are mostly limited to does in farms where bucks are kept (Daniel and Renard, Citation2010).
The availability of semen which can be stored for longer periods would allow an extension of the interval between semen collection and the insemination of females, thus enhancing AI performance, for example in farms without males and located far from semen collection centres (López and Alvariño, Citation1998; Gogol, Citation2013). Unfortunately, storage of rabbit semen for longer than 24 to 48 h causes deterioration of semen quality with a decrease in fertility (Roca et al., Citation2000; López-Gatius et al., Citation2005; Aksoy et al., Citation2008; Rosato and Iaffaldano, Citation2011). Therefore, extending the interval of liquid semen storage beyond 48 h or freezing the semen, remains one of the major goals in the meat rabbit industry. Although semen cryopreservation offers many advantages, the limited survival of sperm after freezing is a major drawback for a widespread use of frozen semen in AI programmes. Only recently a few reports have shown satisfactory results with frozen semen (Iaffaldano et al., Citation2012; Rosato and Iaffaldano, Citation2013). However, the freezing technique is more expensive and requires more equipment than the chilling technique. Thus, also the maintenance of the fertilising capacity of stored rabbit semen for longer than 48 to 72 h remains an important target to reach for the meat rabbit industry. Therefore, attempts are needed to improve semen extenders and storage conditions to prolong the time during which stored semen can maintain its functional status. With regard to the chilled semen conditions, in our previous paper we reported that a temperature of 5°C proved to be more beneficial than 15°C in retaining the overall semen quality during long-term storage (Rosato and Iaffaldano, Citation2011).
However, extenders play a key role in long-term storage of rabbit semen, because they provide the nutrients needed for the metabolic maintenance of sperm cells and control the pH and the osmotic pressure of the medium. Previous studies were made to identify a suitable extender for long-term survival of rabbit spermatozoa (Castellini, Citation1996; Roca et al., Citation2000; Carluccio et al., Citation2004; El-Kelawy et al., Citation2012), but to the best of our knowledge Cortalap® has never been used for long-term storage of rabbit semen, neither has it been compared with other extenders. Therefore, this study has been designed to compare Cortalap® with three other extenders, namely tris-citrateglucose (TCG), Lepus® and Merk III®, to assess the in vitro preservability of rabbit sperm stored for 72 h at 5°C. The best extender identified in vitro was then compared with fresh semen to determine fertility and prolificacy rates in vivo.
Materials and methods
Chemicals
The LIVE/DEAD Sperm Viability Kit was purchased from Molecular Probes Inc. (Eugene, OR, USA). Kanamycin was purchased from Biolife (Biolife S.r.l., Milan, Italy). The Pisum sativum agglutinin-fluorescein isothiocyanate conjugate (PSA-FITC) and all the other chemicals utilised in this study were purchased from Sigma Chemical (St. Louis, MO, USA).
Animals
The animals enrolled in this study were 32 adult rabbit bucks and 60 does of Bianca Italiana breed from the central breeding farm of the Italian Rabbit Breeders National Organization-Breeders Italian Organization (ANCI-AIA), in Volturara Appula (FG), Italy. The rabbits were housed in individual flat-deck cages exposed to a 16 h light/8 h-dark photoperiod and were fed a commercial standard diet with water given ad libitum.
Semen collection and processing
Semen was collected using an artificial vagina. After semen collection, any gel plugs were removed. Ejaculates were pooled to avoid individual differences (four ejaculates/pool; eight pools in total) and delivered to the laboratory in a water bath at 25/30°C. Only ejaculates presenting a white, opalescent appearance and a volume higher than 0.5 mL were utilised and pooled. The sperm concentration was assessed using the improved Neubauer haemocytometer. Each pool of rabbit semen was split into four subsamples and each of them was diluted to a ratio of 1:10 (volume:volume) with four different liquid extenders: TCG, composed of 88 mM of citric acid, 250 mM of tris-hydroxymethy-laminomethane, 47 mM of glucose and 80 mg/L of kanamycin sulphate; Cortalap® (IMV Technologies, L’Aigle, France); Lepus® (IMV Technologies); Merk III® (Minitüb GmbH, Tiefenbach, Germany). The composition of Cortalap®, Lepus® and Merk III® is undisclosed, because of commercial interests. The extended semen samples were stored at 5°C in a refrigerated incubator [M80-TBR basic; MPM instruments, Barnareggio (MI), Italy] for 72 h. Sperm motility, viability, sperm membrane functional competence and acrosome integrity were evaluated in fresh semen (undiluted) and after 4, 24, 48 and 72 h of storage. In total, 272 samples of either fresh or chilled semen were analysed.
Assessment of rabbit semen quality in vitro
Rabbit sperm motility was subjectively assessed by visual examination (Rosato and Iaffaldano, Citation2011). A 10 μL drop of semen was transferred onto a clean glass slide, pre-warmed to 37°C, and covered with a coverslip. The mounted slides were then observed on a warmplate at 400× total magnification with a phase-contrast microscope (Leica Aristoplan; Leica GmbH, Wetzlar, Germany). The percentage of total motility (spermatozoa showing any type of sperm head movement) and forward progressive motility (spermatozoa showing linear movement) were estimated in five microscopy fields.
Sperm viability was measured using the LIVE/DEAD Sperm Viability Kit (Molecular Probes Inc.) containing fluorescent stains SYBR-14 and propidium iodide (PI), following the methods described by Iaffaldano et al. (Citation2010). This procedure was performed on 5 μL of semen, which were added to 39 μL of extender containing 1 μL of SYBR-14 (diluted to 1:100 in dimethyl sulphoxide). Subsequently, the extended semen was incubated at 37°C for 10 min, and then 5 μL of PI were added (diluted to 1:100 in the TCG diluent) followed by incubation at 37°C for 5 more min. Next, 10 μL of this suspension were transferred on microscope slides, covered with coverslips and examined at a magnification of 1000× using a 100×oil immersion objective under epifluorescence microscopy. For each sample, approximately 200 spermatozoa were examined in duplicate aliquots. SYBR-14, a membrane permeant DNA stain, only stains live spermatozoa producing green fluorescence of the nuclei. Propidium iodide stains the nuclei of membrane-damaged cells red. Thus, spermatozoa showing green fluorescence were scored as alive and those showing red fluorescence as dead. The percentage of viable spermatozoa was calculated as number of green cells×100 divided by the total number of sperm cells counted.
To determine sperm osmotic resistance, a hypo-osmotic water test was used. The test was performed by mixing 10 µL of semen with 80 µL of distilled water in an Eppendorf tube, which was incubated for 5 min at 37°C. Subsequently, 10 µL of this mixture were transferred onto a clean glass slide and covered with a thin coverslip before examination under a phase-contrast microscope. Two hundred cells were evaluated by counting in at least five fields at 800× total magnification, and only sperm cells having a curling tail were considered to be HOST-positive. The rationale of the test is based on the assumption that when spermatozoa are exposed to hypo-osmotic media, such as distilled water, an undamaged sperm membrane permits passage of fluid into the cytoplasmic space causing swelling produced by a hypo-osmotic shock and the pressure generated leads to curling of tail fibres. Conversely, the damaged or chemically inactive sperm membrane allows fluid to pass across the membrane without any accumulation and accordingly no cytoplasmic swelling and curling of the tail occurs. The percentage functional integrity of the sperm membrane was calculated as: number of coiled sperm cells equal to 100 divided by the total number of sperm cells counted (Rosato and Iaffaldano, Citation2011).
To determine acrosome integrity, duplicate smears were prepared using a drop of semen from each sample and air-dried. After fixation in methanol for 30 min, the slides were washed with water and air-dried, then incubated with the FITC-PSA conjugate for 30 min at room temperature. The slides were then mounted with 50% glycerol (volume/volume) and cover slipped (Mendoza et al., Citation1992). In each sample, assessment was made of 200 sperm cells at a total 1000× magnification, using an oil immersion objective under epifluorescence illumination. This stain intensely labels the acrosomal region of acrosome-intact sperm cells, which emit a uniform apple-green fluorescence, while acrosome-damaged spermatozoa show scarce or no green fluorescence in the anterior part of the head. The percentage of acrosome intact spermatozoa was calculated as a fraction of the total.
Artificial insemination
Based on the results obtained in vitro, we selected Cortalap® as the best extender and compared its efficacy with fresh semen in an AI trial. In January 2014, 60 multiparous (31 days post-partum) receptive rabbit does were randomly allotted to two groups of 30 does. Animals in the 1st group (control group) were inseminated with a dose of 0.5 mL of fresh semen diluted to 1:10 with Cortalap®, animals in the 2nd group were inseminated with a dose of 0.5 mL of diluted semen to 1:10 and cooled at 5°C for 72 h in Cortalap®. Both the fresh and stored doses contained approximately 32 million sperm cells/0.5 mL.
For oestrus synchronisation, all does were subjected to the following biostimulation protocol: flushing (3 days before insemination), change of cage (3 days before insemination), and extension of the photoperiod from 16 to 24 h of light (2 days before insemination). At the time of insemination, each female was administered an intramuscular injection of buserelin acetate to induce ovulation (1 µg/doe).
Fertility (number of pregnant does/number of inseminations) was determined by abdominal palpation performed in each doe 17 days after AI. At parturition, the following factors were determined: kindling rate (number of does giving birth/number of inseminations), number of kids born (total born/kindling), and number of kids born alive (born alive/kindling).
Statistical analysis
Sperm quality variables (motility, viability, sperm membrane integrity, acrosome and DNA integrity) were compared among the treatments by ANOVA, followed by Duncan’s comparison test. Fertility and prolificacy data between fresh and chilled semen were compared by independent-samples t-test. Differences were considered statistically significant at P<0.05. All statistics were calculated by SPSS (Citation2006).
Results and discussion
In this paper we intended to find a suitable extender for the storage of rabbit sperm up to 72 h by comparing Cortalap® with TCG, Lepus® and Merk III®. Subsequently, the best extender identified in vitro was compared with fresh semen in field trials. reports the quality parameters of fresh semen, which showed a good initial quality. Indeed, about 90% of the sperm population exhibited motility, viability and acrosome integrity, whereas approximately 80% of the sperm population exhibited a forward progressive movement, osmotic resistant plasma membrane and an average sperm concentration of 645×106 mL–1.
As we expected, the preservation at 5°C for 72 h worsened the quality of rabbit semen, which was differently affected depending on the extender used. The results of semen quality during 72 h of chilled storage with different extenders (Lepus®, Cortalap®, TCG and Merk III®) are presented in Figures 1-5.
The effects of different extenders on total and progressive motility of chilled sperm are shown in and . Total and forward progressive motility obtained in semen chilled with Cortalap® were significantly higher (P<.05) at all storage time-points (from 4 to 72 h) with respect to the other extenders. Moreover, we observed that the lower percentage of sperm motility was recorded in semen stored with TCG; instead, Lepus® and Merk III® showed intermediate values without significant statistical difference between them.
A similar trend was observed for viability (). Higher viability values (P<0.05) were found when semen was stored with Cortalap® at 4, 24, 48 and 72 h of storage compared with other extenders. The worst viability was found with TCG, while Lepus® showed significantly higher values than Merk III® at 4 and 48 h of storage.
For the acrosome integrity (), the best values during the 72 h storage period were also obtained with Cortalap® (P<0.05). Moreover, Merk III® and Lepus® showed similar values at 24, 48 and 72 h of storage except at 4 h, where a better acrosome integrity value was recorded with Lepus® (P<0.05).
Sperm membrane integrity, as reflected by the other parameters considered (), was significantly affected by the extender (P<0.05). Functional integrity was significantly higher in Cortalap® with respect to Merk III®, Lepus® and TCG at all storage time-points (P<0.05), while the worst results were found with TCG. Similar values were reported in the comparison between Merk III® and Lepus® at 24, 48 and 72 h except at 4 h, where Lepus® was better preserved than Merk III® (P<0.05).
Our most significant finding was that the Cortalap® extender preserved better the quality of rabbit semen in vitro during 72 h of storage at 5°C, in comparison with Lepus®, Merk III® and TCG. Unfortunately, we did not know the composition of Cortalap® as well as Lepus® and Merk III®, because it is undisclosed due to commercial interests. We can only conclude that Cortalap®, with respect to the other extenders, provides a suitable environment for rabbit spermatozoa stored at 5°C up to 72 h. It is known that an ideal extender should have nutrients as energy source (glucose and fructose are most commonly used), substances that buffer against harmful changes of pH, provide a physiological osmotic pressure and concentration of electrolytes (citric acid, sodium citrate, TES and Tris) and finally also antibiotics which prevent bacterial growth (Roca et al., Citation2000). Our research also showed that the worst semen quality during the 72 h storage period at 5°C was obtained using TCG. Moreover, the semen quality obtained with TCG in this study was lower than that recorded in our previous work (Rosato and Iaffaldano, Citation2011). This could be due to the different rabbit genetic types used. In this paper Lepus® and Merk III® extenders preserved the quality of rabbit semen in the same way, however Carluccio et al. (Citation2004) showed that Merk III® was better than Lepus® during liquid storage of semen at 4°C for 34 h.
reports fertility and prolificacy rates recorded after the AI of does with fresh semen or semen chilled in Cortalap® for 72 h at 5°C. Fertility and kindling rates for the does inseminated with semen chilled with Cortalap® were significantly lower with respect to those inseminated with fresh semen (20 and 20% vs 93.33 and 86.67%, respectively) (P£0.05). Also the number of kids born and the number of young born alive were significantly lower with chilled (2.2±0.3 and 1.8±0.5) than fresh semen (10.1±0.5 and 9±0.6).
Hence, we can conclude that, although Cortalap® preserved semen quality better in vitro after 72 h at 5°C than the other extenders, its application in vivo has not achieved reproductive performance adequately enough to recommend its use in AI programmes. Reproductive performance reflected the quality of fresh and stored semen. Previous papers showed that the most important parameters pertaining to fertility are the number of spermatozoa inseminated and their motility (Castellini and Lattaioli, Citation1999; Brun et al., Citation2002). Alvariño et al. (Citation1996), using semen stored for 24 h, found that, in order to ensure an adequate fertility rate and litter size under field conditions, more than 20 million viable sperm cells/dose are needed. These observations enabled us to understand the reason why, though containing 32 million sperm cells/0.5 mL like the fresh semen dose, our inseminating dose of chilled semen did not allow us to maintain satisfactory reproductive performance: it only contained about 10 million viable and motile spermatozoa.
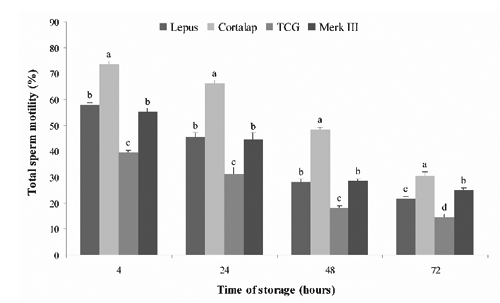
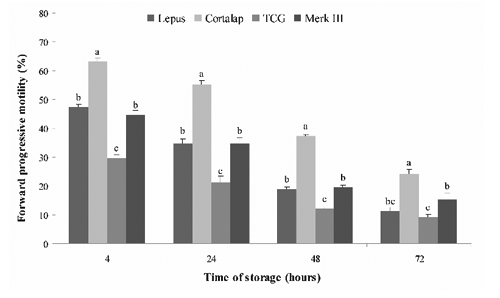
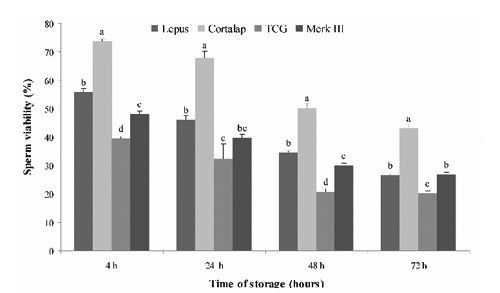
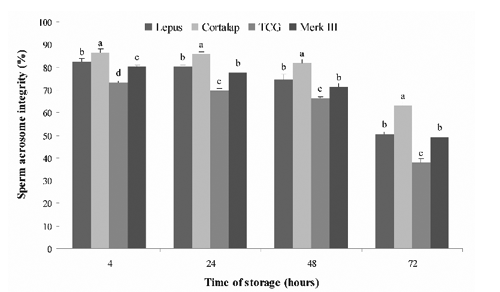
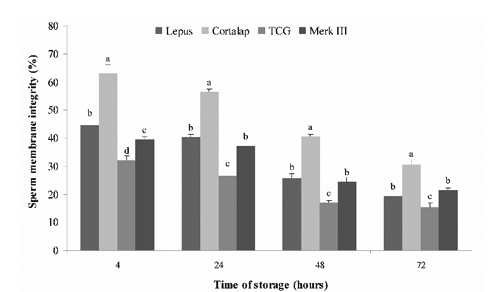
Table 1. Sperm qualitative parameters of freshly collected rabbit semen (n=8).
Table 2. Reproductive performance of rabbit does after artificial insemination with fresh semen or semen chilled in Cortalap®.
Conclusions
In conclusion, our results in vitro indicate that rabbit semen chilled with Cortalap® has shown better quality characteristics (P<0.05) than the other extenders used, because it offered a suitable environment during the 72 h storage at 5°C. Hence, we can infer that, although Cortalap® preserved semen quality better in vitro after 72 h at 5°C than the other extenders, its use in vivo has not guaranteed an adequate reproductive performance and therefore cannot be recommended in AI programmes.
Acknowledgements
The authors thank the President (Dr. Sergio Pompa) and the Director (Dr. Mario Giovannoli) of the Italian Rabbit Breeders National Organization-Breeders Italian Organization (ANCI-AIA) for allowing to use their Central Breeding Farm; Drs. Paola Principe and Michele Schiavitto for technical assistance with semen collection and artificial inseminations; and A.D. Burchell for the English revision of the manuscript.
References
- Aksoy M. Cankat LehimcioğluN. Akman O., 2008. Effect of seminal plasma on functional integrity of rabbit sperm membranes during storage at 4°C or freezing. World Rabbit Sci. 16:1-6.
- Alvariño J.M.R. Lopez F.J. Del ArcoJ. A. Bueno A. Torres R., 1996. Effect of semen concentration on rabbit artificial insemination with fresh or 24 hours stored semen. Proc. 6th World Rabbit Congr., Toulouse, France, 2:33-35.
- Aurich C. Seeber P. Müller-Schlösser F., 2007. Comparison of different extenders with defined protein composition for storage of stallion spermatozoa at 5 degrees C. Reprod. Domest. Anim. 42:445-448.
- Brun J.M. Theau-Clement M. Bolet G., 2002. The relationship between rabbit semen characteristics and reproductive performance after artificial insemination. Anim. Reprod. Sci. 70:139-149.
- Carluccio A. Robbe D. De AmicisI. Contri A. Russo F. Paoletti M., 2004. Artificial insemination in rabbits: laboratory and field trial with three different semen extenders. World Rabbit Sci. 12:65-79.
- Castellini C., 1996. Recent advances in rabbit artificial insemination. Proc. 6th World Rabbit Congr., Toulouse, France, 2:13-26.
- Castellini C. Lattaioli P., 1999. Effect of motile sperms inseminated on reproductive performance of rabbit does. Anim. Sci. 57:111-120.
- Daniel N. Renard J.P., 2010. Artificial insemination in rabbits. Cold Spring Harb. Protoc. 2010: pdb.prot5358.
- El-Kelawy H.M. Tawfeek M.I. El-Gaafary M.N. Ibrahim H., 2012. Viability and fertilizing ability of extended rabbit semen stored at 5°C. pp 285-289 in Proc. 10th World Rabbit Congr., Sharm, El-Sheikh, Egypt.
- Gogol P., 2013. Motility parameters and intracellular ATP content of rabbit spermatozoa stored for 3 days at 15°C. Folia Biol.-Krakow 61:87-91.
- Iaffaldano N. Di IorioM. Rosato M.P., 2012. The cryoprotectant used, its concentration, and the equilibration time are critical for the successful cryopreservation of rabbit sperm: dimethylacetamide versus dimethylsulfoxide. Theriogenology 78: 1381-1389.
- Iaffaldano N. Rosato M.P. Paventi G. Pizzuto R. Gambacorta M. Manchisi A., 2010. The irradiation of rabbit sperm cells with He-Ne laser prevents their in vitro liquid storage dependent damage. Anim. Reprod. Sci. 119:123-129.
- López F.J. Alvariño J.M.R., 1998. Artificial insemination of rabbits with diluted semen stored up to 96 hours. World Rabbit Sci. 6:251-253.
- López-Gatius F. Sances G. Sancho M. Yániz J. Santolaria P. Gutiérrez R. Núñez M. Núñez J. Soler C., 2005. Effect of solid storage at 15°C on the subsequent motility and fertility of rabbit semen. Theriogenology 64:252-260.
- Mendoza C. Correras A. Moos J. Tesarik J., 1992. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J. Reprod. Fertil. 95:755-763.
- Paulenz H. ÅdnøyT. Fossen O.H. Söderquist L., 2010. Effect on field fertility of addition of gelatine, different dilution rates and storage times of cooled ram semen after vaginal insemination. Reprod. Domest. Anim. 45:706-710.
- Roca J. Martínez S. Vázquez J.M. Lucas X. Parrilla I. Martínez E.A., 2000. Viability and fertility of rabbit spermatozoa diluted in Tris-buffer extenders and stored at 15°C. Anim. Reprod. Sci. 64:103-112.
- Rosato M.P. Iaffaldano N., 2011. Effect of chilling temperature on the long-term survival of rabbit spermatozoa held either in a tris-based or a jellified extender. Reprod. Domest. Anim. 46:301-308.
- Rosato M.P. Iaffaldano N., 2013. Cryopreservation of rabbit semen: comparing the effects of different cryoprotectants, cryoprotectant-free vitrification, and the use of albumin plus osmoprotectants on sperm survival and fertility after standard vapor freezing and vitrification. Theriogenology 79:508-516.
- SPSS, 2006. User’s guide, ver. 15.0. SPSS Inc., Chicago, IL, USA.
- Vyt P. Maes D. Dejonckheere E. Castryck F. Van SoomA., 2004. Comparative study on five different commercial extenders for boar semen. Reprod. Domest. Anim. 39:8-12.
- Xu C.L. Zhou J.B. Zhao B.T. Lan G.C. Luo M.J. Chang Z.L. Sui H.S. Tan J.H., 2009. Liquid storage of goat semen in chemically defined extenders. Reprod. Domest. Anim. 44:771-778.
- Zhao B.T. Han D. Xu C.L. Luo M.J. Chang Z.L. Tan J.H., 2009. Protocol optimization for long-term liquid storage of goat semen in a chemically defined extender. Reprod. Domest. Anim. 44:865-872.
