Abstract
The present study was conducted to determine the effect of a yeast cell wall extract (Bio-Mos) and palm kernel expeller (PKE) on the performance, nutrient digestibility, and ileal bacteria population of broiler chickens. A total of 60 1-d-old male broiler chicks (Cobb 500) were fed one of the 3 isonitrogenous and isocaloric diet including a control diet, or a control diet supplemented with 2 g/kg Bio-Mos (1-42 d), and for the third group, the control diet at 1-28 d following a diet containing 200 g/kg of an enzymatically-treated PKE at 29-42 d. The weight gains of birds fed the PKE containing diet (96.17 g/d) were less than other groups (109.10 and 104.42 g/d for the Bio-Mos and control diet, respectively) (P<0.05). Dietary inclusion of PKE increased bird’s feed intake (214.45 g/d) and feed conversion ratio (FCR) (2.23) than the Bio-Mos diet (194.87 and 1.79 g/d for feed intake and FCR, respectively) (P<0.05). The PKE diet had lower digestibility coefficients for dry matter (83.37%), ash and crude protein (78.63%) than the PKE free diets (P<0.05). As a ratio of the ileal total bacteria, there were no differences in the ileal population of Lactobacilli and Enterococcus genus or Enterobacteriaceae among the experimental groups (P>0.05), but the birds fed PKE or Bio-Mos containing diets had a lower population of Escherichia coli than the control group (P<0.05). The results showed that PKE potentially has a prebiotic property for chicken; however, a 200 g/kg dietary inclusion rate of PKE is not commercially recommendable because of its negative effects on the nutrients digestibility.
Introduction
A prebiotic compound is defined as a non-digestible food ingredient that can be utilised by intestinal microflora and have a beneficial effect on the host (Gibson and Roberfroid, Citation1995). The positive effects of mannose-based prebiotics, either mannan-oligosaccharides or mannose, on the immune system of chicken have been well established (Oyofo et al., Citation1989a; Lyons, Citation2002). The pure form (Oyofo et al., Citation1989a) or yeast cell wall-derived (Spring et al., Citation2000) mannose are capable of competitive attachment to the FimH lectin of gram-negative pathogens, resulting in a lower intestinal colonisation of these bacteria (Oyofo et al., Citation1989a, Citation1989b; Spring et al., Citation2000; Baurhoo et al., Citation2007). The commercial product Bio-Mos® (Alltech Inc., Nicholasville, KY, USA), a derivative of the outer cell wall of Saccharomyces cerevisiae, has been suggested to improve growth performance and immune system in broilers (Hooge, Citation2004; Rosen, Citation2007). Bio-Mos contain about 17% of α-mannan, which is possibly its bioactive component (Kwiatkowski and Kwiatkowski, Citation2012).
Many commonly used feeds may have some beneficial prebiotic effects which are independent of their nutritional value. Palm kernel expeller (PKE) is a fibrous byproduct of palm oil industry, which more commonly is used in ruminant nutrition. Cell wall components consists of more than 600 g/kg of PKE and its fibrous component is mainly composed of insoluble mannose-based polysaccharides (Alimon, Citation2004). Jaafar and Jarvis (Citation1992) showed that the cell wall consists of 580 g/kg β-mannan, 120 g/kg cellulose, and 40 g/kg xylan. β-mannan which is also known as β-galactomannan, is a viscose polysaccharide and the presence of large amounts of this compound in feed cause digestive organs hyperplasia, (Ikegami et al., Citation1990) and an increase in energy expenditure in the digestive system. It seems that enzymatic degradation of β-mannan in PKE produces more simple form carbohydrates, including mannan-oligosaccharides or mannose and increase its nutrients digestibility (Dingle, Citation1995).
In a preliminary experiment at the University Putra Malaysia, the authors used the dietary levels of 200 or 300 g/kg of PKE at both the grower and finisher or just the finisher diets of broiler chickens and the result showed that the 300 g/kg dietary PKE in particular when used at an earlier age, were lead to a poorer performance (unpublished data).
Despite the anti-nutritional effects listed for PKE, prebiotic properties have been also reported for the derivatives of β-mannan hydrolysis, and it seems that PKE has the potential to enhance the chicken immune system as does mannan derived from yeast cell wall (Allen et al., Citation1997). However, there is not enough data on the potential prebiotic effects of PKE in birds digestive tract. The present study was conducted to compare the effects of PKE at the maximum dietary allowance (200 g/kg), to Bio-Mos as a standard mannanoligisacharid supplement at the recommended level (2 g/kg), on the growth performance and ileal microbial populations in broiler chickens.
Materials and methods
High-performance liquid chromatography analysis of the experimental diets
The following high-performance liquid chromatography (HPLC) protocol was used to determine the main mannan-oligosaccharides concentration in the experimental diets. Oligosaccharides were assayed using 2690 Waters HPLC (Milford, MA, USA) and COS-MOSIL Sugar-D column (250×4.6 mm i.d., 5 m; Nacalai Tesque Inc., Kyoto, Japan). The mobile phase consisted of acetonitrile and water (at the ratio of 65:35) with a flow rate of 0.7 mL/min. Reflective index detector (Waters 2414; Waters) was used to detect oligosaccharides. The sample injection volume was 20 L and the running time was 45 min. Different concentrations of mannan-oligosaccharides (mannobiose, mannotriose, mannotetraose, mannopentaose, and mannohexaose; Megazyme, Wicklow, Ireland) were used as standards for calibration of the system.
Birds and diets
Animal care guidelines were used according to the European guide recommendations for animal use for experimental and other scientific purposes (European Commission, Citation2007). For the study, day old male broiler chicks (Cobb 500) were obtained commercially. A total of 60 chicks with body weight uniformity (42 g) were allocated to 15 cages (4 chicks each). The chicks were maintained at 23L:1D photo schedule. Diets and fresh water were provided ad libitum. For starter and grower periods (1 to 28 d), the chicks were fed on a standard commercial diet which was supplemented with 2 g/kg Bio-Mos for 5 cages. At 29 d of age, the experimental birds were fed one of the 3 isonitrogenous and isocaloric experimental finisher diets, including a control diet, a control diet supplemented with 2 g/kg Bio-Mos (for the previously Bio-Mos supplemented cages), and a diet containing 200 g/kg of an mannanasetreated PKE (the processed product were obtained from the company) (). All the diets were formulated according to the Cobb 500 guidelines. The quite low metabolisable energy (ME) content of the finisher diets (2990 kcal/kg) was due to the rather low ME content of PKE and the isonitrogenous nature of the experimental diets, so the nutrients requirements were multiplied by a 0.8 coefficient. Nevertheless, the ME/crude protein (CP) ratios were in line with the Cobb 500 guidelines suggestion. Celite® (Celite Corp., Santa Barbara, CA, USA) was added at 15 g/kg to all of finisher diets as an acid insoluble ash marker. At 28 and 42 d of age, the birds were weighed and feed consumption was recorded to determine the feed conversion ratios (FCRs) for finisher phase. Mortality was recorded and FCR was corrected for mortality.
Digestibility trial
At 42 d of age, 3 birds per pen were slaughtered and contents of the distal ileum (second half of the ileal content from the yolk stalk to the ileocecal junction) were gently expressed with double distilled water and collected and frozen. The digesta samples were dried at 60°C in an oven (Universal Oven UNP 200®; Memmert, Schwabach, Germany). Dry matter (DM), ether extracts (EE), and ash content in the digesta and diets were assayed according to AOAC (Citation1990). The acid insoluble ash (AIA) was determined according to Van Keulen and Young (Citation1977). The apparent digestibility of nutrients was calculated by using the following formula:
apparent digestibility of nutrients (%)=(nutrients/AIA)diet-(nutrients/AIA)digesta/(nutrients/AIA)diet×100
where, nutrients/AIA=the ratio of nutrients to insoluble ash.
Ileal bacteria quantification
The birds were slaughtered at the age of 42 d and ileal digesta were collected from 12 birds per treatment. Within each treatment, 10 g of the digesta from 3 birds were pooled and used for a total of 4 replicates per treatment for DNA extraction. Approximately 2 g of the pooled digesta was placed in a 2 mL microcentrifuge tube and stored at -20°C until DNA extraction.
DNA extraction
DNA was extracted from digesta samples and pure cultures using the QIAamp DNA Stool Mini Kit® (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s protocols. The DNA was then stored at -20°C for future use. The extracted DNA from pure cultures was used to produce a high concentration of the target DNA using conventional PCR and to prepare a standard curve. The PCR products were purified using the MEGAquick-spin® (Intron Biotechnology Inc., Sungnam, Korea), and the purity and concentration of DNA in each sample were measured using a Nanodrop ND-1000® spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA). The number of copies of a template DNA per mL of elution buffer was calculated using the Staroscik formula (Staroscik, Citation2012). Standard curves were prepared by serially diluting the PCR products from pure cultures of each bacterial group.
Quantitative real time polymerase chain reaction
The following primers (10 ng/mL concentration) were used to quantify different bacteria populations (First BASE Laboratories, Selangor, Malaysia): F-5 -CGGCAACGAGCGCAACCC-3 and R-5 -CCATTGTAGCACGTGTGTAGCC-3 (Denman and McSweeney, Citation2006) for total bacteria, F-5 -CATCCAGTGCAAACCTAAGAG-3 and R-5 -GATCCGCTTGCCTTCGCA-3 (Wang et al., Citation1996) for Lactobacilli, F-5 -GTGTGATATCTACCCGCTTCGC-3 and R-5 - AGAACGCTTTGTGGTTAATCAGGA-3 (Frahm and Obst, Citation2003) for Escherichia coli, F-5 - CCCTTATTGTTAGTTGCCATCATT-3 and R-5 - ACTCGTTGTACTTCCCATTGT-3 (Rinttila et al., Citation2004) for Enterococcus genus, F-5 - CATTGACGTTACCCGCAGAAGAAGC-3 and R-5 -CTCTACGAGACTCAAGCTTGC-3 (Bartosch et al., Citation2004) for Enterobacteriaceae family. Real-time PCR was performed with the BioRad CFX96 Touch® (BioRad, Hercules, CA, USA) using optical grade plates.
Five ng of digesta DNA were added to a 25 L PCR reaction in a SYBR green assay (iQTMSYBR Green Supermix®; BioRad). Each reaction included 12.5 L SYBR Green Supermix, 1 L of each primer, 1 L of each DNA sample, and 9.5 L H2O. The reaction conditions for amplification of DNA were 94°C for 5 min and then 40 cycles of 94°C for 20 s, 58°C (Lactobacillus) or 60°C (other bacteria) for 30 s and 72°C for 20 s. To confirm the specificity of amplification, a melting curve analysis was carried out after the last cycle of each amplification. The expected size of amplified fragments was 566 bp for Eubacteria, 341 bp for Lactobacillus group, 82 bp for Escherichia coli, 144 bp for Enterococcus genus, and 195 bp for Enterobacteriaceae family which were verified on a 2% (W/V) agarose gel for 40 min at 80 V.
Real-time PCR data for each bacteria quantity were obtained as follows: plasmid DNA of Methanobrevibacter ruminantium, obtained from cloning process using the TOPO TA cloning Kit® (Invitrogen, Carlsbad, CA, USA), was used to prepare the standard for total bacteria. To prepare the standards for other groups of bacteria, DNA extracts from pure cultures of Lactobacillus brevis, Escherichia coli, Enterococcus faecium, and Enterobacter cloacae were used. To calculate the amount of DNA in digesta samples, calibration standards constructed by amplifying known amounts of target DNA and were used to convert the Ct values into quantities of DNA. The Ct values for the calibration standards were regressed onto the log10 DNA, allowing a different equation for each run. The functions describing the relationship between Ct (threshold cycle) and x (log copy number) for the different assays were Ct=-0.327x+ 15.191; R2=0.97 for total bacteria; Ct=-0.266x+9.5329; R2=0.99 for Lactobacilli; Ct=-0.2991x+10.98; R2=0.99 for Escherichia coli; Ct=-0.2904x+ 10.354; R2=0.99 for Enterococcus genus and Ct=-0.2761x+10.784; R2=0.98 for Enterobac - teriaceae. The estimated values were expressed as a bacterial cell number per mL of the liquid phase of digesta. The standard curves presented a dynamic range of 5 to 6 orders of magnitude and a strong linear correlation (R20.98). The final data were calculated as a ratio of absolute quantities of each bacterial group to the total bacteria.
Statistical analysis
The performance data were analysed using 4 replicates (whole birds of each pen were the experimental unit for performance data), and 12 replicates (three selected birds per pen) were used for statistical analysis of nutrient digestibility data. Data were subjected to analysis of variance using the Anova procedure of SAS® (SAS, Citation2001), and significant differences among treatment means were determined at P<0.05 by Duncan’s Multiple Range Test (Duncan, Citation1955).
Results
Mannan-oligosaccharides content of the experimental diets
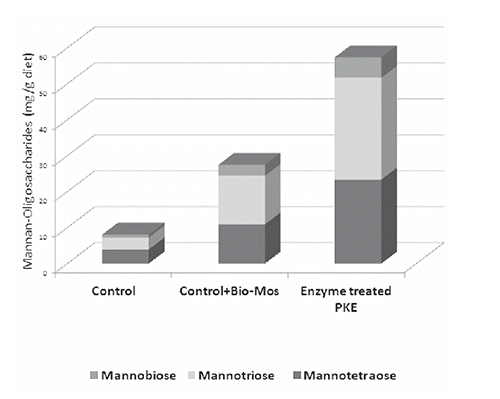
The mannan-oligosaccharides content of the experimental diets is presented in . The PKE containing experimental diets, showed the most mannan-oligosaccharides concentration and, as was expectable, the lowest mannan-oligosaccharides concentrations were observed in the control diet. Mannot riose constituting the major component, and mannotetraose and mannobiose at lower orders. Mannopentose and mannohexose were not detected in the experimental diet samples.
Performance traits
The effects of dietary Bio-Mos® and PKE on broiler performance are shown in . The weight gain of birds fed the PKE containing diet were less than other groups (P<0.05). Dietary inclusion of PKE increased bird’s feed intake and FCR than the Bio-Mos® supplemented diet (P<0.05). However, daily feed intake and FCR in the control group did not differ from those in other groups (P<0.05).
The digestibility coefficients
The PKE containing diet had lower digestibility coefficients for DM, ash and CP than the PKE free diets (P<0.05). The digestibility of EE fraction did not affect by the dietary treatments (P<0.05). The digestibility coefficients for DM, crude ash and CP were similar for the control and Bio-Mos® supplemented diets (; P<0.05).
Table 1. Composition of the experimental diets.
Table 2. Effect of palm kernel expeller and Bio-Mos® supplement on the performance trait of broiler chickens (29 to 42 days of age).
Table 3. Effect of palm kernel expeller and Bio-Mos® supplement on the digestibility coefficients for dry matter, crude ash, crude protein and ether extract in broiler chickens.
Table 4. Effect of palm kernel expeller and Bio-Mos® supplement on the bacterial populations of ileal digesta in broiler chickens.
Ileum bacteria population
The bacterial populations of ileal digesta as a ratio of the total bacteria population are shown in . There were no differences in the ileal population of Lactobacilli and Enterococcus genus or Enterobacteriaceae among the experimental groups. However, the birds fed PKE or Bio-Mos diets had a lower population of Escherichia Coli than the control group (P<0.05).
Discussion
The HPLC analysis of the experimental diets confirms that the mannan-oligosaccharides concentration of the diet containing 200 g/kg enzymatic treated PKE, was even more than the Bio-Mos supplemented diet. This can justify the previous reports on the health promoting effects of PKE in poultry (Allen et al., Citation1997). In this study, the Bio-Mos supplement was included in the experimental diets from the starter phase, since it is generally accepted that the dietary mannan-oligosaccharides more considerably change the intestinal metabolism at the early ages of bird’s life (Yang et al., Citation2009). However, as noted earlier, because of the fibrous nature of PKE, its inclusion in the chicken’s diets at the starter or grower phases could adversely affect bird’s performance. In line with the findings of the present study, the feed intake of birds fed a palm kernel meal based diet has been usually higher than those in control counterparts in the previous reports (Onwudike, Citation1986; Sundu et al., Citation2006), Furthermore, the harmful effects of PKE on the diet’s nutrient digestibility in this study have been reported by some authors (Panigrahi and Powell, Citation1991; Onifade and Babatunde, Citation1998; Sundu et al., Citation2006).
The contraction of the gizzard, proventriculus, and duodenum are totally coordinated and hard and fibrous feedstuffs may increase the contraction of the gizzard and speed up the peristaltic movement of digesta in either duodenum or whole small intestine (Duke, Citation1986). Broiler chickens are of a limited ability to digest dietary fibre, such as β-mannan, because of the absence of any mannan-degrading enzymes in the digestive tract of birds (Sundu et al., Citation2006). There are reports on the faster passage rate of food in the digestive tract of birds fed PKE-containing diets (Onifade and Babatunde, Citation1998). The higher passage rate is in line with the lower nutrients digestibility coefficients observed for PKE containing diet in the current study.
The PKE used in this study was an enzymatically-treated type and the β-mannan fraction was considerably degraded to mannanoligosaccharides which lack the fibrous characteristics. However, the PKE sample used in this study was contained about 7% shell and it seems that the gritty lignified shell of PKE may contribute to an increased passage rate of the digesta increased feed intake and lower nutrient digestibility in the digestive tract.
The low digestibility of PKE, associated with a higher intake of the PKE containing diet, brings about a considerable higher FCR for PKE fed birds in this study. The lower performance of the broiler chickens fed the PKE containing diet can be also attributed to its chemical analysis. The crude fibre of PKE, ranging from 160 to 180 g/kg, and the metabolisable energy of PKE for poultry is rather low (1500-1800 kcal/kg; Alimon, Citation2004). Nwokolo et al. (Citation1976) and Onwudike (Citation1986) showed that the average availability of amino acids in PKE was 85% which was lower than that in most oilseed meals.
The effect of palm kernel meal to change the population of intestinal bacteria has been reported by Fernandez et al. (Citation2002). Lactobacilli are common inhabitants of the crop and ileum in broiler chickens, whereas obligate anaerobes predominate in the cecum (Gong et al., Citation2002; Guan et al., Citation2003; Lu et al., Citation2003).
Muthusamy et al. (Citation2011) used enzymatically hydrolysed whole Saccharomyces cerevisiae yeast and the pellets of yeast cell wall in the diets of broiler chickens and found a higher Lactobacillus population in the duodenal and jejunal digesta as compared with the control. However, there is an inconsistency in the effects of mannan-oligosaccharides on intestinal lactobacilli population in broilers (Spring et al., Citation2000; Fernandez et al., Citation2002; Biggs et al., Citation2007) and turkeys (Fairchild et al., Citation2001; Sims et al., Citation2004). Some reports indicate that Lactobacillus may improve broiler performance (Jin et al., Citation1996). The family of Enterobacteriaceae includes many genera and strains colonising the small and large intestine, and includes members of the non-pathogenic autochthonous (commensal) microbiota as well as pathogens, especially Escherichia coli. Most Enterococcus species are mainly opportunistic pathogens. In poultry, enterococci are frequently isolated from dead-in-shell and 1-day-old chicks, often affecting the yolk sac (Cortes et al., Citation2004; Deeming, Citation2005).
In the present study the ileal Lactobacilli, Enterococcus or Enterobacteriaceae family populations was not influenced by the diets rich in mannan-oligosaccharides (additive Bio-Mos or PKE). However, there were comparable reducing effects of the Bio-Mos supplemented diet and the diet containing enzymatically-treated PKE on the intestinal Escherichia coli population. Escherichia coli is the principal pathogenic organism implicated in cellulitis, the major cause of carcass condemnation at the processing of plants (Messier et al., Citation1993). Mannan-oligosaccharides have been reported to have receptor sites for the fimbriae of Escherichia coli which results in an elimination of these particular bacteria as the digesta flows out (Yang et al., Citation2009). Then, inclusion of PKE in broiler chickens’ diets represents a potential nutritional strategy that can control intestinal colonisation of some pathogenic bacteria, thereby conferring intestinal health benefits to the host.
Conclusions
The withdrawal of growth promoting antibiotics from the broiler chicken feed needs the industry to find different alternatives to these products. The PKE sample used in this study can exert some effects comparable to those of prebiotics. However, it seems that dietary PKE can even affect the performance negatively, so that a 200 g/kg dietary inclusion level of PKE is not commercially recommendable. Moreover, the effects of the PKE on bird’s intestinal bacteria populations and digestion efficiency are often conflicting and further work needs to find a balance between the prebiotic properties and the quite low nutritive value of PKE in broiler chickens diet formulation.
Acknowledgments
this study was supported by the Ministry of Education Malaysia, under the Long Term Research Grant (LRGS 2/2012).
References
- AlimonA.R., 2004. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev. 40:12-14.
- AllenV.M.Fernandez F.Hinton M.H., 1997. Evaluation of the influence of supplementing the diet with mannose or palm kernel meal on salmonella colonization in poultry. Brit. Poultry Sci. 38:485-488.
- AOAC, 1990. Official method of analysis. 15th ed., Association of Official Analytical Chemists, Washington, DC, USA.
- BartoschS.Fite A.Macfarlane G.T.McMurdo M.E., 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microb. 70:3575-3581.
- BaurhooB.Letellier A.Zhao X.Ruiz-Feria C.A., 2007. Cecal populations of lactobacilli and bifidobacteria and Escherichia coli populations after in vivo Escherichia coli challenge in birds fed diets with purified lignin or mannanoligosaccharides. Poultry Sci. 86:2509-2516.
- BiggsP.Parsons C.M.Fahey G.C., 2007. Effects of several oligosaccharides on growth performance, nutrient digestibilities, and caecal microbial populations in young chicks. Poultry Sci. 86:2327-2336.
- CortesC.R.Tellez IsalasG.Lopez CuelloC.Villaseca-Flores J.M.Anderson R.C.Eslavacampos C., 2004. Bacterial isolation rate from fertile eggs, hatching eggs, and neonatal broilers with yolk sac infection. Rev. Latinoam. Microbiol. 46:12-16.
- Deeming D.C., 2005. Yolk sac, body dimensions and hatchling quality of ducklings, chicks and poults. Brit. Poultry Sci. 46:560-564.
- Denman S.E.McSweeney C.S., 2006. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol. Ecol. 58:572-582.
- Dingle J.G., 1995. The use of enzymes for better performance of poultry. pp 1-9 in 6th Proc. Queensland Poultry Science Symp., Gatton Australia.
- DukeG.E., 1986. Alimentary canal: anatomy, regulation, of feeding, and motility. In: Sturkie P.D. (ed.) Avian physiology. Pringer-Verlag, New York, NY, USA, pp 269-288.
- DuncanD.B., 1955. Multiple range and multiple F tests. Biometrics 11:1-42.
- European Commission, 2007. Commission recommendation on guidelines for the accommodation and care of animals used for experimental and other scientific purposes, 2007/526/EC. In: Official Journal, L 197/1, 30/07/2007.
- FairchildA.S.Grimes J.L.Jones F.T.Wineland M.J.Edens F.W.Sefton A.E., 2001. Effects of hen age, Bio-Mos and flavomycin, on poult susceptibility to oral Escherichia coli challenge. Poultry Sci. 80:562-571.
- FernandezF.Hintaon M.Van GilsB. 2002. Dietary mannan oligosaccharides and their effect on chicken caecal microflora in relation to salmonella enteritidis colonization. Avian Pathol. 31:49-58.
- FrahmE.Obst U., 2003. Application of the fluorogenic probe technique (TaqMan PCR) to the detection of Enterococcus spp. and Escherichia coli in water samples. J. Microbiol. Meth. 52:123-131.
- GibsonG.R.Roberfroid M., 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412.
- GongJ.Forster R.J.Yu H.Chambers J.R.Sabour P.M.Wheatcroft R.Chen S., 2002. Diversity and phylogenetic analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 41:171-179.
- GuanL.L.Hagen K.E.Tannock G.W.Korver D.R.Fasenko G.M.Allison G.E., 2003. Detection and identification of Lactobacillus species in crops of broilers of different ages by using PCR-denaturing gradient gel electrophoresis and amplified ribosomal DNA restriction analysis. Appl. Environ. Microb. 69:6750-6757.
- HoogeD.M., 2004. Meta-analysis of broiler chicken pen trials-evaluating dietary mannan oligosaccharide, 1993-2003. Int. J. Poult. Sci. 3:163-174.
- IkegamiS.Tsuchihashi F.Harada H.Tsuchihashi N.Nishide E.Innami S., 1990. Effect of viscous indigestible polysaccharides on pancreatic-biliary secretion and digestive organs in rats. J. Nutr. 120:353-360.
- JaafarM.D.Jarvis M.C., 1992. Mannans of oil palm kernels. Phytochemistry 31:463-464.
- JinL.Z.Ho Y.W.Abdullah N.Ali M.A.Jalaludin S., 1996. Influence of dried Bacillus subtilis and Lactobacilli cultures on intestinal microflora and performance in broilers. Asian-Austral. J. Anim. 9:397-403.
- KwiatkowskiS.Kwiatkowski S.E., 2012. Yeast (Saccharomyces cerevisiae) glucan polysaccharides: occurrence, separation and application in food, feed and health industries. In: Karunaratne D.N. (ed.) The complex world of polysaccharides. Tech Publ., Rijeka Croatia, pp 47-70.
- LuJ.Idris U.Harmon B.Hofacre C.Maurer J.J.Lee M.D., 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microb. 69:6816-6824.
- LyonsT.P., 2002. Navigating from niche markets to mainstream: a feed industry Kakumei. pp 1-16 in Proc. Alltech’s 16th Annual Asia Pacific Lecture Tour, Beijing, China.
- MessierS.Quessy S.Robinson Y.Devriese L.A.Hommez J.Fairbrother J.M., 1993. Focal dermatitis and cellulitis in broiler chickens: bacteriological and pathological findings. Avian Dis. 37:839-844.
- MuthusamyN.Haldar S.Ghosh T.K.Bedford M.R., 2011. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. Brit. Poultry Sci. 52:694-703.
- NwokoloE.N.Bragg D.B.Saben H.S., 1976. The availability of amino acids from palm kernel, soybean, cotton seed and rape seed meal for the growing chick. Poultry Sci. 55:2300-2304.
- OnifadeA.A.Babatunde G.M., 1998. Comparison of the utilization of palm kernel meal, brewers dried grains and maize offal by broiler chicks. Brit. Poultry Sci. 39:245-250.
- OnwudikeO.C., 1986. Palm kernel as a feed for poultry. 2. Diets containing palm kernel meal for starter and grower pullets. Anim. Feed Sci. Technol. 16:187-194.
- OyofoB.A.Deloach J.R.Corrier D.E.Norman J.O.Ziprin R.L.Mollenhauer H.H., 1989a. Prevention of Salmonella typhimurium colonization of broilers with D-mannose. Poultry Sci. 68:1357-1360.
- OyofoB.A.Droleskey R.E.Norman J.O.Mollenhauer H.H.Ziprin R.L.Corrier D.E.Deloach J.R., 1989b. Inhibition by mannose of in vitro colonization of chicken small intestine by Salmonella typhimurium. Poultry Sci. 68:1351-1356.
- PanigrahiS.Powell C.J., 1991. Effects of high inclusion of Palm kernel meal in broiler chick diets. Anim. Feed Sci. Technol. 34:37-47.
- RinttilaT.Kassinen A.Malinen E.Krogius L.Palva A., 2004. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 97:1166-1177.
- RosenG.D., 2007. Holo-analysis of the efficacy of Bio-Mos in broiler nutrition. Brit. Poultry Sci. 48:21-26.
- SAS, 2001. User’s guide: statistics, version 9.2. SAS Inst. Inc., Cary NC, USA.
- SimsM.D.Dawson K.A.Newman K.E.Spring P.Hooge D.M., 2004. Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poultry Sci. 83:1148-1154.
- SpringP.Wenk C.Dawson K.A.Newman K.E., 2000. The effects of dietary mannan oligosaccharide on caecal parameters and the concentration of enteric bacteria in the caeca of salmonella challenged broiler chicks. Poultry Sci. 79:205-211.
- StaroscikA., 2012. Calculator for determining the number of copies of a template. Available from: http://cels.uri.edu/gsc/cndna.html
- SunduB.Kumr A.Dingle J., 2006. Palm kernel meal in broiler diets: effect on chicken performance and health. World. Poultry Sci. J. 62:316-325.
- Van Keulen J.Young B.A., 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44:282-287.
- WangR.F.Cao W.W.Cerniglia C.E., 1996. PCR detection and quantitation of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microb. 62:1242-1247.
- YangY.Iji P.A.Choct M., 2009. Dietary modulation of gut microflora in broiler chickens: a review of the role of six kinds of alternatives to in-feed antibiotics. World. Poultry Sci. J. 65:97-114.