Abstract
The American lobster (Homarus americanus) is one of the most important crustacean resources in North America. In Italy and Europe, this fishery product is available throughout the year and it has a high and increasing commercial demand. American lobsters are traditionally marketed live and stocked, without feed, in temperature controlled recirculating systems for several weeks before being sold in the market places. The current Italian legislation does not fix a maximum length of time for the crustacean confinement and specific welfare requirements. In the present research, a 4-week experiment was carried out using 42 adult H. americanus reared in 4 recirculating aquaculture tanks. After one month of confinement, mean glucose, protein and total haemocyte count levels in the hemolymph of H. americanus were stable and similar (P>0.05) to the values observed at the beginning of the experiment. Results of the proximate analysis of the abdominal muscles of H. americanus showed no significant differences in concentrations of crude protein, lipid and ash during the trial. At the end of the experiment, the sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting analysis revealed a marked degradation of the muscle myofibrillar proteins. A number of fragments, possibly from myosin, were evident in the range between 50 and 220 kDa between time t0 and t28. Results of this study show that the main hemolymphatic variables and degradation analysis of the muscle myofibrillar proteins can be used as sensitive indicators of the crustacean stress response to confinement and starvation.
Introduction
Lobster fishery (Homarus americanus) is one of the most important economic activities in United States and Canada (mainly Northeast America and Atlantic Canada). In North America, total landings have substantially increased in the last decades (Phillips et al., Citation2013), while, on the other hand, total number of lobster catches in the Mediterranean countries (Italy, Turkey, Croatia, etc.) has been very low (close to zero) (Phillips et al., Citation2013). Lobsters are traditionally marketed live and kept in recirculating aquaria for several weeks in non-feeding conditions. The current Italian legislation does not oblige retail operators to sale live animals. However, in the guidelines of the Italian law it is explicitly suggested to sell live animals in order to improve their qualitative characteristics (Candotti, Citation2007). Furthermore, for many consumers, to buy live animals is perceived as a good and fair practice. At the retail level, lobsters are kept in temperature controlled aquaria at very low temperatures (<8°C) in order to reduce metabolic processes and cannibalism. According to some studies, transportation and stocking conditions may cause stress to animals and reduce their welfare (Beard and McGregor, Citation2004; Danford et al., Citation2001; Lorenzon et al., Citation2000). Rapid environmental changes may cause stressful conditions for crustaceans, increasing the vulnerability to bacteria and reducing the immune response (Mercier et al., Citation2006). Jones (Citation2009) showed a significant correlation between fishing method and survival rate of lobsters during the stocking phase. The physiological response of lobsters to external stressors mobilizes energy substrates (Zhou et al., Citation2011). Hyperglycemia and release of lactate in the hemolymph are the typical crustaceans responses (Aparicio-Simon et al., Citation2010). Several studies have shown an increase in glucose concentrations in the hemolymph under stress conditions such as handling (Bergmann et al., Citation2001), changes in salinity (Spaargen and Haefiner, Citation1987), diseases and pollutants (Lorenzon et al., Citation2004), temperature changes during transportation (Lorenzon et al., Citation2007), and the confinement for long periods with other individuals (Ridgway et al., Citation2006). The release of crustacean hyperglycemic hormone (CHH) is the most important mediating neuroendocrine mechanism involved in the stress response of crustaceans and it is modulated by different neuromodulators such as catecholamines, dopamines, norepinephrines and epinephrines (Aparicio-Simon et al., Citation2010). Other variables involved in the stress response are the concentration of the total protein in the hemolymph (Jones, Citation2009; Lorenzon et al., Citation2007) and the total haemocyte count (THC) (Lorenzon et al., Citation2007). The aim of this study was to evaluate the effect of confinement for one month and feed deprivation on some properties of the hemolymph and muscle degradation pattern of American lobsters.
Materials and methods
Animals and experimental design
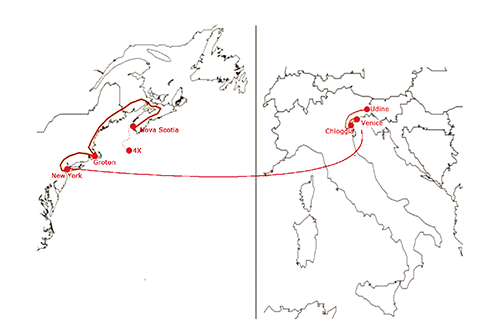
H. americanus specimens were caught in the North Atlantic waters, specifically in the area of fisheries 4X, off the coast of Nova Scotia (Canada), in the period between the 31st December 2012 and 10th February 2013 and exported by the Newell Lobster factory (Yarmouth, Canada) to Groton (CT, USA). Lobsters were dry transported by airplane to Italy, departing from the airport J.F. Kennedy, New York, in April 2013. After their arrival in Venice, they were transported to Chioggia to the CAM srl factory. On 16th April, lobsters were picked up and taken to Udine. The map of the route is shown in . The research study was performed in the aquaria of the Food Science Department, University of Udine, Italy for one month. Thirty-six adult Homarus americanus males (initial weight of approximately 530 g) were reared in four temperature controlled and recirculating aquaculture tanks (0.56 m2 each, 10% water volume replacement per day) using natural marine water. Aeration diffusers were provided within each tank. Lobsters were kept without food for four weeks. The stocking density of lobsters was of 16/m2 (similar to the average values used in Italy in the retail markets). Claws of lobsters were tied to avoid injuries and no shelters were provided in the tanks. During the experiment, water parameters (temperature, O2, pH, N-NH4, N-NO2, N-NO3, salinity) were analyzed (APHA, Citation1980) daily. A daily photoperiod of 12 h was maintained all throughout the experiment. Light was supplied by a 40 W white fluorescent tube (350 lux at the water surface). Tanks were covered on the top to reduce light intensity. At the arrival, all lobsters were acclimated in separate tanks for 48 h in the aquaria of the Food Science Department. Every week, all animals were weighed and nine lobsters (all animals of one tank) sacrificed.
Proximate analysis
From each animal about 50 g of muscle was sampled for proximate analysis (AOAC, Citation1995). The quantitative determination of crude protein, moisture and ash from the muscle was obtained after homogenization of the samples with an ultra turrax (Ika Laboratories, Staufen Germany). The content in total lipids was determined by extraction with chloroform: methanol (2:1 v/v) according to the Folch method (Folch et al., Citation1957).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis
Abdominal muscle samples were extracted for the protein degradation analysis in sodium dodecyl sulfate (SDS) solution containing 100 mM dithiotreitol. Extracts were analyzed using SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting with anti-actin antibody.
Determination of hemolymph parameters
Hemolymph samples were withdrawn from the pericardial sinus of each animal using a 2.5-mL syringe at the beginning of the experiment and every week. Samples were tested for glycemia, total protein content and THC. Each sample was centrifuged for 2 min at 5000 rpm and the supernatant was transferred and kept at -20°C until the analyses. Glucose content was measured from the supernatant using One touch® II meter (Lifescan, Milpitas, CA, USA) and a commercial kit test. The concentration of total protein was determined by means of the screen point method (Hospitex Diagnostics srl, Osmannoro-Sesto Fiorentino, Italy). The number of haemocytes were determined using 400 µL of hemolymph fixed with 1600 µL of 1% formalin in phosphate buffered saline and stored at +4°C.
Statistical analysis
All the statistical analysis were performed using the SPSS 14 package for windows. Water quality data were subjected to ANOVA and Duncan’s multiple range test was used to compare the means (SPSS, Citation2007). All the other growth and welfare parameters were analyzed by means of the Student’s test (SPSS, Citation2007). Levene’s test was used to assess the homogeneity of variances.
Results and discussion
Water quality analysis
Water quality parameters measured during the experiment are presented in . Results of water analysis showed no significant differences in temperature, pH, ammonia and nitrate [with the only exception of nitrite concentrations at the end of the third week: P<0.05; 16 df; standard error of variance (SE)=0.45] concentrations in the closed recirculating system.
Growth parameters
During the experiment, the total mean body weights of lobsters increased slightly compared to the initial values (). This result was probably due to the combined effects of water loss during the transportation phase (exposure to air) and water intake of animals during the confinement period. Although the change in live body weights was not statistically significant, the general trend was a slight constant increase of weights during the experiment.
Hemolymph parameters
Glucose, total protein concentrations and THC in the hemolymph of H. americanus during the experiment are shown in to 4, respectively. After one month of confinement, mean glucose, protein and THC levels in the hemolymph of H. americanus were similar (P>0.05) to the initial values. Although the overall mean differences of glucose and total protein concentrations were not significant at the statistical analysis, during the experiment the highest values were observed after two weeks, while the lowest value of THC after one week (P<0.01; 40 df; SE=268529; 1st week vs other treatments). This pattern seems to indicate a temporary situation of stress for animals, showing both the mobilization of energy (glucose) and total protein (presumably due to the synthesis of hemocyanin). The improvement of glucose concentrations in the 3rd and 4th week could indicate a lower organic load of the biological filter and/or that lobsters were no longer capable to deplete the glycogen stores. In the literature, some threshold values are reported indicating stress in crustaceans: glucose>15 mg/dL, total protein>6 g/dL, and THC<1500 mm2/10,000 (Lorenzon et al., Citation2004, Citation2007, Citation2008).
Gluconeogenesis, the metabolic process that allows the formation in the hepatopancreas of glucose, starts, in conditions of energy deficit, from non-carbohydrate precursors. Among these, the most important are amino acids, producing lactate via the anaerobic metabolism which is after converted into pyruvate. The latter is converted back into glucose by reversing the steps of glycolysis. The neo-synthesis of sugar is fundamental because, by this way, quick energy is provided in the form of ATP through the metabolic pathways of glycolysis and oxidative phosphorylation. The release of glucose from the reserve sites is coordinated by the neuroendocrine system, in particularly from the XO-SG complex located in the eyestalk (Govind, Citation1992). Among the hormones produced and released, CHH is responsible for the regulation of glucose concentration in the hemolymph (Fingerman, Citation1987; Webster, Citation1996; Chang et al., Citation1998; Lorenzon et al., Citation2004, Citation2007). In many species, it was observed that the first response to stress is the mobilization of glucose. The immune system of crustaceansis is mainly represented by haemocytes, cellular component of the hemolymph, divided in three types: hyaline cells, involved in phagocytosis, granular and semigranular hemocytes. Granules contain elements of the prophenolooxidase system, an enzymatic cascade system responsible for the production of melanin, involved in the mechanisms of defense (Söderhäll and Smith, Citation1986; Smith and Söderhäll, Citation1991). In the literature, the role of hemolymphatic total protein in crustaceans is well documented (Brouwer et al., Citation2004). This metabolite can therefore be used as indicator of the environmental stress (Brouwer et al., Citation2004). Many authors agree that the key component is the hemocianine (Hc), the respiratory pigment, representing 80 to 90% of the total protein in the hemolymph of crustaceans (Chen and Cheng, Citation1995; Watt et al., Citation1999; Chausson et al., Citation2004). Although the physiological stress induces also the mobilization of other proteins, including the metallothionein and the stress proteins (Cimino et al., Citation2002; Ravaux et al., Citation2007), Hcs are widely used for monitoring the welfare condition of crustaceans. Hemocianines are oligomeric proteins formed by the aggregation of subunits whose molecular weights are between 65 and 90 kDa. As pointed out by Dolashka-Angelova et al. (Citation2001), most of Hcs are glycoproteins, wherein the carbohydrate component varies from species to species. It has also been observed that the number of subunits constituting the Hcs has an interspecific variability (Giomi and Beltramini, Citation2007). With respect to this, Hodgson and Spicer (Citation2001), analyzing the electrophoretic pattern of hemolymph,found that Hcs of different species of decapods have a number of subunits between six and ten. The prolonged exposure of animals to stress conditions may determine the appearance of undesirable effects such as the tissue oxidation with effects on the quality of the final product (Hermes-Lima and Ginger-Savin, Citation2002). Several antioxidant enzymes such as the superoxide dismutase, catalase and glutathione-S-transferase have been identified as a possible key elements in crustacean defense systems (against the free radicals) (Halliwell and Gutteridge, Citation2007). These enzymes remove or transform the free radicals in less toxic metabolites (Halliwell and Gutteridge, Citation2007). When the production of antioxidants is not sufficient to contrast the production of free radicals, the result is the oxidative stress, i.e. the increase of oxidative damage at the cell level (Almeida et al., Citation2005). These damages are the oxidation of DNA, lipids and proteins that can alter the normal cellular functions (Halliwell and Gutteridge, Citation2007). In a study on crab Paralomis granulosa, the antioxidative enzyme activity was used as an indicator of the cell damage caused by different types of air exposure (Romero et al., Citation2007). In another study, Romero et al. (Citation2011) examined the antioxidant activity in the same crab Paralomis granulosa after the exposure to air for 6 h at 7°C and re-submersion in the water. The highest concentrations of antioxidant enzymes were detected in the gills after the water re-submersion. In the same research, an additional experiment was carried out to test whether the stress due to air exposure (for increasing times), affected the flavor of the final product. Despite observing some differences in the flavour of the meat, it was not possible to accurately distinguish the different treatments.
Proximate analysis
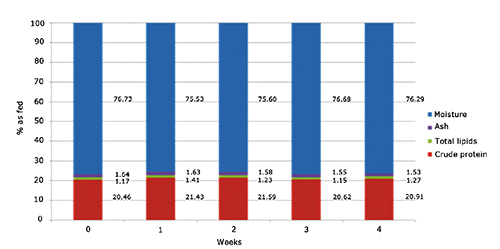
The proximate composition of muscle at the end of the experiment is reported in . Results of the proximate analysis showed no significant differences in the concentrations of crude protein (P>0.05; 40 df; SE=0.72), total lipids (P>0.05; 40 df; SE=0.01) and ash (P>0.05; 40 df; SE=0.01) in the muscle of H. americanus at the end of the experiment. Some studies reported a change in the chemical composition of the muscle tissue as a result of the different stocking conditions (Barrente et al., Citation2009; Fang et al., Citation1992). For instance, Fang et al. (Citation1992) observed a variation in the concentration and composition of free amino acids (FAA) in crustaceans according to the salinity of the water. The pool and the concentration of the FAA in the muscle may influence the quality and flavour of the final product (Wang et al., Citation2004; Zhou et al., Citation2011).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis
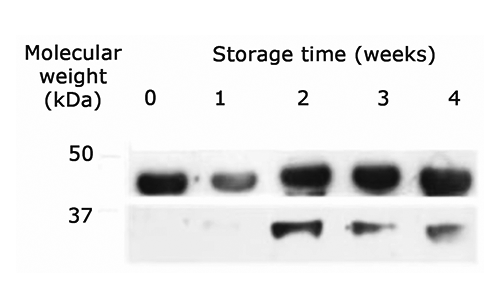
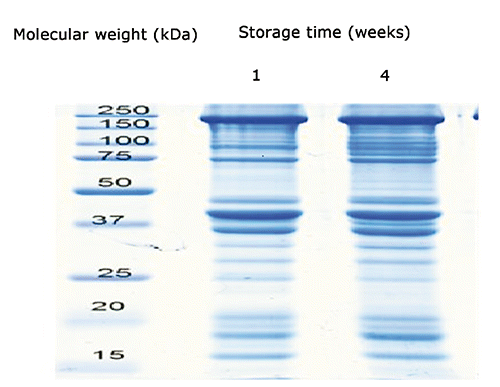
At the end of the experiment, the SDS-PAGE analysis revealed a marked degradation of the myofibrillar proteins. The degradation of the muscle protein was analysed through the identification of actin and myosin extracts and their possible products of proteolysis over time ( and ). A number of fragments, possibly from myosin, was evident in the range between 50 and 220 kDa between the time t0 and t28. A proteolytic product of 37 kDa, recognized by the anti-actin antibody, was also detected. The formation of new bands is probably due to the protein degradation under the fasting process. The Western Blot method against the C-terminal actin was also helpful in identifying those proteolytic fragments derived from the native actin. In vitro experiments have clearly shown that the myosin of lobsters is particularly susceptible to proteolytic attacks (Siemankowski et al., Citation1980). In the present experiment, after 28 days, it was evident a new band of 37 kDa, identified as a C-terminal fragment of the actin protein. These results suggest that, during the stocking phase, the muscle of lobsters undergoes degradation of both thick and thin filaments. Such degradation may be due to stress induced by fasting, which is well known to induce muscle degradation both in mammals and fish (Ilian and Forsberg, Citation1992; Preziosa et al., Citation2013). The muscle of lobsters contains different isoforms of calpain (Gornik et al., Citation2010) and ubiquitin/proteasome, which mediate the turnover of myofibrillar proteins. Salem et al. (Citation2005) observed an increase of mRNA of Capn1 and Capn2 after a fasting period of 35 days in rainbow trout. Other systems can also be activated by non-feeding conditions resulting in the degradation of the fibrillar proteins (Mykles, Citation1997).
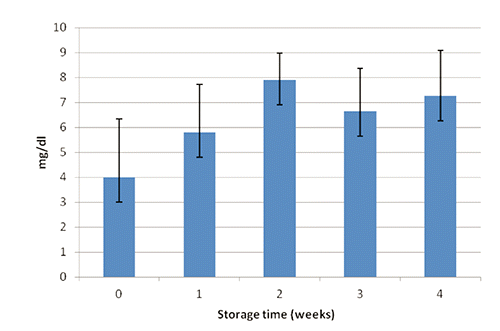
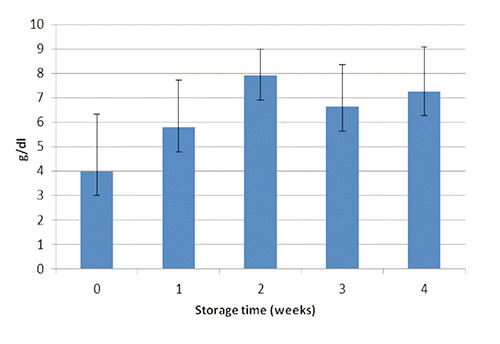
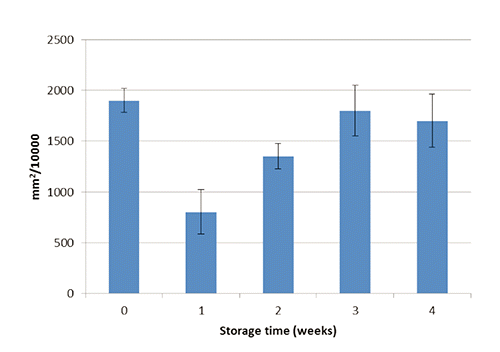
Table 1. Changes in water quality parameters during the trial.
Table 2. Variation of body weights of lobsters during the experiment compared to the initial value.
Conclusions
Crustaceans are able to display a wide range of adaptation strategies to survive and grow. The overall knowledge of these complex biological systems could be of importance in order to identify useful welfare indicators. In this study, some hemolymphatic variables and muscle degradation analysis (SDS-PAGE and Western blotting) resulted as sensitive indicators of stress in H. americanus. Furthermore, in the present research no statistical effects of starvation and storage of H. americanus were observed on body chemical composition. Further research work will be needed in the near future on several quality parameters related to freshness, such as sensory evaluations and k value. Starting from these results, several practical guidelines may be applied by retail operators in order to develop an optimal stock management system of H. americanus.
References
- Almeida E.A. Dias BainyA. C. Dafire A.L. Gomes O.F. Medeiros M.H.G. Di MascioP., 2005. Oxidative stress in digestive gland and gill of the brown mussel (Perna perna) exposed to air and re-submersed. J. Exp. Mar. Biol. Ecol. 318:21-30.
- AOAC, 1995. Official methods of analysis. 16th ed., Association of Official Analytical Chemists, Arlington, VA, USA.
- Aparicio-Simon B. Pinon M. Ricotta R. Ricotta I.S., 2010. Neuroendocrine and metabolic response of Pacific white leg shrimp Litopenaeus vannamei exposed to acute handling stress. Aquaculture 298:308-314.
- APHA, 1980. Standard methods for the examination of water and wastewater. 15th ed. American Public Health Association, Washington, DC, USA.
- Barrente S. Marques A. Teixeira B. Vaz-Pires R. Nunes M.L., 2009. Nutritional quality of the edible tissues of European lobster Homarus gammarus and American lobster Homarus americanus. J. Agr. Food Chem. 57:3645-3652.
- Beard T.W. McGregor D., 2004. Storage and care of live lobster. CEFAS, Lowestoft, UK.
- Bergmann M. Taylor A.C. Moore P.G., 2001. Physiological stress in decapod crustaceans Munida rugosa and Liocarcinus depurator discarded in the Clyde Nephrops fishery. J. Exp. Mar. Biol. Ecol. 259:215-229.
- Brouwer M. Larkin P. Brown-Peterson N. King C. Manning S. Denslow N., 2004. Effects of hypoxia on gene and protein expression in the blue crab, Callinectes sapidus. Mar. Environ. Res. 58:787-792.
- Candotti P., 2007. Sofferenza di aragoste ed astici vivi legate con chele e su letto di ghiaccio. Ministero della Salute ed., Roma, Italy.
- Chang E.S. Keller R. Chang S.A., 1998. Quantification of crustaceans hyperglycemic hormone by ELISA in haemolymph of the lobster Homarus americanus, following various stress. Gen. Comp. Endocr. 111:359-366.
- Chausson F. Sanglier S. Leize E. Hagège A. Bridges C.R. Sarradin P.M. Shillito B. Lallier F.H. Zal F., 2004. Respiratory adaptations to the deep-sea hydrothermal vent environment: the case of Segonzacia mesatlantica, a crab from the Mid-Atlantic Ridge. Micron 35:31-41.
- Chen J.C. Cheng S.Y., 1995. Hemolymph oxygen content, oxyhemocyanin, protein levels and ammonia excretion in the shrimp Penaeus monodon exposed to ambient nitrite. J. Comp. Physiol. B 164:530-535.
- Cimino E.J. Owens L. Bromage E. Anderson T.A., 2002. A newly developed ELISA showing the effect of environmental stress on levels of hso86 in Cherax quadricarinatus and Penaeus monodon. Comp. Biochem. Phys. A 132:591-598.
- Danford A.R. Uglow R.F. Garland J., 2001. Effect of long-haul international transport on lobster hemolymph constituents and nitrogen metabolism. pp 103-106 in Proc. 2nd Int. Conf. and Exhibit. on Marketing and Shipping Live Aquatic Products, Seattle, USA.
- Dolashka-Angelova P. Beltramini M. Dolashki A. Salvato B. Hristova R. Voelter W., 2001. Carbohydrate composition of Carcinus aestuarii Hemocyanin. Arch. Biochem. Biophys. 389:153-158.
- Fang L.S. Tang C.K. Lee D.L. Chen I.M., 1992. Free amino acid composition in muscle and hemolymph of the prawn Penaeus monodon in different salinities. Nippon Suisan Gakk. 58:1095-1102.
- Fingerman M., 1987. The endocrine mechanism of crustaceans. J. Crustacean Biol. 7:1-24.
- Folch J. Lees M. Sloane-Stanley G.H., 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 226:497-509.
- Giomi F. Beltramini M., 2007. The molecular heterogeneity of hemocyanin: its role in the adaptive plasticity of Crustacea. Gene 398:192-201.
- Gornik S.G. Westrop G.D. Coombs G.H. Neil D.M., 2010. Molecular cloning and localization of a calpain-like protease from the abdominal muscle of Norway lobster Nephrops norvegicus. Mol. Biol. Rep. 37:2009-2019.
- Govind C.K., 1992. Nervous system. In: HaarisonF.W. HumersA.G. ( eds.) Microscopic anatomy of invertebrates. Wiley Inc., New York, NY, USA, pp 395-438.
- Halliwell B. Gutteridge J.M.K., 2007. Free radicals in biology and medicine. 4th ed. Oxford University Press, Oxford, UK.
- Hermes-Lima M. Ginger-Savin T., 2002. Review: animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp. Biochem. Phys. C 133:537-556.
- Hodgson E. Spicer J.I., 2001. Subunit composition of crustacean haemocyanins are species-specific: evidence from non-decapod species. Comp. Biochem. Phys. A 128:873-888.
- Ilian M.A. Forsberg N.E., 1992. Gene expression of calpains and their specific endogenous inhibitor, calpastatin in skeletal muscle of fed and fasted rabbits. Biochem. J. 287:163-171.
- Jones C.M., 2009. Advances in the culture of lobsters. In: BurnellG. AllanG.L. ( eds.), New technologies in aquaculture: improving production efficiency, quality and environmental management. Woodhead Publ. and CRC Press, Cambridge, UK, pp 204-210.
- Lorenzon S. Edomi P. Giulianini P.G. Mettulio R. Ferrero E.A., 2004. Variation of crustacean Hyperglycemic hormone (CHH) level in the eyestalk and hemolymph of the shrimp Palaemon elegans following stress. J. Exp. Mar. Biol. Ecol. 207:4205-4213.
- Lorenzon S. Francese M. Ferrero E.A., 2000. Heavy metal toxicity and differential effects on the hyperglycemic stress response in the shrimp Palaemon elegans. Arch. Environ. Con. Tox. 39:167-176.
- Lorenzon S. Giulianini P.G. Libralato S. Martinis M. Ferrero E.A., 2008. Stress effect of two different transport systems on the physiological profiles of the crab Cancer pagurus. Aquaculture 278:156-163.
- Lorenzon S. Giulianini P.G. Martinis M. Ferrero E.A., 2007. Stress effect of different temperatures and air exposure during transport on physiological profiles in the American lobster Homarus americanus. Comp. Biochem. Phys. A 147:94-102.
- Mercier L. Palacios E. Campa-Cordova A.I. Tovar-Ramirez D. Hernandez-Herrera R. Ricotta I.S., 2006. Metabolic and immune responses in Pacific white leg shrimp Litopeneaus vannamei exposed to a repeated handling stress. Aquaculture 258:633-640.
- Mykles D.L., 1997. Crustacean muscle plasticity: molecular mechanisms determining mass and contractile properties. Comp. Biochem. Phys. B 117:367-378.
- Phillips B.F. Wahle R.A. Ward T.J., 2013. Lobsters as part of marine ecosystems. A review. In: PhillipsB.F. ( ed.) Lobsters: biology, management, aquaculture and fisheries. Wiley and Sons Ltd., Chichester, UK, pp 1-35.
- Preziosa E. Liu S. Terova G. Gao X. Liu H. Kucuktas H. Terhune I. Liu Z., 2013. Effect of nutrient restriction and re-feeding on calpain family genes in skeletal muscle of channel catfish (Ictalurus punctatus). PLoS One 8:e59404.
- Ravaux J. Toullec J.Y. Léger N. Lopez P. Gaill F. Shillito B., 2007. First hsp70 from two hydrothermal vent shrimps, Mirocaris fortunata and Rimicaris exoculata: characterization and sequence analysis. Gene 386:162-172.
- Ridgway I.D. Taylor A.C. Atkinson R.J.A. Stentiford G.D. Chang E.S. Chang S.A. Neil D.M., 2006. Morbidity and mortality in Norway lobster, Nephrops norvegicus: physiological, immunological and pathological effects of aerial exposure. J. Exp. Mar. Biol. Ecol. 328:251-265.
- Romero M.C. Ansaldo M. Lovrich G.A., 2007. Effect of aerial exposure on the antioxidant status in the sub-antarctic stone crab Paralomis granulosa (Decapoda: Anomura). Comp. Biochem. Phys. C 146:54-59.
- Romero M.C. Tapella R. Sotelano M.P. Ansaldo M. Lovrich G.A., 2011. Oxidative stress in the subantartic false king crab Paraiomis granulosa during air exposure and subsequent re-submersion. Aquaculture 319:205-210.
- Salem M. Nath J. Rexroad C.E. Killefer J. Vao J., 2005. Identification and molecular characterization of the rainbow trout calpains (capn1 and capn2): their expression in muscle wasting during starvation. Comp. Biochem. Phys. B 140:63-71.
- Siemankowski R.F. Zobel C.R. Manuel H., 1980. Comparative studies on the structure and aggregative properties of the myosin molecule. II. The substructure of the lobster myosin molecule. Biochim. Biophys. Acta 622:25-35.
- Smith V.J. Söderhäll K., 1991. A comparison of phenoloxidase activity in the blood of marine invertebrates. Dev. Comp. Immunol. 15:251-261.
- Söderhäll K. Smith V.J., 1986. Prophenoloxidase-activating cascade as a recognition and defense system in arthropods. In: GuptaA.P. ( ed.) Haemocytic and humoral immunity in arthropods. Wiley, NY, USA, pp 251-285.
- Spaargaren D.H. Haefiner P.A., 1987. The effect of environmental osmotic condition on blood glucose levels in the brown shrimp, Crangon crangon (L.). Comp. Biochem. Phys. A 87:1045-1050.
- SPSS, 2007. Statistics base user’s guide, ver. 17.0. SPSS Inc., Chicagom, IL, USA.
- Wang W.-N. Wang A.L. Bao L. WangJ.-P. Lui Y. Sun R.Y., 2004. Changes of protein-bound and free amino acids in the muscle of the freshwater prawn Macrobrachium nipponense in different salinities. Aquaculture 233:561-571.
- Watt A.J.S. Whiteley N.M. Taylor E.W., 1999. An in situ study of respiratory variables in three British sublittoral crabs with different routine rates of activity. J. Exp. Mar. Biol. Ecol. 239:1-21.
- Webster S.G., 1996. Measurement of crustacean hyperglycemic hormone levels in the edible crab cancer pagurus during emersion stress. J. Exp. Biol. 199:1579-1585.
- Zhou M. Wang A.L. Xian J.A., 2011. Variation of free amino acid and carbohydrate concentrations in white shrimp, Litopenaeus vannamei: effects of continuous cold stress. Aquaculture 3l7:182-185.